Abstract
Wideband acoustic transfer function (ATF) measurements of energy reflectance (ER) and admittance magnitude (∣Y∣) were obtained at varying static ear-canal pressures in 4-, 12-, and 27-week-old infants and young adults. Developmental changes in wideband ATF measurements varied as a function of frequency. For frequencies from 0.25 to 0.75 kHz there was as much as a 30% change in mean ER and ∣Y∣ with changes in static ear-canal pressure between 4 and 24 weeks of age. From 0.75 to 2 kHz, the effects of pressure produced a small number of significant differences in ER and ∣Y∣ with age, suggestive of a developmentally stable frequency range. Between 2 and 6 kHz, there were differential effects of pressure for the youngest infants; negative pressures caused increased ER and ∣Y∣ and positive pressures caused decreased ER and ∣Y∣; the magnitude of this effect decreased with age. Findings from this study demonstrate developmental differences in wideband tympanometric ATF measurements in 4-, 12- and 24-week-old infants and provide additional insight on the effects of static ear-canal pressure in the young infant’s ear. The maturational effects shown in the experimental data are discussed in light of known age-related anatomical changes in the developing outer and middle ear.
INTRODUCTION
Acoustic ear-canal measurements are dependent on the physical properties of the external and middle ear, properties that undergo developmental changes in human infants over the first several months of life. Some of these changes include (1) an overall increase in the size of the ear canal and middle-ear space, (2) a decrease in the length of the cartilaginous portion of the ear-canal wall due to growth of the bony portion of the canal wall, (3) changes in ossicular bone density due to loss of residual mesenchyme and ossification, and (4) changes in the orientation of the tympanic membrane and ossicular chain (Ikui et al., 1997; Ruah et al., 1991; Saunders et al., 1983). These anatomical changes in infant ears are thought to contribute to conventional single-frequency tympanometry patterns that are not adultlike over the first 6 months of life (Holte et al., 1991; Hunter and Margolis, 1992; Paradise et al., 1976). The general conclusion from these developmental studies is that the overall impedance characteristics of infants’ ears at low frequencies are dominated more by the effects of mass, in contrast to the middle-ear system in children and adults which is dominated by the effects of stiffness (Holte et al., 1991; Meyer et al., 1997; Roush et al., 1995). However, the extent to which specific developmental changes in the infant external and middle ears contribute to specific variations in acoustic middle-ear measurements is not fully understood. A multifrequency approach to middle-ear assessment would be ideal for infants in the neonatal to 7 month age range in light of the shifting maturational gradient of acoustic ear-canal-response properties (Feeney and Sanford, 2005; Holte et al., 1991; Keefe et al., 1993; Meyer et al., 1997).
From both research and clinical perspectives, it is important to distinguish between variations in middle-ear measurements attributable to developmental effects and those attributable to dysfunction or pathology. Furthermore, understanding how developmental changes influence acoustic response properties of the infant ear have important implications for interpretation of middle-ear assessment measures as well as behavioral and physiologic measures of infant hearing (Holte et al., 1991; Keefe et al., 1993; Roush et al., 1995; Holmer, 2001n1). While more recent studies report normative data for 1 kHz probe-tone tympanometry for infants younger than 7 months of age (Alaerts et al., 2007; Baldwin, 2006; Calandruccio et al., 2006; Kei et al., 2003; Margolis et al., 2003), a complete definition of what constitutes a normal or disordered infant middle ear is currently lacking.
Wideband acoustic transfer function (ATF) measurements, such as energy reflectance (ER) and acoustic admittance, are emerging as attractive alternatives to traditional admittance tympanometric measurements (Feeney et al., 2003; Keefe et al., 2000; Keefe et al., 1993; Keefe et al., 2003; Piskorski et al., 1999; Voss and Allen, 1994). ER is the ratio of sound power reflected from the middle ear to the incident sound power presented by a probe stimulus in the ear canal. ER at ambient ear-canal pressure has been used to examine normal and disordered middle-ear function (Allen et al., 2005; Feeney et al., 2003), the acoustic stapedial reflex (Feeney and Keefe, 1999; Feeney and Keefe, 2001; Feeney et al., 2004), neonatal middle-ear function (Keefe et al., 1993; Keefe et al., 2000), and middle-ear development (Keefe et al., 1993; Keefe and Levi, 1996; Keefe and Abdala, 2007). Specifically, Keefe et al. (1993) investigated the effects of middle-ear maturation on wideband ATF measurements in adults and in five groups of infants and children (1, 3, 6, 12, and 24 months of age). Age-related variations in ER over a frequency range of 0.125–10.7 kHz were reported by Keefe et al. (1993), with ER patterns changing most significantly between 1 and 6 months of age. The greatest changes in ER occurred at frequencies below 0.5 kHz where ER increased by up to 30% between 1 and 6 months of age. Keefe et al. (1993) concluded that significant resonant amplification of ear-canal wall motion occurred for lower frequencies in young infants and that these effects may account for variability encountered with 226 Hz admittance tympanograms in infants.
Additional studies have expanded the use of wideband ambient pressure ATF measurements to include measurements made over a range of static ear-canal pressures (ATF tympanometry) (Keefe and Levi, 1996; Keefe and Simmons, 2003; Margolis et al., 1999). Margolis et al. (1999) obtained conventional multi-frequency-admittance tympanometry and wideband ATF tympanometry results from 20 adults. At frequencies above 2 kHz, multifrequency admittance tympanograms did not follow an orderly pattern, unlike ER tympanograms, which progressed in an orderly fashion as a function of frequency from 0.25 through 11.3 kHz. The authors suggested that this orderly behavior might prove useful for the assessment of pathologic ears. Of particular clinical interest were data presented by Margolis et al. (1999) from one 10-year-old boy with recurrent otitis media. The boy presented with a unilateral 35 dB conductive hearing loss, negative tympanometric peak pressure (TPP) (−250 daPa), and normal 226 Hz static acoustic admittance (SAA). Otoscopy revealed a retracted tympanic membrane, but no evidence of middle-ear effusion. ATF measures at ambient pressure resembled ER typically observed in normal ears under pressurized conditions. However, when the ear canal was pressurized to compensate for the negative middle-ear pressure, an abnormal ER pattern was obtained, indicating the possibility of an additional middle-ear disorder, concurrent with negative middle-ear pressure. Margolis et al. (1999) concluded that due to its apparent sensitivity to middle-ear disorders, ER tympanometry could be a useful tool for clinical middle-ear assessment.
Keefe and Simmons (2003) investigated the test performance of 226 Hz tympanometry, energy transmittance (which they defined as 1 minus ER) at ambient ear-canal pressure, and energy transmittance at static ear-canal pressures as predictors of conductive hearing loss in children 10 years of age and older. Data were obtained from 42 normal-functioning ears and 18 ears with conductive hearing loss. For a fixed specificity of 0.90, the sensitivity of these measures to detect conductive hearing loss was 0.28 for SAA (226 Hz), 0.72 for ambient pressure transmittance, and 0.94 for pressurized transmittance (ATF tympanometry), suggesting that wideband ATF tympanometry may be a useful diagnostic tool.
Based on these results which demonstrate high sensitivity of wideband ATF measurements to changes in middle-ear characteristics due to middle-ear pathology, it is hypothesized that maturational effects of the infant ear canal and middle ear may be better characterized using wideband ATF measurements. Investigation of these responses in young infants is of interest in light of the need for better understanding of infant middle-ear development and for a valid test of middle-ear function for young infants. However, to date, no published studies have described young infant middle-ear characteristics using wideband ATF responses under pressurized ear-canal conditions. The current study was designed to further investigate developmental changes in acoustically based middle-ear measurements in infants and to characterize wideband ATF tympanometry results in infants of 4, 12 and 24 weeks of age.
METHOD
Subjects
One hundred and one infants were recruited for the present study. Cross-sectional data are reported for 60 infants (one ear from each infant), with 20 infants in each age group (4, 12, and, 24 weeks). Mean age and standard deviation (SD) and gender distribution (F=female, M=male) for each age group are as follows: 4 weeks: 4.5 (SD=0.34) weeks, 8F and 12M; 12 weeks: 12.0 (SD=0.34) weeks, 6F and 14M; and 24 weeks: 24.1 (SD=0.31) weeks, 14F and 6M. Infant participant selection criteria included (1) full term birth (40 week gestation, ±2 weeks), (2) birth history free of complications, (3) good health on the test date, (4) no risk factors for hearing loss, (5) no history of otitis media, and (6) clear entrance to the ear canals assessed by visual examination. An approximately equal number of left (L) and right (R) ears were included for each age group (4 weeks: 9 R, 11 L; 12 weeks, 9 R, 11 L, and 24 weeks: 12 R and 8 L. Assignment of right and left ears for data analysis was counterbalanced, alternating with each subject. Because data were not obtained from all ears of every infant, proportions of left and right ears were not equal. Infants were recruited with the assistance of a database maintained at the University of Washington and funded through a grant from the National Institutes of Health.
Screening of infants for inclusion in the study consisted of distortion product otoacoustic emissions (DPOAEs) and 1 kHz admittance tympanometry. DPOAE (2f1-f2) levels for f2 frequencies of 2, 3, and 4 kHz needed to meet criteria of ⩾6 dB signal-to-noise ratio and an absolute level of −10 dB sound pressure level (SPL) or higher. At the time data were collected for the present study, two published studies reported normative data for infants using 1 kHz tympanometry (Margolis et al., 2003; Kei et al., 2003). Based on the 10th–90th percentiles for positive tail peak-compensated SAA reported by these studies, a SAA criterion of ⩾0.3 mmho was used as an infant inclusion criterion. Data from 41 infants were not included in the final analysis due to failure to obtain a complete data set. Reasons for not obtaining a complete data set included cooperation issues such as fussiness or excessive movement (27 infants), failure to meet tympanometric criteria (7 infants), failure to pass DPOAE screening (3 infants), or experimenter error in data acquisition (4 infants). A complete data set was obtained in as short as 20 min if the infant was cooperative. However, additional time was often required for the infant to go to sleep, be fed, or become settled. It was not required that the infant be asleep for testing, and many infants were tested while awake.
Twenty-one adult participants were recruited using flyers posted at various locations on the University of Washington campus. The final adult data set included a total of 20 adults (15 female, 5 male) with a mean age of 24.6 (SD=2.9) years. Adult participation selection criteria included (1) good general health, (2) a negative history of chronic middle-ear disease, (3) 226 Hz tympanometric criteria within normal limits (Margolis and Heller, 1987), (4) hearing within normal limits (air conduction thresholds ⩽15 dBHL and air bone gap ⩽10 dB), and (5) having ear canals free of wax and tympanic membranes free of scarring as assessed using otoscopy. One subject was excluded due to history of a middle-ear disorder, which was revealed after testing was completed. All screening and experimental measurements were obtained from subjects while seated in a double-walled sound treated booth.
Instrumentation and signal processing
Experimental measurements were made using a Welch Allyn prototype diagnostic middle ear (DME) analyzer system. A similar PC-based prototype system had been used for conducting ATF measurements in adults and children (Keefe and Simmons, 2003). The PC housed a multichannel digital signal processor, with one channel providing the wideband probe signal output and the other channel digitizing the response from the probe microphone. A second output channel from the DME system was connected to a modified Grason Stadler immittance instrument (GSI-33) enabling the static ear-canal pressure to be controlled via the DME system.
The DME probe consisted of a custom made receiver∕microphone assembly leading to a probe tip with three separate ports for receiver output, microphone input, and pressure adjustments. Modified (shortened length) GSI tympanometry ear tips were placed on the end of the probe tip to allow for a pressure seal when placed in the participant’s ear canal.
The broadband stimuli were output from a digital-to-analog converter at a 24 kHz sampling rate. The broadband acoustic chirps used for infant measurements had a duration of 40 ms with a frequency bandwidth extending from 0.25 to 8 kHz. The stimuli used with adults were acoustic clicks with bandwidths of 0.25–8 kHz. The broadband click stimuli were presented at a rate of 11.7 per second. The output levels of the clicks and chirps were 57 and 60 dB SPL, respectively, as measured in a 2 cm3 coupler. The differences in stimuli used for infants and adults were based on conventions of the DME prototype (separate probe calibration procedures existed for adults and infants using click and chirp stimuli, respectively). Differences in ER measurements arising from using different stimuli were not expected as long as adequate calibrations were obtained (see Sec. 2C for details). The microphone signal was digitized at a 24 kHz sampling rate using an analog-to-digital converter, high-pass filtered to eliminate low-frequency noise below 0.25 kHz and stored for data analysis. The software incorporated a noise-rejection algorithm, such that only ear-canal-response levels that were at or below a specific criterion were included in the signal averaging. A time-averaged-waveform response (an average of eight stimuli) for each individual pressure condition was obtained and stored for analyses.
Calibration procedure and reflectance calculations
The ER and admittance measurements were made based on a calibration procedure in which the Thevenin source impedance and source pressure of the DME probe were calculated. For a detailed review of the calibration procedure theory for ATF measurements see Keefe and Simmons (2003). Calibration procedures were performed only at ambient pressure. This decision was based on previous work that confirmed that only slight changes in calibration results occurred under pressurized conditions (Keefe and Simmons, 2003). The daily calibration procedure for the DME probe involved calculating the Thevenin source impedance and pressure by modeling the acoustic wave propagation inside two rigid-walled cylindrical calibration tubes. Each tube was open at one end and closed at the other end with a steel plug. During calibration measurements, the DME probe was coupled to a given tube using a modified GSI rubber ear tip. With the probe placed in the tube opening, the broadband stimuli were presented, and the response was fitted to a model of acoustic wave propagation for each smooth-walled cylindrical tube. This method estimated the Thevenin equivalent parameters of the probe system. The DME software executed all calculations involved in the calibration procedure. Sets of “small” and “large” calibration tubes were used to obtain Thevenin equivalent parameters for infant and adult wideband ATF measurements, respectively. The infant calibration tubes had lengths of 236.7 and 61 mm, each with a diameter of 4.8 mm, which is a close approximation to ear-canal diameter in infants. The adult calibration tubes had lengths of 296.2 and 84 mm, each with a diameter of 7.9 mm, which is a close approximation to ear-canal diameter in adults. To assess the validity of the calibration procedure, a software-implemented root mean squared (rms) reflectance error function was generated to determine the error in the acoustical estimate of the length of each cylindrical tube relative to the acoustic wave propagation model. A rms reflectance error of ⩽0.01 was required for a successful calibration. A successful calibration measurement was completed within 2–3 min.
The DME software used the source impedance and pressure from the calibration procedure and subsequent ear-canal measurements to calculate the pressure reflection coefficient (Rp). Rp was calculated as
| (1) |
where Z is the impedance measured in the ear canal and Zc is the characteristic impedance of the ear canal (Keefe et al., 1992). The characteristic impedance of the ear canal is needed to calculate Rp and is equal to
| (2) |
where ρ (rho) is the density of air, c is the phase velocity of sound in air, and S is the area of the ear canal. Since an actual area measurement of the ear canal was not practical, the DME system utilized an estimate of ear-canal area equal to that of each calibration tube to calculate Zc. The squared magnitude of the pressure reflectance,
| (3) |
was used to calculate ER.
Wideband tympanometry measurements
External-ear-canal pressure changes were accomplished using the GSI-33 pressure pump, controlled by the DME system. Measurements with the DME system were made at static pressures of −200, −100, −50, −25, 0, +25, +50, +100, and +200 daPa relative to TPP values obtained during screening measurements with 226 Hz or 1 kHz tympanometry for adults and infants, respectively. For instance, if a participant presented with a TPP of −10 daPa, the extreme static pressures were set to −210 and +190 daPa, with interim static pressures adjusted accordingly. An ambient pressure ATF measurement was obtained before making the static pressure measurements. Following placement of the ear tip and during the initial ambient ATF test, the DME system performed an acoustic leak check. An acoustic leak was determined by an estimation of an appropriate ear-canal volume. If a leak was detected, which was usually the result of an inadequate probe fit in the ear, the experimenter was prompted by the DME software to check the probe fit and run the test again. When an appropriate fit was obtained, all ambient and static pressure conditions were then run. Measurements from the single ambient and nine ear-canal pressure conditions constituted one “trial” and took approximately 1 min to complete. However, if an infant was sufficiently noisy, averaging would be suspended by the DME system’s artifact rejection algorithm; therefore, some trials with infant participants took longer to complete. The probe was kept in place for the entire trial. In the event a probe seal was lost during a particular trial, the entire trial was repeated.
Coupler measurements
Over the course of five months of data collection, wideband ER measurements were made periodically (11 times) in a Zwislocki coupler (Knowles Electronics, DB-100 with DB-050 canal extension) to ensure consistency of stimulus presentation and response characteristics. These measurements were made in a sound treated booth, with the probe inserted into the Zwislocki coupler, terminated by a microphone, and placed on a foam rubber pad. Table 1 presents ER SD values by frequency for 11 coupler measurements. Small SD values (all less than 5%) suggest minimal change in probe calibration characteristics over the course of the study.
Table 1.
Energy reflectance (ER) standard deviation (SD) values at octave frequencies for 11 ER measurements made with the same probe over the course of five months in a Zwislocki coupler (Knowles Electronics, DB-100 with DB-050 canal extension).
| Frequency(Hz) | ER SD |
|---|---|
| 250 | 0.016 |
| 500 | 0.026 |
| 1000 | 0.011 |
| 2000 | 0.026 |
| 4000 | 0.013 |
| 8000 | 0.046 |
RESULTS
Multiple acoustic variables may be derived from wideband ATF measurements and examination of more than one variable is useful when interpreting wideband ATF results. ER and acoustic admittance measurements, including admittance magnitude (∣Y∣), admittance phase, and conductance (G), are examined in the following sections.
Wideband energy reflectance
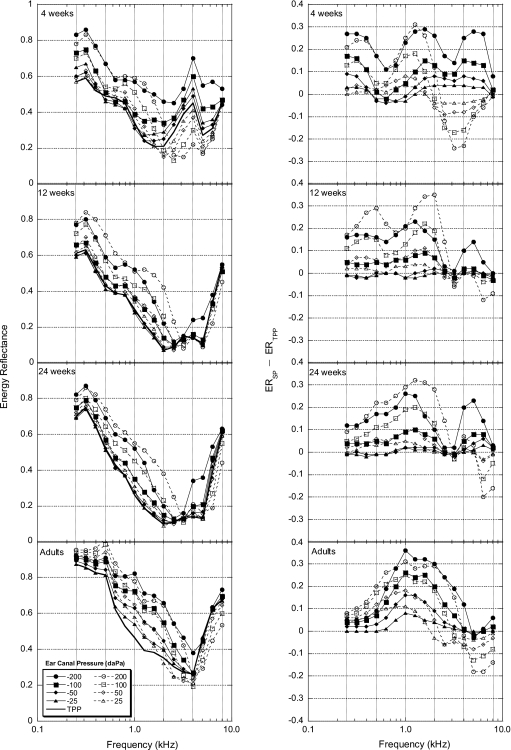
Figure 1 (left panel) illustrates one-third-octave averaged ER results for each age group at nine ear-canal pressures (−200, −100, −50, −25, 0, +25, +50, +100, and +200 daPa, relative to TPP). In addition, ER pressure differences are presented in Fig. 1 (right panel). ER pressure differences are defined as ER at a given static pressure condition (ERSP) minus ER at TPP (ERTPP). Therefore, a positive value on the y axis for Fig. 1 (right panel) indicates an increase in ER at a given static pressure relative to ER at TPP, while a negative value indicates a decrease in ER relative to ER at TPP.
Figure 1.
Group mean one-third-octave energy reflectance (ER) and ER differences (left and right panels, respectively) plotted as a function of frequency for all age groups (N=20 for each group). ER pressure differences are defined as ER at an individual static pressure (ERSP) minus ER at TPP (ERTPP).
Ear-canal pressure generally resulted in increased ER relative to TPP. Examination of 0.25 kHz in Fig. 1 (right panel) for each age group shows that the degree of change in ER with pressure steadily decreased as age increased, with an approximately 30% change in ER at 0.25 kHz for 4-week-old infants and less than a 10% change at the same frequency for adults. ER difference patterns for 4-week-old infants exhibited a multipeaked effect across frequency, with maxima in the ER difference functions occurring near 0.25 and 1.5 kHz. This multi-peaked pattern became less distinct for the 12- and 24-week-old infants and shifted to a single-peaked function in the adult data. The greatest pressure induced increases in ER for all age groups occurred at frequencies between 0.75 and 2 kHz (see Fig. 1, right panel). For 4-week-old infants, between 2 and 6 kHz, and to a lesser extent 8 kHz, the effects of positive and negative ear-canal pressures of the same magnitude (i.e., ±25, ±50, etc.) were opposite in nature, with negative pressures causing increased ER and positive pressures resulting in decreased ER. This effect was generally limited to more extreme ear-canal pressures (i.e., ±200 and ±100 daPa) for frequencies greater than 5 kHz for the two older infant age groups. For adults, on average, both positive and negative pressures resulted in similar ER increases. However, some positive pressures, for frequencies above 2 kHz, resulted in decreased ER while negative pressures had minimal effects.
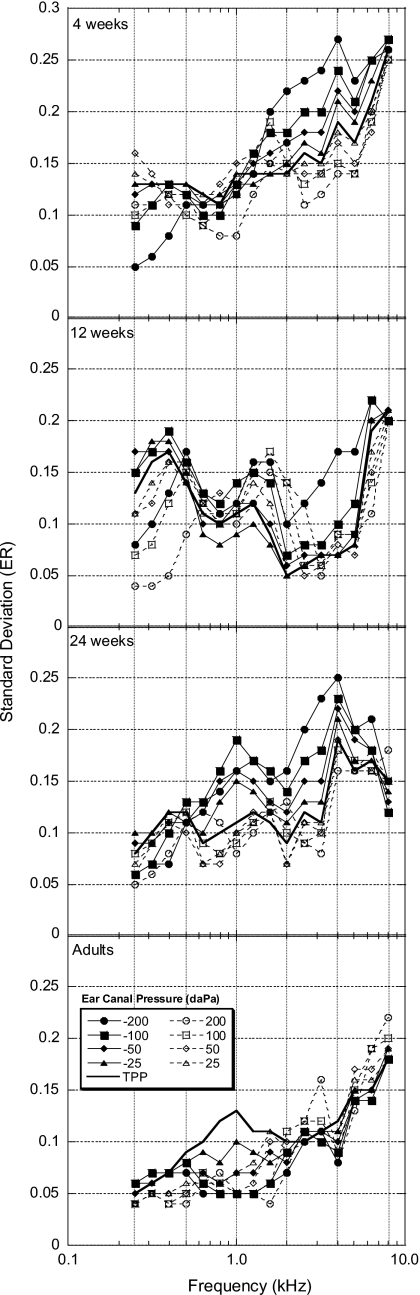
As a measure of variability, ER SDs were calculated (see Fig. 2). Overall, across frequency and pressure conditions, the adult data showed the smallest SDs. With the exception of the 12-week-old infants, SDs generally increased as frequency increased. For the infant groups, negative ear-canal pressures tended to cause SDs to increase, relative to SDs at TPP. Some pressure conditions (mostly positive) caused SDs to decrease for the infant groups while nearly all pressure conditions for the adult group resulted in either no change or decreases in SD relative to SDs at TPP.
Figure 2.
Energy reflectance (ER) standard deviations (SDs) as a function of frequency for all age groups. The parameter is ear-canal pressure.
Statistical analyses
Separate two-way analyses of variance (ANOVAs) were calculated at 16 one-third-octave center frequencies to examine the effects of age and pressure on ER pressure differences. An alpha level of 0.05 was used for all analyses. Age and pressure were significant factors at all frequencies. Post hoc analyses (Tukey tests) were computed to test for significant differences between age groups. Statistically significant differences in changes to ER as a result of ear-canal pressure were observed between infants and adults. Table 2 shows the ages and frequency∕pressure conditions for which infant ER pressure differences were significantly different from adults. At frequencies below 0.5 kHz ER pressure differences for the youngest infants were significantly different from adults, with most differences found at extreme (±200 daPa) ear-canal pressures. For this same frequency region, no significant differences in ER pressure differences were found between 24-week-old infants and adults for any pressure condition. Significant age and pressure effects were more prevalent at frequencies between 0.63 and 1.26 kHz with more significant differences detected between infants and adults. At frequencies from 1 to 6.35 kHz there tended to be greater differences between adults and infants for negative-pressure differences than for positive-pressure differences.
Table 2.
Results of post hoc analyses (Tukey tests) indicating the ages (in weeks) at which infant ER pressure differences (ERat a given static ear-canal pressure minus ER at TPP) were significantly different from adults (α=0.05). A blank space indicates the lack of a statistically significant difference between any of the infant groups and the adult group.
| Frequency(Hz) | Static ear-canal pressure (daPa) relative to tympanometric peak pressure (TPP) | |||||||
|---|---|---|---|---|---|---|---|---|
| −200 | −100 | −50 | −25 | 25 | 50 | 100 | 200 | |
| 250 | 4 12 | 4 | 4 | 4 12 | ||||
| 315 | 4 12 | 4 | 4 | 4 12 | ||||
| 397 | 4 12 | 4 | 4 12 | |||||
| 500 | 4 | 4 12 24 | 4 12 24 | 4 | 12 | |||
| 630 | 4 12 24 | 4 12 24 | 4 | 4 12 24 | 4 12 24 | 4 12 24 | 4 | |
| 794 | 4 12 | 4 12 24 | 4 12 24 | 4 12 | 4 12 | 4 12 | 4 12 | 4 12 |
| 1000 | 4 12 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 12 | 12 | 12 |
| 1260 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 | |||
| 1587 | 12 24 | 4 12 24 | 12 24 | 12 | 4 | |||
| 2000 | 12 24 | 12 24 | 12 24 | 12 | 12 | 4 | 4 | 4 |
| 2520 | 12 24 | 12 24 | 12 24 | 24 | 4 | 4 | ||
| 3175 | 12 24 | 12 24 | 4 | 12 24 | 4 | 4 12 24 | ||
| 4000 | 4 | 4 | 4 | 4 | 12 24 | 12 24 | 4 12 24 | 4 24 |
| 5040 | 4 12 24 | 4 24 | 4 24 | 4 | 12 24 | 12 24 | 12 24 | 4 12 24 |
| 6350 | 4 24 | 4 | 4 24 | 24 | 4 | |||
| 8000 | 4 | 4 | 4 | |||||
Next an analysis was made of significant ER differences between infant groups using post hoc analyses (Tukey tests). Table 3 shows ages (in weeks) for which there were significant differences in ER for different frequency∕pressure conditions. The majority of significant age and pressure effects were found between 4-week-old infants and the two older infant groups. Specifically, below 0.5 kHz, significant differences were found across nearly all frequency∕pressure conditions. At frequencies between 0.79 and 1.58 kHz, age and pressure were found to be significant factors in only three frequency∕pressure conditions. However, this is a frequency region where there were many adult-infant differences. For the frequency region from 2.5 to 6.35 kHz, the effects of pressure for 4-week-old infants were significantly different from the effects of pressure for 12- and 24-week-old infants. In contrast, across this same frequency range (with the exception of 6.35 kHz), there were no significant differences for the effects of pressure between 12- and 24-week-old infants. In fact, for all frequency∕pressure conditions, only nine conditions exhibited significant differences between 12- and 24-week-old infants, with approximately half of them occurring below 0.8 kHz for pressures at +100 and +200 daPa and the other half at 6.35 kHz.
Table 3.
Results of post hoc analyses (Tukey tests) indicating the ages (in weeks) at which infant ER pressure differences (ER at a given static ear-canal pressure minus ER at TPP) were significantly different from each other (α=0.05). A blank space indicates the lack of a statistically significant difference between any of the infant groups.
| Frequency(Hz) | Static-ear-canal pressure (daPa) relative to tympanometric peak pressure (TPP) | |||||||
|---|---|---|---|---|---|---|---|---|
| −200 | −100 | −50 | −25 | 25 | 50 | 100 | 200 | |
| 250 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–24 | 4–24 12–24 | ||
| 315 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–24 12–24 | 4–12 12–24 | ||
| 397 | 4–12 | 4–12 | 12–24 | |||||
| 500 | 4–12 | 4–12 | ||||||
| 630 | 12–24 | 4–24 | 4–12 4–24 | |||||
| 794 | 4–24 | |||||||
| 1000 | ||||||||
| 1260 | ||||||||
| 1587 | 4–12 | 4–12 | ||||||
| 2000 | 4–24 | 4–12 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | |||
| 2520 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 | 4–12 4–24 | 4–12 4–24 |
| 3175 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 |
| 4000 | 4–12 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 |
| 5040 | 4–12 | 4–12 | 4–12 | 4–12 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 |
| 6350 | 4–12 | 4–12 12–24 | 4–12 12–24 | 12–24 | 12–24 | 4–24 | ||
| 8000 | 4–24 | 4–12 4–24 | ||||||
Wideband admittance
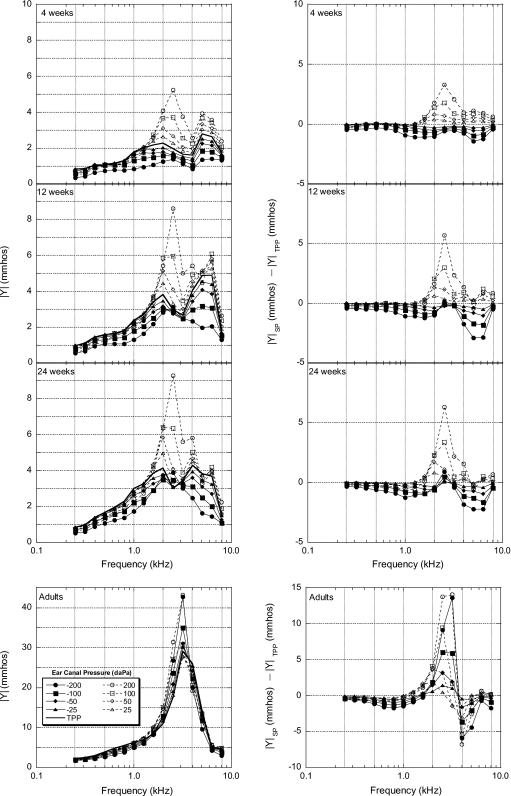
One-third-octave averaged wideband admittance magnitude (∣Y∣) values in mmhos are presented in Fig. 3 (left panel). Like ER pressure differences in Fig. 1, ∣Y∣ pressure differences were also calculated and are presented in Fig. 3 (right panel). It should be noted that, unlike ER measurements which are relatively unaffected by probe position in the ear canal, admittance measurements are affected by the immittance qualities of the air space between the probe and the tympanic membrane (Stinson et al., 1982). Therefore, some of the absolute admittance differences observed between groups are due to differences in ear-canal volumes (Keefe et al., 1993). Figure 3 (left panel) shows double-peaked ∣Y∣ transfer functions for infants in the 1.5–6 kHz range compared to a single-peaked transfer function for the adults. Also, as age increased, ∣Y∣ increased, with as much as a 27 mmho increase in peak ∣Y∣ between 4-week-old infants and adults. Negative pressures, with few exceptions, created decreased ∣Y∣ in the infant groups. The most pronounced effect of pressure on ∣Y∣ for the infant groups was for positive pressures (mainly 100 and 200 daPa) from 1.5 to 4 kHz, with as much as a 6 mmho increase in magnitude across this frequency range. Especially for the 24-week-old infants, this pressure induced increase in ∣Y∣ created a transfer function similar in shape to the adults.
Figure 3.
Group mean one-third-octave admittance magnitude (∣Y∣) and ∣Y∣ pressure differences in mmhos (left and right panels, respectively) plotted as a function of frequency for all age groups (N=20 for each group). ∣Y∣ pressure differences are defined as ∣Y∣ at an individual static pressure (∣Y∣SP) minus ∣Y∣ at the TPPs (∣Y∣TPP). Note the difference in scales on the y axes for infants and adults.
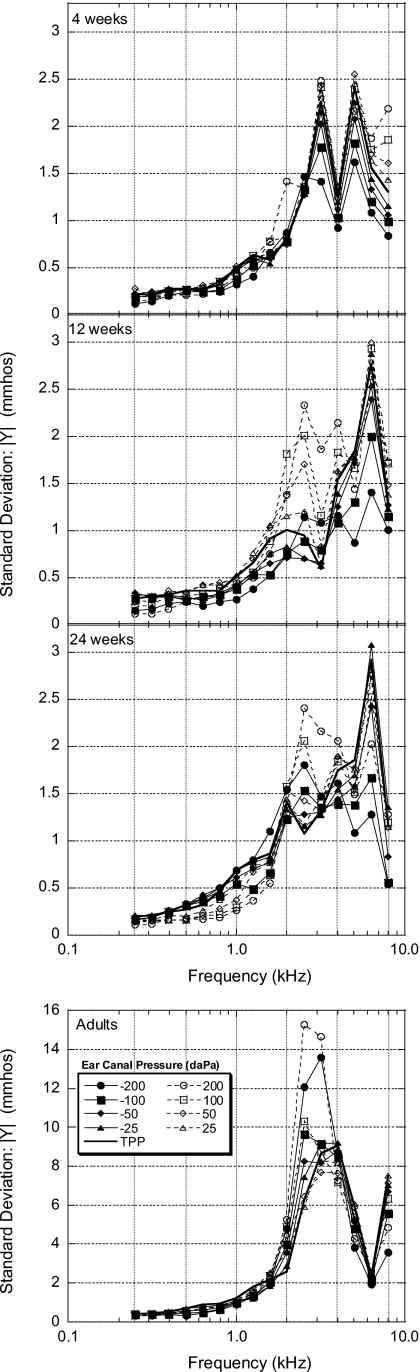
As a measure of the variability in ∣Y∣, SDs were calculated across subjects for each pressure condition and at each frequency (see Fig. 4). Overall, there was a nonmonotonic increase in SD as frequency increased. Gradually increasing SDs, with similar values across pressure conditions, were found for all age groups for frequencies from 0.25 to 1.5 kHz. Infant groups exhibited a double-peaked SD function for frequencies between 2 and 6 kHz similar to mean ∣Y∣ values. While the lower-frequency SD peak for 4-week-old infants showed similar variability across pressure conditions, the lower-frequency SD peak for 12- and 24-week-old infants was dominated by positive ear-canal pressures.
Figure 4.
Admittance magnitude ∣Y∣ standard deviations (SDs) as a function of frequency for all age groups. The parameter is ear-canal pressure. Note the difference in scales on the y axes for infants and adults.
Statistical analyses
Separate two-way ANOVAs and post hoc analyses (Tukey tests) were calculated at 16 one-third-octave center frequencies for admittance data. Tables 4, 5 show the frequency∕pressure regions where changes in ∣Y∣, relative to TPP, were statistically significant. Table 4 shows results for comparisons between adult and infant groups and Table 5 shows the results for comparisons between infant groups. The patterns of significant differences for ∣Y∣ are similar to those of ER (see Table 2), with the midfrequency region for both ATF measures showing fewer significant differences between ages. However, more differences between 12- and 24-week-old infants were found for ∣Y∣ pressure differences compared to ER pressure differences, especially at 1.58 and 2 kHz. Compared to ER pressure differences between infants and adults shown in Table 2, significantly different ∣Y∣ pressure differences shown in Table 4 tend to group more toward frequencies ranging from 1.26 to 5.4 kHz.
Table 4.
Results of post hoc analyses (Tukey tests) indicating the ages (in weeks) at which infant admittance magnitude (∣Y∣) pressure differences (∣Y∣ at a given static ear-canal pressure minus ∣Y∣ at TPP) were significantly different from adults (α=0.05). A blank space indicates the lack of astatistically significant difference between any of the infant groups and the adult group.
| Frequency(Hz) | Static ear-cannal prassure (daPa) relative to tympanometric peak pressure (TPP) | |||||||
|---|---|---|---|---|---|---|---|---|
| −200 | −100 | −50 | −25 | 25 | 50 | 100 | 200 | |
| 250 | 4 12 24 | 4 | 4 12 | |||||
| 315 | 4 12 24 | 24 | 4 12 | |||||
| 397 | 4 | 4 | 4 | 12 | 4 | 4 | ||
| 500 | 4 | 4 | 4 | 12 | 4 | 4 | 4 | |
| 630 | 4 | 4 12 24 | 4 | 12 | 12 | 12 | 4 | |
| 794 | 4 12 | 4 12 | ||||||
| 1000 | 4 | 4 | 4 24 | |||||
| 1260 | 4 12 24 | 4 12 24 | 4 | 4 24 | 4 24 | |||
| 1587 | 4 12 | 4 12 | 4 12 | 4 12 | ||||
| 2000 | 4 12 | 4 12 | 4 12 | 4 12 | 24 | 24 | 24 | 4 24 |
| 2520 | 4 12 | 4 12 | 4 12 24 | 4 12 | 4 24 | 4 | 4 12 24 | 4 12 24 |
| 3175 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 | 4 12 24 | 4 12 | 4 | 4 |
| 4000 | 4 12 24 | 4 24 | 4 12 24 | 4 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 |
| 5040 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 | 4 12 24 |
| 6350 | 4 24 | |||||||
| 8000 | ||||||||
Table 5.
Results of post hoc analyses (Tukey tests) indicating the ages (in weeks) at which infant admittance magnitude (∣Y∣) pressure differences (∣Y∣ at a given static ear-canal pressure minus ∣Y∣ at TPP) were significantly different from each other (α=0.05). A blank space indicates the lack of a statistically significant difference between any of the infant groups.
| Frequency(Hz) | Static ear-canal pressure (daPa) relative to tympanometric peak pressure (TPP) | |||||||
|---|---|---|---|---|---|---|---|---|
| −200 | −100 | −50 | −25 | 25 | 50 | 100 | 200 | |
| 250 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–24 | 4–24 | |||
| 315 | 4–24 | 4–12 4–24 | ||||||
| 397 | 4–12 | 4–12 4–24 | 4–12 | |||||
| 500 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–24 | 4–12 4–24 | |||
| 630 | 4–24 | 12–24 | 4–24 12–24 | |||||
| 794 | 12–24 | 12–24 | ||||||
| 1000 | ||||||||
| 1260 | 4–12 4–24 | |||||||
| 1587 | 4–12 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 |
| 2000 | 4–12 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 12–24 | 4–24 | |||
| 2520 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–24 | ||||
| 3175 | 4–12 4–24 | 4–24 | 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | |
| 4000 | 4–12 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | 4–12 4–24 | |||
| 5340 | ||||||||
| 6350 | 4–12 | 4–12 | 4–12 | 4–12 | ||||
| 8000 | ||||||||
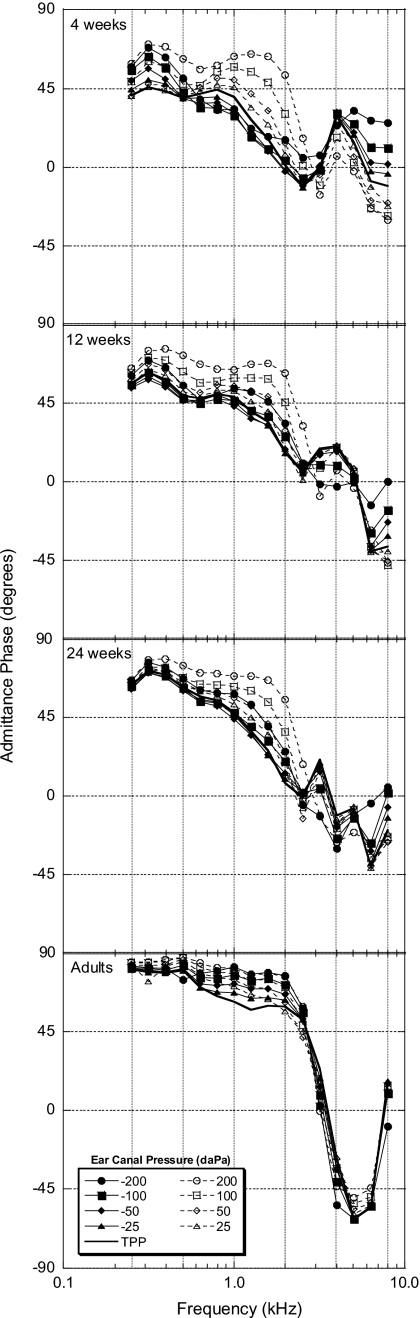
Admittance phase
Mean one-third-octave admittance phase is illustrated in Fig. 5. The phase at TPP (solid line) for the infant groups revealed a general negatively directed monotonic shift from stiffness to mass controlled phase as frequency increased from 0.25 to 2 kHz, with nonmonotonic changes in phase for frequencies from 2 to 8 kHz. Adult phase followed a similar pattern out to 5 kHz, and then shifted toward a positive direction out to 8 kHz. At frequencies below 1 kHz for the 4-week-old infants, admittance phase at TPP was near +45°, indicating equal contributions of conductance and susceptance components. Admittance phase angle shifted in a positive direction with increasing age at frequencies below 1 kHz for the infant groups, indicating increased stiffness with increasing age. Data in Fig. 5 illustrate differences in the effects of ear-canal pressure on phase, relative to phase measurements at TPP. Positive ear-canal pressures created the greatest change in phase for the infant groups, with the largest changes occurring between 1 and 3 kHz. The most common effect of static ear-canal pressure changes for the infants was an increase in phase, indicating an increase in the stiffness component. Ear-canal pressure had smaller effects on phase for the adult group, with positive and negative ear-canal pressures effecting similar changes in phase.
Figure 5.
Group mean one-third-octave admittance magnitude (∣Y∣) phase in degrees as a function of frequency for all age groups (N=20 for each group).
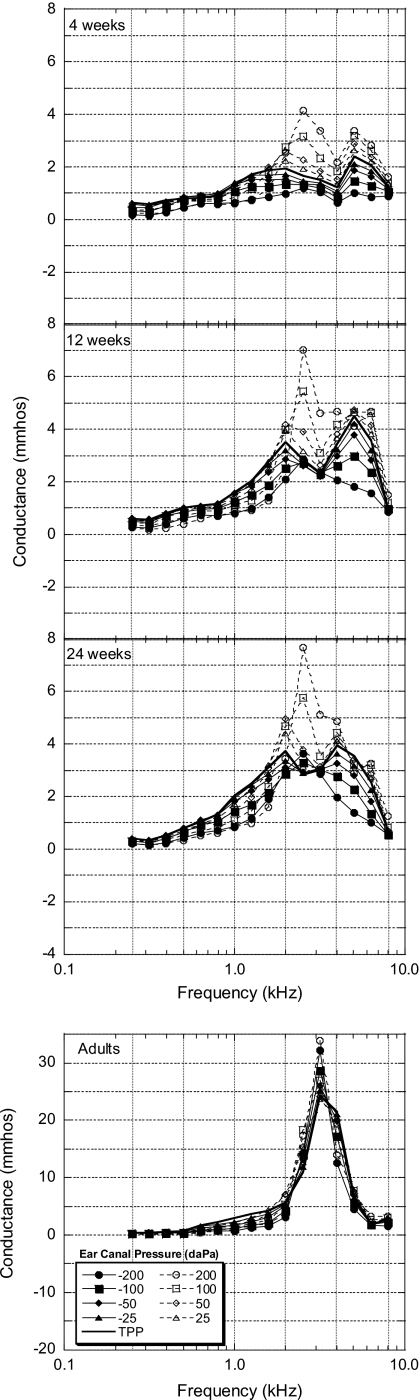
Conductance
Mean one-third-octave conductance (G), the real component of admittance, is presented in Fig. 6. Four-week-old infant G values from 0.5 to 2 kHz were reduced (<3 mmho) with the introduction of either negative or positive ear-canal pressures. At frequencies greater than 2 kHz for the 4-week-old group, positive pressures created the greatest changes in G, but only by amounts no greater than 3 mmho. The effects of positive pressures were larger (up to 5 mmho) in the 12- and 24-week-old infants. In contrast, both positive and negative ear-canal pressures had similar effects across frequency for the adult group.
Figure 6.
Group mean one-third-octave conductance values (mmhos) plotted as a function of frequency for all age groups (N=20 for each group). Note the difference in scales on the y axes for infants and adults.
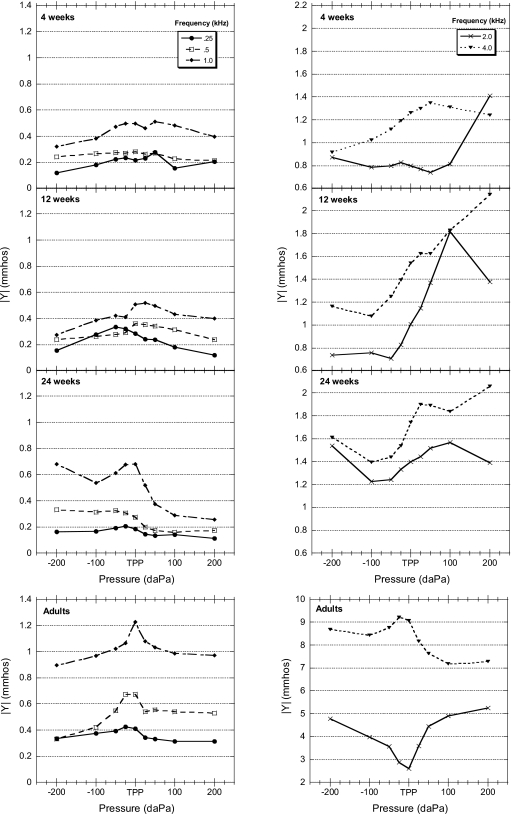
Wideband admittance tympanograms
Mean wideband ∣Y∣ tympanograms are plotted at octave frequencies from 0.25 to 4 kHz in Fig. 7. The tympanograms for the adult group (bottom panels) were single peaked from 0.25 to 1 kHz, followed by a 2 kHz tympanogram with a “notch” (an abrupt decrease in admittance followed by an increase as a function of pressure), and then returning to single-peaked functions at 4 kHz. ∣Y∣ tympanograms for 12- and 24-week-old infants were similar in morphology to the adult tympanograms with respect to the absence of notching up to 2 kHz. In contrast, the 4-week-old infant data showed a single-peaked tympanogram at 0.25 kHz with broad notching appearing at 0.5 kHz and a single-peaked tympanogram at 1 kHz. The tympanograms at these frequencies were consistent with previous research in infants (Holte et al., 1991). Wideband admittance tympanograms for 4 week old at 2 and 4 kHz exhibited different patterns than those observed in adults. Compared to the adult tympanograms, infant tympanograms were less symmetrical around TPP and the negative ∣Y∣ tails exhibited lower ∣Y∣ values relative to positive ∣Y∣ tails.
Figure 7.
Group mean one-octave admittance magnitude (∣Y∣), in mmhos, plotted as a function of pressure (daPa) for all age groups (N=20 for each group). Note the difference in scales on the y axes for infants and adults.
DISCUSSION
Wideband energy reflectance
Ambient pressure conditions
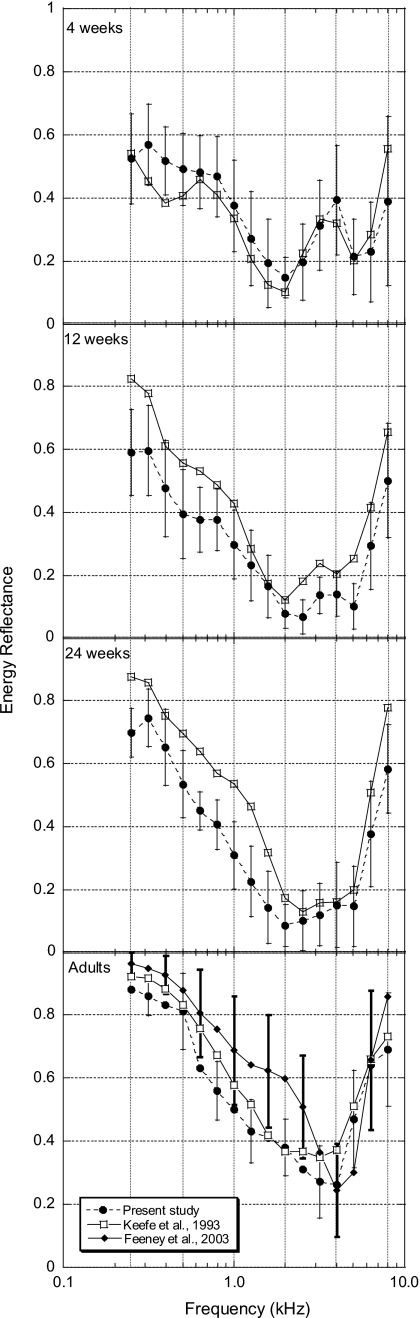
As ER measurement methods evolve, it is important to compare results and identify similarities and differences across studies. Mean one-third-octave ambient ER data for 1-, 3-, and 6-month-old infants and adults from Keefe et al. (1993) and for adults from Feeney et al. (2003) are plotted along with mean one-third-octave ambient data from the present study in Fig. 8 (error bars indicate ±1 standard deviation from the mean). The ambient ER measurements from the present study illustrated in Fig. 8 were obtained prior to the ER measurements obtained at static ear-canal pressures. While there are some overall magnitude differences in ER results between the different studies at each age, the patterns of the ER responses from the present study are consistent with data from Keefe et al.(1993) and Feeney et al. (2003). The largest differences between ER results for the two older infant groups and adults are mostly found below 2 kHz. The differences at lower frequencies may have been influenced by different methods used to estimate ear canal area. Keefe et al. (1993) used an acoustic estimate, while data from the present study were calculated using a set value based on the diameter of the calibration tubes (see Sec. 2C). Variation in ER measurements can occur if the ear canal is of a different diameter than the calibration tubes. The diameter of the calibration tubes used for infant measurements was 4.8 mm, close to the ear-canal diameter of 1-month-old infants (4.4 mm) as reported by Keefe et al. (1993); this age group had the best ER agreement between studies. The same ear-canal diameter (4.4 mm) used in measurements made in 12- and 24-week-old infants, with larger ear-canal diameters, could contribute to the lower ER results, relative to data from Keefe et al. (1993).
Figure 8.
Group mean one-third-octave ambient energy reflectance ER as a function of frequency for all ages for the present study (N=20 for each group) and for infants and a group of adults from Keefe et al. (1993). The numbers of subjects for each age group in Keefe et al. (1993) are as follows: 1 month=15, 3 months=18, 6 months=11, and adults=10. The bottom panel also includes young adult ER data (N=40, 75 ears) from Feeney et al. (2003). Error bars indicate ±1 SD from the mean. Note that to avoid overlapping error bars in the lower panel, adult SD data are plotted for alternating frequencies for the present study and Feeney et al. (2003).
Static pressure conditions
Results from the present study illustrate maturational effects on acoustic ear-canal measurements made under pressurized ear-canal conditions. ER results for 4-week-old infants, especially in the frequency regions below 0.75 kHz and between 2 and 6 kHz, are evidence of a middle-ear system that responds differently to changes in ear-canal pressure than older infants and adults. The magnitude of ER change with positive and negative ear-canal pressures at and below 0.5 kHz was greatest for the 4-week-old infants. This effect may be the result of a more compliant energy absorbing ear-canal wall (Keefe et al., 1993), which becomes stiffer with the introduction of ear-canal pressure and in turn reflects more acoustic energy. The gradual decrease in magnitude of this effect with age may reflect the developmental change from a cartilaginous to ossified infant ear canal (Saunders et al., 1983). The decrease in ER in response to positive pressures between 2 and 6 kHz for the 4-week-old infants is evidence of a more energy-absorbent infant ear under positive-pressure conditions. The effects of pressure on ER for 12- and 24-week-old infants were similar to each other and, while still different than adults, were more adultlike with respect to their overall patterns than those of the 4-week-old group.
In general, the results observed in the adult group were similar to wideband ATF data presented in previous work (Keefe and Simmons, 2003; Margolis et al., 1999), showing increases in ER with pressure up to approximately 3 kHz, with a slight decrease in ER with positive pressures above 3 kHz. In fact, for all age groups, there were only minimal decreases in ER with the introduction of negative ear-canal pressures.
Wideband admittance
Changes in ∣Y∣ at TPP with age in the present study are consistent with previous work in infants that has shown a general trend of increasing ∣Y∣ as a function of age (Holte et al., 1991; Meyer et al., 1997). With regard to changes in ∣Y∣ resulting from changes in static ear-canal pressures, positive pressures had the greatest effect on ∣Y∣, with similar overall response patterns across infant groups (see Fig. 3). Positive pressures for frequencies from 1 to 4 kHz created more adultlike (peaked) ∣Y∣ functions. Negative ear-canal pressures had a different effect for the infant groups when compared to the adult group, especially between 2 and 4 kHz. For example, in the infant groups, negative pressures always caused ∣Y∣ to decrease, whereas positive pressures caused both increases and decreases in ∣Y∣ depending on frequency. This differential pressure effect suggests that the characteristics of young infant ears are different than the ears of older infants and adults. Possible explanations for these differences are discussed further below.
Admittance phase results at TPP from the present study are in general agreement with data from Keefe et al. (1993) and Holte et al. (1991). The youngest infants in Keefe et al. (1993), Holte et al. (1991), and the present study (Fig. 4) present with nonmonotonic changes in admittance phase as a function of frequency. This pattern is suggestive of ear-canal and∕or middle-ear resonance effects, possibly due to vibration of the ear-canal wall and∕or immature tympanic membrane or middle-ear characteristics. Increased ear-canal wall compliance may partly explain the multiple resonances observed in the infant groups, and the gradual decline of the resonance at 0.5 kHz with increasing age may reflect developmental changes in ear-canal compliance properties. The phase function zero crossing, indicative of equal contributions of outer and middle-ear compliance (+ phase) and mass (− phase) components, increases with age, shifting from 2 kHz for the 4-week-old infants to 4 kHz for the adults. The larger adult ear-canal volume (resulting in increased compliance) is the most likely contributor to the higher-frequency ∣Y∣ phase zero crossing in adults. However, increased middle-ear or ear-canal stiffness in adults could also contribute to this upward frequency shift.
Conductance values (G) are of interest inasmuch as they play a part in the power (P) input into the middle ear, as shown through the relationship
where p represents sound pressure amplitude. Adult conductance values, obtained using a similar calibration technique (Thevenin source parameters), presented by Keefe et al. (1993) and Neely and Gorga (1998) are similar to those obtained in the present study. Examination of conductance values at TPP in Fig. 6 reveals increases in conductance as age increases, with the largest increases occurring above 2 kHz. Based on evidence showing behavioral threshold differences between infants and adults (Olsho, 1988), Holmer (2001) examined the relationship between middle-ear conductance measured at ambient ear-canal pressure and behavioral thresholds in 11- and 24-week-old infants and adults. While Holmer found large changes in behavior thresholds (up to 30 dB SPL) she reported only small changes in conductance between infants and adults at 0.5, 1, and 2 kHz. At 4 kHz, however, Holmer observed that as age increased, conductance increased, but behavioral thresholds decreased, suggesting a relationship between improved behavioral thresholds and increases in middle-ear conductance. Specifically, Holmer reported that between 11-week-old infants and adults, conductance increased by a mean of 8 dB (ranging from approximately 2 to 10 dB re: 1 mmho) and thresholds decreased by 27 dB. Holmer noted that while the developmental changes in conductance do not account for all of the infant∕adult differences in behavioral thresholds at 4 kHz, they appear to be responsible for part of them. Conductance values at TPP for 4 kHz in the present study also showed age-related increases, in particular, conductance values increased from 4 mmho at 12 weeks to 20 mmho for adults. These conductance values at 4 kHz, converted to decibels in the same manner as the data from Holmer (10 log10 g re: 1 mmho), were 6 and 13 dB for 12-week-old infants and adults, respectively. This 7 dB difference in conductance at 4 kHz between 12-week-old infants and adults for the present study is within 1 dB of the mean differences reported by Holmer, suggesting consistency in results across studies for these ages.
ATF for G and ∣Y∣ each exhibited multipeaked structures. Similar multipeaked structures were obtained by Keefe et al. (1994) for diffuse-field pressure transfer functions from a reverberant field to the infant ear canal and for diffuse-field absorption cross-section levels (DACLs). Keefe et al. (1994) calculated the DACLs using their diffuse-field pressure transfer function data along with G data from Keefe et al. (1993). The DACLs, defined as a quantitative measure of the acoustic response of the external ear and power transfer into the middle ear, were compared across infants and adults (see Keefe et al., 1994, Fig. 14). Conductance ATFs from the present study (Fig. 6) are similar in shape to DACL functions shown by Keefe et al. (1994) and also follow similar transitions with age. Functions from both studies for 4-week-old infants had two peaks (at 2 and 5 kHz), and as age increased, the higher-frequency peak gradually shifted downward in frequency, possibly representing a downward shift in the ear-canal or middle-ear resonant frequency. In addition, the overall level and magnitude of the functions increased as age increased. For the infant groups, with few exceptions, only positive pressures created increased G or increased power transfer into the middle ear. However, both positive and negative pressures resulted in increased G for the adult group, mainly around the peak of the G transfer function.
Wideband admittance tympanograms
Wideband ∣Y∣ tympanograms constructed from the present data showed increases in absolute ∣Y∣ with age (see Fig. 7). Holte et al. (1991) reported similar developmental changes in multifrequency admittance tympanograms for infants less than 5 month old. Holte et al. (1991) investigated changes from 0.226 to 0.9 kHz and found increasing ∣Y∣ as a function of age for frequencies above 0.226 kHz. Examination of tympanometric shape in the present study reveals a broad notch in the 4-week-old admittance tympanograms at 0.5 kHz. This is consistent with infant admittance tympanometry patterns presented by Holte et al. (1991), which were suggestive of canal-wall resonance in the low-frequency region hypothesized to be related to the more compliant nature of the infant ear-canal wall. While tympanogram notching at low frequencies is not observed for the 12- and 24-week-old infants (see Fig. 7), there is some evidence of notching at 2 kHz. The adult group also exhibited a notch in the tympanogram at 2 kHz. The frequency region where admittance notching is observed in adults is consistent with previous research, showing the 10th–90th percentile range for adult middle-ear resonant frequency of 0.8–2 kHz (Margolis and Goycoolea, 1993).
Differences between ∣Y∣ at extreme positive and negative ear-canal pressures (±200 daPa) have been reported in previous studies in young infants for 226 Hz and 1 kHz tympanograms (Kei et al., 2003; Margolis et al., 2003). Results from the present study show similar results and present new data that show a continuation of this pattern up to 4 kHz. If the negative tails of the 12- and 24-week-old infants’ 2 kHz tympanograms were adjusted upward to be equal in magnitude with the positive tails, they would appear fairly similar in shape to the adult 2 kHz tympanogram, including the broad notching around TPP (see Fig. 7). The cause of this differential pressure effect is unclear, but Margolis et al. (2003) suggested that decreased admittance at the negative tympanogram tail could be due to a partial “collapse” of the ear canal under negative pressure, resulting in reduced ear-canal volume, while positive ear-canal pressure could effectively increase the canal volume, resulting in increased admittance. Supporting this assumption is data from Holte et al. (1991) who obtained video recordings of the effects of pneumatic otoscopy pressure pulses (approximating ±250–300 daPa) presented to infant ears. They reported mean changes in ear-canal diameter (relative to resting diameter) of 18.3% and −28.2% in 1–7 day old infants for positive- and negative-pressure pulses, respectively. These effects decreased with age and were negligible in infants of 51–66 days old. Holte et al. (1991) did report asymmetrical monotonically increasing tympanograms (from negative to positive decapascals) in some infants but did not specifically investigate any relationships between tympanometric symmetry and degree of change in ear-canal diameter. It is also possible that the mass characteristics of the young infant middle ear (e.g., tympanic membrane and ossicles) are more easily manipulated with changes in static ear-canal pressure compared to older children and adults and that shifts in mass with negative pressure could possibly explain this sloping pattern in admittance tympanograms, especially since the effects of mass are manifested at higher frequencies and the positive∕negative admittance differential seen in these young infants’ becomes more pronounced as frequency increases.
Hypotheses of anatomical effects
Based on anatomical changes in the infant ear and knowledge regarding the effects of mass and stiffness on acoustic ear-canal measurements, inferences can be made as to what anatomical changes might account for some of the developmental differences observed in the present study. The large mean ER increase with age below 0.7 kHz seen in the infant groups, especially for the extreme pressure conditions, may be due to the stiffening of the compliant infant ear-canal wall. As age increased, the effect of static pressures for frequencies below 0.7 kHz decreased and was small in adults (see Fig. 1). While specific points in the timeline of infant ear-canal development have not been fully described, the decreasing effect of pressure with age could reflect the effects of a maturing (ossifying) ear canal, which becomes more resistant to changes in pressure with age (Saunders et al., 1983). Qi et al. ( 2006) developed a nonlinear finite-element model of the newborn (22 days old) ear-canal wall to investigate the effects of static pressures on infant ear-canal volume. Their model predicts a nonlinear elastic behavior of ear-canal wall displacement in response to high static pressures. Based on their model, maximal displacement plotted as a function of positive and negative ear-canal pressures would result in an asymmetrical sigmoid shaped tympanometric function. ∣Y∣ tympanogram data for frequencies above 2 kHz from the present study for all infants generally agree with the model prediction (Fig. 7). However, at frequencies below 2 kHz, the result would be a symmetrically shaped tympanometric function. It is possible that pressure induced changes in infant ear-canal volume, as modeled by Qi et al. (2006), could account for the sigmoid shaped ∣Y∣ tympanograms seen at frequencies above 2 kHz in 4-week-old infants. A strict comparison between the model from Qi et al. (2006) and data from the present study is limited since the experimental data are not measurements of ear-canal wall effects alone and include tympanic membrane and middle-ear effects. Qi et al. (2006) acknowledge their model as a beginning step and that further work is needed to include the tympanic membrane and middle ear to better understand development of the complete infant conductive system.
Because of the substantial contribution of the ossicles to the mass effects in middle-ear function, it would follow that acoustic effects at high frequencies could be due to changes in the properties of the ossicular chain (Saunders et al., 1983). Eby and Nadol (1986) reported that while the neonatal ossicles have obtained adult dimensions, they are histologically immature and composed mostly of cartilage. In addition, temporal bone data suggest that mass loading of the ossicles could be due to residual mesenchyme adhering to the ossicular chain. This residual embryonic tissue has been observed in infants as late as 5 months of age (Spector and Ge, 1981) and could influence the high-frequency ATF responses observed in young infants. This developmental timeline is consistent with data from the present study that show a differential pressure effect at frequencies above 2 kHz for 4-week-old infants, which lessens in magnitude for 12- and 24-week-old infants.
Age-related changes in the distance from the tympanic membrane to the footplate of the stapes have also been reported and are thought to be due to a change in orientation of the ossicular chain as the tympanic membrane rises from a nearly horizontal position, relative to the axis of the ear canal, to a more vertical adultlike position (Eby and Nadol, 1986). This change in ossicular chain orientation may also contribute to the markedly different high-frequency ER responses (above 2 kHz) in 4-week-old infants from the present study.
It is possible that the result of negative ear-canal pressures in 4-week-old infants may be to lessen the efficiency of the middle-ear system by functionally disarticulating the ossicular chain as the tympanic membrane is pulled outward into the ear canal. This effect could account for the increased ER with increasing negative pressure. The coupling of the ossicular chain is thought to be less rigid in young infants and may be affected more by the outward or perhaps more downward pull of negative ear-canal pressure on the infant tympanic membrane than in the adult (Saunders et al., 1983). Conversely, positive ear-canal pressure may enhance ossicular coupling due to the ossicles being “pushed” more closely together, creating a more acoustically efficient middle ear, resulting in decreased ER for frequencies from 2 and 6 kHz (see Fig. 1).
CONCLUSIONS AND CLINICAL IMPLICATIONS
Data from the present study provide insight into the effects of ear-canal pressure on acoustic measurements in adult and infant ears. Both infant and adult ATF measurements were influenced by the introduction of ear-canal pressure. While ATF results for all infants at ambient and pressurized conditions were different from adult measurements, data from 4-week-old infants were the least adultlike, showing the greatest changes in ER across frequency. The different effects of positive and negative ear-canal pressures on wideband ATFs may be a reflection of functional differences in the infant ear canal and middle-ear system and could potentially serve as a marker for maturation of the infant ear canal and middle ear.
The range of frequencies from 0.8 to 2 kHz, where few significant differences for ATF tympanometry were found for the three infant age groups, may be a frequency region of interest in future studies due to its apparent maturational stability. In addition, Feeney and Sanford (2005) reported that robust acoustic reflex responses and good signal-to-noise ratios were found for young infants in this same general frequency region. For frequencies between 2 and 4 kHz, positive pressures usually resulted in decreased ER and conversely increased ∣Y∣, effectively creating a more efficient middle-ear transmission system perhaps due to a better coupling of the ossicles in the pressurized infant middle ear and thus more closely matching the impedance of the ear canal. This hypothesis could be tested by examining changes in otoacoustic emission (OAE) levels or auditory brainstem response (ABR) thresholds with changes in ear-canal pressures as a function of infant age.
Because of the large differences in wideband ER and ∣Y∣ between 4- and 12-week-old infants, measurements taken at additional ages could be used to more closely define middle-ear maturation between these two ages. Results from the present study demonstrate that the effects of ear-canal pressure on ATF measurements are different for infants than adults. The maturational changes in middle-ear characteristics observed in the present study may explain much of the previously reported variability in 226 Hz immittance characteristics in infants. (Hunter and Margolis, 1992; Paradise et al., 1976; Sprague et al., 1985). A wideband approach to infant middle-ear assessment would provide a broader “window” with which to observe differences due to middle-ear development and possibly differences due to middle-ear pathology. While ambient ATF data from Keefe et al. (1993) show increasingly similar ER for infants above 6 months of age and adults, differences, especially between approximately 1.5 and 6 kHz, still exist, and ATF tympanometry studies for infants above 6 months of age may reveal additional developmental information. Ear-canal and middle-ear development might also be studied by conducting a longitudinal study to examine individual differences in the developmental time course.
Additional work is also needed to determine normative data sets for disordered ears of young infants since age-related differences in ambient and pressurized ER and admittance have been observed in a population with normal middle-ear status. Based on data from Keefe and Simmons (2003), which demonstrated that pressurized transmittance accurately predicts conductive hearing loss, investigations of pressurized transmittance in disordered infant ears would be an appropriate next step.
Results from the present study confirmed the findings of Holmer (2001), showing increases in conductance with age. This information is beneficial in terms of improving our understanding of infant auditory development and for future studies of auditory development. Investigations in young infants employing objective measures of auditory sensitivity, such as ABR or OAEs should take into account the possible effects that external- and middle-ear developments may have on the results. Since universal newborn hearing screening programs employ one or both of these procedures (ABR∕OAE), potential middle-ear influences are important factors to consider (Keefe et al., 2003). In addition, a valid middle-ear test for neonates and young infants could potentially help identify false-positive hearing screening results, which are often attributed to middle-ear or ear-canal factors (Keefe et al., 2000; Vander Werff et al., 2007). It would also be of interest to obtain wideband tympanometric ATF measurements in a neonatal population to identify possible developmental differences in these very young infants. Since the age at which infants are seen in early hearing detection and identification programs varies, having an accurate age appropriate tool for identification of middle-ear status would prove useful over the course of rescreening and follow-up as well as provide more accurate interpretation of OAE and ABR test results.
Based on results from the present study, which showed developmental effects across the frequency range from 250 to 8000 Hz in infants, a wideband ATF approach to infant middle-ear assessment is advantageous. However, there are several challenges facing new and evolving wideband ATF measurements. First, since wideband ATF technology is still evolving, new equipment and techniques are being updated and changed. While it appears that results from different studies using different equipment/techniques have been consistent, methodological variables need to be considered as future investigations reference past experimental evidence.
From a clinical standpoint, if wideband ATF measurements are to be useful, data analysis and interpretation must be relatively straightforward. Traditional multifrequency tympanometry, which provides much more information than single-frequency admittance tympanometry, has not enjoyed widespread use. This possibly stems from an inability to quickly and efficiently interpret the results (Fowler and Shanks, 2002). While research findings suggest that valuable diagnostic information can be obtained using ambient and pressurized wideband ATF measurements, quick and efficient interpretation is important if it is to be used as an effective clinical tool. From the large amount of data that can be obtained with wideband ATF measurements, predictive variables should be identified in an effort to develop middle-ear tests with appropriate sensitivity and specificity (Keefe and Simmons, 2003). Future studies of normal and disordered middle-ears could address issues of improving sensitivity and specificity of these emerging middle-ear tests, while also achieving objectivity in test interpretation.
ACKNOWLEDGMENTS
The authors thank Douglas H. Keefe for providing software for the measurement of wideband acoustic transfer functions and for helpful discussions throughout the processes of data collection, analyses, and manuscript preparation. Data collection for this study took place at the Virginia Merrill Bloedel Hearing Research Center, University of Washington, Seattle, WA. This research and manuscript preparation was supported by grants from the National Institutes of Health (NIH), National Institute on Deafness and other Communication Disorders (NIDCD) to the University of Washington (F31-DC007296 and P30-DC004661), and Boys Town National Research Hospital (T32-DC000013).
Portions of this work were presented at the American Auditory Society, Scientific and Technology Meeting, Scottsdale, AZ, March 2006 and the 30th Meeting of the Association for Research in Otolaryngology, Denver, CO, February 2007.
Footnotes
Holmer, N. M. (2001). “The effect of middle ear conductance on behavioral pure-tone thresholds of 11 and 24 week old infants and adults,” Unpublished Master’s thesis, University of Washington.
References
- Alaerts, J., Luts, H., and Wouters, J. (2007). “Evaluation of middle ear function in young children: Clinical guidelines for the use of 226- and 1,000-Hz tympanometry,” Otol. Neurotol. 28, 727–732. [DOI] [PubMed] [Google Scholar]
- Allen, J. A., Jeng, P. S., and Levitt, H. (2005). “Evaluation of human middle ear function via an acoustic power assessment,” J. Rehabil. Res. Dev. 10.1682/JRRD.2005.04.0064 42, 63–78. [DOI] [PubMed] [Google Scholar]
- Baldwin, M. (2006). “Choice of probe tone and classification of trace patterns in tympanometry undertaken in early infancy,” Int. J. Audiol. 45, 417–427. [DOI] [PubMed] [Google Scholar]
- Calandruccio, L., Fitzgerald, T. S., and Prieve, B. A. (2006). “Normative multifrequency tympanometry in infants and toddlers,” J. Am. Acad. Audiol 17, 470–480. [DOI] [PubMed] [Google Scholar]
- Eby, T. L., and Nadol, J. B., Jr. (1986). “Postnatal growth of the human temporal bone. Implications for cochlear implants in children,” Ann. Otol. Rhinol. Laryngol. 95, 356–364. [DOI] [PubMed] [Google Scholar]
- Feeney, M. P., Grant, I. L., and Marryott, L. P. (2003). “Wideband energy reflectance measurements in adults with middle-ear disorders,” J. Speech Lang. Hear. Res. 46, 901–911. [DOI] [PubMed] [Google Scholar]
- Feeney, M. P., and Keefe, D. H. (1999). “Acoustic reflex detection using wide-band acoustic reflectance, admittance, and power measurements,” J. Speech Lang. Hear. Res. 42, 1029–1041. [DOI] [PubMed] [Google Scholar]
- Feeney, M. P., and Keefe, D. H. (2001). “Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance and power,” Ear Hear. 10.1097/00003446-200108000-00006 22, 316–332. [DOI] [PubMed] [Google Scholar]
- Feeney, M. P., Keefe, D. H., and Sanford, C. A. (2004). “Wideband reflectance measures of the ipsilateral acoustic stapedius reflex threshold,” Ear Hear. 10.1097/01.aud.0000145110.60657.73 25, 421–430. [DOI] [PubMed] [Google Scholar]
- Feeney, M. P., and Sanford, C. A. (2005). “Detection of the acoustic stapedius reflex in infants using wideband energy reflectance and admittance,” J. Am. Acad. Audiol 16, 278–290. [DOI] [PubMed] [Google Scholar]
- Fowler, C. G., and Shanks, J. E. (2002). “Tympanometry,” in Handbook of Clinical Audiology, edited by Katz J. (Lippincott, New York: ), pp. 175–204. [Google Scholar]
- Holte, L., Margolis, R. H., and Cavanaugh, R. M., Jr. (1991). “Developmental changes in multifrequency tympanograms,” Audiology 30, 1–24. [DOI] [PubMed] [Google Scholar]
- Hunter, L. L., and Margolis, R. H. (1992). “Multifrequency tympanometry: Current clinical application,” Am. J. Audiol. 1, 33–43. [DOI] [PubMed] [Google Scholar]
- Ikui, A., Sando, I., and Fujita, S. (1997). “Postnatal change in angle between the tympanic annulus and surrounding structures: Computer-aided three-dimensional reconstruction study,” Ann. Otol. Rhinol. Laryngol. 106, 33–36. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., and Abdala, C. (2007). “Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears,” J. Acoust. Soc. Am. 10.1121/1.2427128 121, 978–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H., Bulen, J. C., Arehart, K. H., and Burns, E. M. (1993). “Ear-canal impedance and reflection coefficient in human infants and adults,” J. Acoust. Soc. Am. 10.1121/1.407347 94, 2617–2638. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Bulen, J. C., Campbell, S. L., and Burns, E. M. (1994). “Pressure transfer function and absorption cross section from the diffuse field to human infant ear canal,” J. Acoust. Soc. Am. 10.1121/1.408380 95, 355–371. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Folsom, R. C., Gorga, M. P., Vohr, B. R., Bulen, J. C., and Norton, S. J. (2000). “Identification of neonatal hearing impairment: ear-canal measurements of acoustic admittance and reflectance in neonates,” Ear Hear. 10.1097/00003446-200010000-00009 21, 443–461. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., and Levi, E. (1996). “Maturation of the middle and external ears: Acoustic power-based responses and reflectance tympanometry,” Ear Hear. 10.1097/00003446-199610000-00002 17, 361–373. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Ling, R., and Bulen, J. C. (1992). “Method to measure acoustic impedance and reflection coefficient,” J. Acoust. Soc. Am. 10.1121/1.402733 91, 470–485. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., and Simmons, J. L. (2003). “Energy transmittance predicts conductive hearing loss in older children and adults,” J. Acoust. Soc. Am. 10.1121/1.1625931 114, 3217–3238. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Zhao, F., Neely, S. T., Gorga, M. P., and Vohr, B. R. (2003). “Ear-canal acoustic admittance and reflectance effects in human neonates. I. Predictions of otoacoustic emission and auditory brainstem responses,” J. Acoust. Soc. Am. 10.1121/1.1523387 113, 389–406. [DOI] [PubMed] [Google Scholar]
- Kei, J., Allison-Levick, J., Dockray, J., Harrys, R., Kirkegard, C., Wong, J., Maurer, M., Hegarty, J., Young, J., and Tudehope, D. (2003). “High-frequency (1000 Hz) tympanometry in normal neonates,” J. Am. Acad. Audiol 14, 20–28. [DOI] [PubMed] [Google Scholar]
- Margolis, R. H., Bass-Ringdahl, S., Hanks, W. D., Holte, L., and Zapala, D. A. (2003). “Tympanometry in newborn infants—1 kHz norms,” J. Am. Acad. Audiol 14, 383–392. [PubMed] [Google Scholar]
- Margolis, R. H., and Goycoolea, L. M. (1993). “Multifrequency tympanometry,” Ear Hear. 14, 408–413. [DOI] [PubMed] [Google Scholar]
- Margolis, R. H., and Heller, J. W. (1987). “Screening tympanometry: Criteria for medical referral,” Audiology 26, 197–208. [DOI] [PubMed] [Google Scholar]
- Margolis, R. H., Saly, G. L., and Keefe, D. H. (1999). “Wideband reflectance tympanometry in normal adults,” J. Acoust. Soc. Am. 10.1121/1.427055 106, 265–280. [DOI] [PubMed] [Google Scholar]
- Meyer, S. E., Jardine, C. A., and Deverson, W. (1997). “Developmental changes in tympanometry: A case study,” Br. J. Audiol. 31, 189–195. [DOI] [PubMed] [Google Scholar]
- Neely, S. T., and Gorga, M. P. (1998). “Comparison between intensity and pressure as measures of sound level in the ear canal,” J. Acoust. Soc. Am. 10.1121/1.423876 104, 2925–2934. [DOI] [PubMed] [Google Scholar]
- Olsho, L. W., Koch, E. G., Carter, E. A., Halpin, C. F., and Spetner, N. B. (1988). “Pure-tone sensitivity of human infants,” J. Acoust. Soc. Am. 10.1121/1.396630 84, 1316–1324. [DOI] [PubMed] [Google Scholar]
- Paradise, J. L., Smith, C. G., and Bluestone, C. D. (1976). “Tympanometric detection of middle ear effusion in infants and young children,” Pediatrics 58, 198–210. [PubMed] [Google Scholar]
- Piskorski, P., Keefe, D. H., Simmons, J. L., and Gorga, M. P. (1999). “Prediction of conductive hearing loss based on acoustic ear-canal response using a multivariate clinical decision theory,” J. Acoust. Soc. Am. 10.1121/1.426713 105, 1749–1764. [DOI] [PubMed] [Google Scholar]
- Qi, L., Liu, H., Lutfy, J., Funnell, W. R., and Daniel, S. J. (2006). “A nonlinear finite-element model of the newborn ear canal,” J. Acoust. Soc. Am. 10.1121/1.2363944 120, 3789–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush, J., Bryant, K., Mundy, M., Zeisel, S., and Roberts, J. (1995). “Developmental changes in static admittance and tympanometric width in infants and toddlers,” J. Am. Acad. Audiol 6, 334–338. [PubMed] [Google Scholar]
- Ruah, C. B., Schachern, P. A., Zelterman, D., Paparella, M. M., and Yoon, T. H. (1991). “Age-related morphologic changes in the human tympanic membrane. A light and electron microscopic study,” Arch. Otolaryngol. Head Neck Surg. 117, 627–634. [DOI] [PubMed] [Google Scholar]
- Saunders, J. C., Kaltenback, J. A., and Relkin, E. M. (1983). “The structural and functional development of the outer and middle ear,” in Development of Auditory and Vestibular Systems, edited by Romand R. and Romand M. R. (Academic, New York: ), pp. 3–25. [Google Scholar]
- Spector, G. J., and Ge, X. X. (1981). “Development of the hypotympanum in the human fetus and neonate,” Ann. Otol. Rhinol. Laryngol. Suppl. 90, 1–20. [PubMed] [Google Scholar]
- Sprague, B. H., Wiley, T. L., and Goldstein, R. (1985). “Tympanometric and acoustic-reflex studies in neonates,” J. Speech Hear. Res. 28, 265–272. [DOI] [PubMed] [Google Scholar]
- Stinson, M. R., Shaw, E. A., and Lawton, W. B. (1982). “Estimation of acoustical energy reflectance at the eardrum from measurements of pressure distribution in the human ear canal,” J. Acoust. Soc. Am. 10.1121/1.388257 72, 766–773. [DOI] [PubMed] [Google Scholar]
- Vander Werff, K. R., Prieve, B. A., and Georgantas, L. M. (2007). “Test-retest reliability of wideband reflectance measures in infants under screening and diagnostic test conditions,” Ear Hear. 28, 669–681. [DOI] [PubMed] [Google Scholar]
- Voss, S. E., and Allen, J. B. (1994). “Measurement of acoustic impedance and reflectance in the human ear canal,” J. Acoust. Soc. Am. 10.1121/1.408329 95, 372–384. [DOI] [PubMed] [Google Scholar]