Abstract
Cell culture models can provide information pertaining to the effective dose, toxiciology, and kinetics, for a variety of neuroactive compounds. However, many in vitro models fail to adequately predict how such compounds will perform in a living organism. At the systems level, interactions between organs can dramatically affect the properties of a compound by alteration of its biological activity or by elimination of it from the body. At the tissue level, interaction between cell types can alter the transport properties of a particular compound, or can buffer its effects on target cells by uptake, processing, or changes in chemical signaling between cells. In any given tissue, cells exist in a three-dimensional environment bounded on all sides by other cells and components of the extracellular matrix, providing kinetics that are dramatically different from the kinetics in traditional two-dimensional cell culture systems. Cell culture analogs are currently being developed to better model the complex transport and processing that occur prior to drug uptake in the CNS, and to predict blood-brain barrier permeability. These approaches utilize microfluidics, hydrogel matrices, and a variety of cell types (including lung epithelial cells, hepatocytes, adipocytes, glial cells, and neurons) to more accurately model drug transport and biological activity. Similar strategies are also being used to control both the spatial and temporal release of therapeutic compounds for targeted treatment of CNS disease.
Keywords: Blood-brain barrier, Cell Culture Model, TEER, Hydrogel, Microfluidic
I. Introduction
The development of realistic, reproducible, and validated cell culture models holds the potential to greatly improve the in vitro evaluation of neuroactive compounds. Cell culture models are commonly used to estimate the pharmacological properties of a compound including mode of action, interaction, dose, and kinetics. However, it is difficult to predict how such compounds will perform in vivo based on in vitro data. Although advances in cellular and molecular biology have streamlined the in vitro screening of drugs via high-throughput methods, most in vitro studies cannot take into account the complex cellular, tissue and organ system interactions that occur in vivo. The interactions that occur within and between the liver, adipose tissue, kidney and other tissues lead to metabolic, storage and clearance properties that are dramatically different from the properties observable in most simplified cell culture systems.
What makes the prediction of pharmacological properties for a given compound even more difficult for central nervous system therapeutics is the specialized barrier that is formed by the interactions between neural cells and vascular endothelial cells. The blood-brain barrier is a selectively permeable tissue that prevents most polar compounds with molecular masses greater than 500 Daltons from entering the brain tissue [1–3]. The barrier properties of the brain capillary networks are determined by the formation of tight junctions between endothelial cells, as well as by the specialized transport mechanisms and cellular metabolism of endothelial and glial cells [4, 5]. Many compounds that display promising therapeutic results in vitro are larger than 500 Da and water soluble, and hence they display limited efficacy in vivo, because they are unable to enter brain tissue in significant amounts.
More realistic cell culture models must be developed for the purposes of screening compounds for blood-brain barrier permeability and development of technologies by which the brain can be accessed. Recent advances in microfabrication and nanotechnology have allowed researchers to develop new methods for fabricating and probing in vitro biological systems for use as accurate cell culture models. Many systems utilize microfluidics to simulate the transport properties of blood vessels on a physiologically relevant scale. Hydrogel matrices can be used to reconstruct more realistic cellular microenvironments for co-culture systems and transport studies. Other technologies including microelectrodes can be used to monitor cell culture systems in real time. Engineering a more realistic cellular environment will facilitate the development of new therapeutic strategies and will aid in the discovery of new classes of therapeutics. Through a combination of multiple cell culture systems, including liver, adipose, and kidney into an on-chip design, it may also be possible to study the organ interactions in a biological representation of theoretical pharmacokinetic models.
This review will discuss in detail the complex cellular and tissue interactions that determine drug efficacy and permeability at the blood-brain barrier. Particular emphasis will be placed on (1) the development of realistic cell culture systems for modeling the blood-brain barrier, (2) technologies that facilitate development of these models, and (3) the potential integration of these models into cell culture analog (CCA) systems. Strategies for drug delivery across the blood-brain barrier as well as technologies that can be used for controlled spatial and temporal delivery of neuroactive compounds to the central nervous system will also be discussed.
II. Complex organ and tissue interactions in vivo: systemic organs and the blood-brain barrier
Difficulties in the prediction of drug properties in vivo from in vitro data result from the complex interactions between cells, tissues, and organs, as well as differences in cell behavior and metabolism in the native cellular microenvironment. While kinetic models can be useful for predictions of dose, based on body volume and mode of delivery, such models are only mathematical representations of the key organ systems of the body. In order to be accurate, kinetic models require anticipation of all possible drug mechanisms.
A. Metabolism, storage, and elimination by organ systems
1. Delivery
Neuroactive compounds can enter the body by transdermal absorption [6–8], inhalation [9–11], ingestion [12, 13], intravenous injection [14, 15] or direct delivery to target tissue [16, 17]. Direct delivery provides a guaranteed method for administration of an active compound to the brain at a known concentration. However, this method often requires craniotomy, risks damaging healthy brain tissue, and does not permit long term drug delivery. All other delivery options are indirect and involve exposure of the compound to other organ systems in which it can become metabolically processed, stored, or a cause of side effects.
Having entered systemic circulation, a compound can either enter tissue or be eliminated from the body. The process of uptake can be explained by means of kinetic models typically based on one or two compartments [18–20]. One-compartment models, which obey first-order kinetics, can be explained as follows. After a drug is administered, the kinetics are determined by the drug concentration in blood plasma, the initial dose, the volume of target tissue through which the drug is to be distributed, and the rate at which it is eliminated. Two-compartment models are more realistic, taking into consideration the equilibration and distribution of the drug into peripheral tissues. In two-compartment models, the volume of peripheral tissue through which the drug is distributed, as well as forward and reverse rate constants for drug entry into the tissue, are included [18,20].
However, pharmacokinetic prediction becomes more difficult as more factors and components are taken into consideration. In the blood stream, a fraction of the drug dose can bind plasma proteins or erythrocytes and thus not be available for target tissue absorption, metabolism, or elimination [21–23]. Delivery through ingestion is complicated by the fact that the compound must be broken down and absorbed by the gastrointestinal tract before it enters circulation. Assuming full breakdown and absorption by the small intestine, the compound would enter the portal vein, where it would be transported directly to the liver and subjected to extensive metabolism before reaching systemic circulation. Even by intravenous delivery, a large fraction of drug is not absorbed into the target tissue on the first pass through circulation, and is subject to metabolism or elimination. In general, the less direct and invasive the method of delivery, the less chance the compound will enter the target tissue. Therefore, the delivery method for any neuroactive compound can dramatically affect both the amount and activity of the compound in circulation before it reaches the brain. Levels of a compound can be further reduced due to impermeability at the blood-brain barrier, thereby necessitating significantly higher initial therapeutic doses to be administered. For example, dexamethasone, a glucocorticoid metabolized in the liver and commonly used to reduce inflammation resulting from central nervous system tumors or meningitis, must be used at therapeutic levels that have the potential to cause side effects in many systemic organs [24, 25]. Prolonged high-dose treatment with dexamethasone or other corticosteroids has been linked to a variety of side effects including insulin resistance, Cushing’s syndrome, and gastric ulcer [26].
2. Storage and metabolism
One important reservoir for drugs, particularly lipophilic drugs that can cross the blood-brain barrier, is adipose tissue. In adipose tissue, circulation is generally lower than in peripheral tissues such as muscle. Therefore, drugs and toxins can persist longer before being eliminated or metabolized in organs.
Table 1 includes some of the key enzymes involved in drug metabolism in various organs and the brain. Although almost all organs and tissues are capable of metabolizing drugs, the liver is a focus for metabolism and bioconversion [19]. The rate at which a drug is metabolized by the liver is dependent upon hepatic clearance, blood flow, and metabolism via the cytochrome P450 system [18, 27, 28]. A variety of liver enzymes are responsible for altering the chemical structure of, and producing metabolites from, neuroactive compounds [29–31]. The process by which a metabolite is produced can involve multiple steps and enzymes. Compounds can undergo oxidation, reduction, hydrolysis, hydration, or isomerization [19]. One of the most important enzyme systems in the oxidative metabolism of drugs and other xenobiotics is the cytochrome P450 system (CYP450). CYP450 enzymes are found in lung, intestine, liver, adipose and brain tissue, and is capable of catalyzing oxidative reactions on many different substrates [30, 32–34]. Phase 1 oxidative metabolism serves to alter the chemical specificity and function of the molecules and can render certain compounds more or less active, or target them for further metabolism.
Table 1. Key enzymes in Phase 1 and Phase 2 xenobiotic metabolism.
Key enzymes are displayed with corresponding tissue expression information and a selection of known subtypes. General catalytic functions are assigned. The cytochrome P450 Phase 1 enzymes, as well as SULT, UGT, and GST Phase 2 enzymes, play critical roles in the detoxification and metabolism of a wide variety of both endogenous and xenobiotic substances. Bracketed numbers denote references.
| Enzyme | Tissue Expression | Subtypes | Function | Ref. |
|---|---|---|---|---|
| Phase I | ||||
| Cytochrome P450 | Brain
Intestine Kydney Liver Lung Skin |
1A1, 1A2, 1B1, 2C8, 2C9, 2C18, 2C19, 2D4, 2D6, 2E1, 3A4, 3A5, 3A7, 4A9, 4A11
2C8, 2C9, 2C19, 2D6, 2J2, 3A4, 3A5 1A1, 1A2, 1B1, 2A6, 2B1, 2B2, 2B6, 2C8, 2D6, 2C11, 2E1, 3A1, 3A2, 3A4, 3A5, 4A2, 4A3, 4A11 1A1, 1A2, 2A6, 2B6, 2B7, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 4A9, 4A11 1A1, 1A2, 1B1, 2A6, 2A13, 2B6, 2C9, 2C19, 2D6, 2E1, 2F1, 2S1, 3A4, 3A5, 4B1 1A1, 1A2, 1B1, 2A6, 2B6, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5 |
Inducible enzymes that catalyze monooxygenase reactions on many organic endogenous and xenobiotic compounds. | [31, 32, 34–49] |
| Flavin monooxygenase | Liver, Kidney, Skin | 1, 3, 4, 5 | Catalyze monooxygenase reactions on nitrogens or amine groups of many xenobiotic substances. | |
| Peroxidases | Liver | Act on organic lipid peroxidases. | ||
| Amine Oxidases | Liver | Oxidize primary amines and diamines. | ||
| Dehydrogenases | Liver, Skin | Catalyze the oxidation of alcohols, aldehydes, and acetaldehydes for detoxification. | ||
| Molybdenum hydroxylase | Liver | Aldehyde oxidases, Xanthine oxidases | Oxidize heterocyclic compounds and aldehydes. | [50] |
| Esterase/Amidase | Brain, Liver, Skin | Hydrolyze xenobiotic esters and amides. | ||
| Epoxide hydrolase | Liver, Lung, Skin | Convert epoxides formed by the metabolism of aromatic compounds into trans-dihydrodiols. | ||
| NAD(P)H:quinone reductase | Liver, Skin | Catalyzes oxidoreductive reaction using NADPH for endogenous quinones and some xenobiotics. | ||
| Phase 2 | ||||
| Sulfotransferase (SULT) | Brain
Intestine kidney Liver Lung Skin |
1A1, 1A3, 1E1, 2A1, 4A1
1B1, 1C1, 1C2, 1E1, 2B1 A1, 1A3, 1E 2A1 1A, 1B, 1C, 1E, 2A, 2B 1A1, 1A2 1A1, 1A3 |
Transfer sulfate groups from 3′-Phosphoadenosine-5′-phosphosulfate to protein or lipids. | [29, 42, 48, 49] |
| UDP-glucouronosyl transferase (UGT) | Brain
Intestine kidney Liver Lung Skin |
1A6, 2B7
1A1, 1A3, 1A6, 1A8, 2B7 1A6, 2B7, 2B15, 2B17 1A1, 1A3, 1A4, 1A6, 1A9, 2A1, 2B4, 2B7, 2B15, 2B17 1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2A1, 2B4, 2B7, 2B10, 2B11, 2B15, 2B17, 2B28 2B15 |
Conjugate sugar moieties to many xenobiotics to increase aqueous solubility | [29, 42, 48, 49, 51] |
| Glutothione-S-Transferase (GST) | Brain
Intestine Kidney Liver Lung Skin |
pi
alpha, pi, mu, theta alpha, pi, theta alpha, pi, mu, theta, omega, zeta, sigma pi pi |
Conjugate glutathione to many xenobiotics to increase aqueous solubility | [29, 42, 48, 49, 52–54] |
| Amino Acid Conjugation | Liver | Conjugation to glycine or other amino acids to exogenous carboxylic acids for excretion. | ||
| N-acetyltransferase (NAT) | Liver, Skin | NAT1, NAT2 | Transfer acetyl groups from acetyl-co-A to a variety of aromatic compounds. | |
| Methyltransferase | Liver | O-, S-, N- | Transfer methyl group from donor to receptor. | |
| 5-nucleotidase | Brain, Liver | Dephosphorylation of deoxy- and ribo- nucleoside monophosphates. | ||
| Alkaline phosphatase | Brain, Kidney, Liver | Dephosphorylation of proteins and nucleotides. | ||
| γ-Glutamyl Transpeptidase (GGTP) | Brain, Kidney, Liver | Glutathione metabolism and transfer of amino acids across plasma membrane. |
Often the products of Phase 1 enzyme metabolism undergo a second round of metabolism (Phase 2), whereby they are conjugated to water soluble functional groups. Compounds can be conjugated to glutathione, amino acids, or fatty acids; they can undergo condensation reactions; or they can be acetylated. Glutathione-S-transferases (GSTs), present in the cytosolic, mitochondrial, and microsomal compartments of cells, are some of the most important types of Phase 2 enzymes, responsible for conjugation of glutathione to many xenobiotics, including cancer therapeutics [54–58]. While GSTs function to detoxify many carcinogens, they are also present in many drug-resistant gliomas, where they undesirably metabolize cancer therapeutics such as l,3-bis(2-chloroethyl)-l-nitrosourea, cisplatin, and temozolomide [59–62].
By-products of these two enzymatic phases can be further modified so as to enable transport out of cells and eventually out of the body. For example, γ-glutamyl transpeptidase (GGTP) and dipeptidases catalyze the extracellular cleavage of glutathione groups added by GST to xenobitoic compounds, recovering cysteines for reuse in cellular metabolism and preparing the metabolite for elimination by the renal system [63]. GGTP expression is high in brain capillary endothelial cells and has also been associated with drug-resistant malignant gliomas [64, 65].
3. Elimination
Elimination of drugs occurs primarily by way of the gastrointestinal and renal systems. In the kidney, circulating drugs and drug metabolites are filtered and removed from circulation, are filtered and returned to circulation, or continue through circulation unfiltered. Assuming that the kidneys are capable of removing any drug that enters, the clearance rate is directly proportional to the flow rate, and indirectly proportional to the volume of distribution [19]. Both of these factors are involved in determining the half-life of a drug [19]. The polarity of the drug is an important factor in renal elimination, since this characteristic determines the solubility in water. Most drugs are rendered more water soluble when they are converted to metabolites, resulting in increased elimination by specialized transporters found in renal tissue [66, 67]. Some drugs can also be absorbed by the lungs and eliminated via exhalation. Ingested drugs are often eliminated before reaching circulation by way of the gastrointestinal system.
B. The blood-brain barrier: development, structure, transport, and metabolism
Drug delivery and targeting to the central nervous system are complicated by the complexity and heterogeneity of brain tissue. The brain contains, in addition to a variety of specialized types of neurons, several types of astrocytes, microglia, oligodendrocytes, vascular cells, and pial cells. Gene expression, chemical signaling, and distribution of individual cell types are tightly regulated during development [68–70]. The presence of these cells and their relative proportions vary between brain regions [71]. However, typically there is a 10:1 astrocyte:neuron ratio in the brain [72]. The brain is a highly interconnected organ, such that treatment in one region can give rise to unpredictable changes in the transmission of both chemical and electrical signals of another region. In the management of psychiatric illness, global changes in brain function in response to medication produce side effects including lethargy, disturbances in coordination, and dulling of affect, and these effects often lead to noncompliance in dosing. Therapeutics used for treatment of local diseases and disorders of the brain often result in global changes in brain function; and thus accordingly, targeting to local areas is of a high priority to reduce therapeutic side effects. Many psychotropic therapeutics, including lithium, tricyclic antidepressants, and clozapine, are metabolized before entering the brain, and the existence of genetic polymorphisms in several cytochrome P450 subtypes necessitate the use of therapeutic drug monitoring to manage appropriate dose [73]. What makes the brain an even more difficult target for drug therapy is the selective transport barrier that is constituted by the interactions between vascular endothelial cells and the neural cells of brain tissue.
1. The blood-brain barrier
The central nervous system is separated from systemic circulation by physical, metabolic, and transport barriers. Under normal conditions, many small molecules and almost all large or polar molecules, toxins, pathogens, and circulating cells are excluded from entering the brain tissue. Such exclusion serves to protect the brain and maintain homeostasis. However, if brain pathologies develop that require treatment with drugs or antibiotics, the exclusion of large molecules becomes a serious problem. When drugs are being developed and tested for treatment of neurological diseases, it is important to ascertain at an early stage whether a particular drug will cross the blood-brain barrier. In systemic vessels, endothelial cells are closely associated with one another, but in most cases they do not express continuous tight junctions; rather, fenestrations exist between adjacent cells, allowing most molecules to pass into the surrounding tissue [74]. In the brain, however, neighboring endothelial cells interact with one another to form tight junctions that prevent molecules larger than 500 Da, and also many polar molecules, from passing [74]. The interactions between neighboring cells form a barrier through which only small molecules, lipophilic molecules, or those with specific transport mechanisms can pass [1].
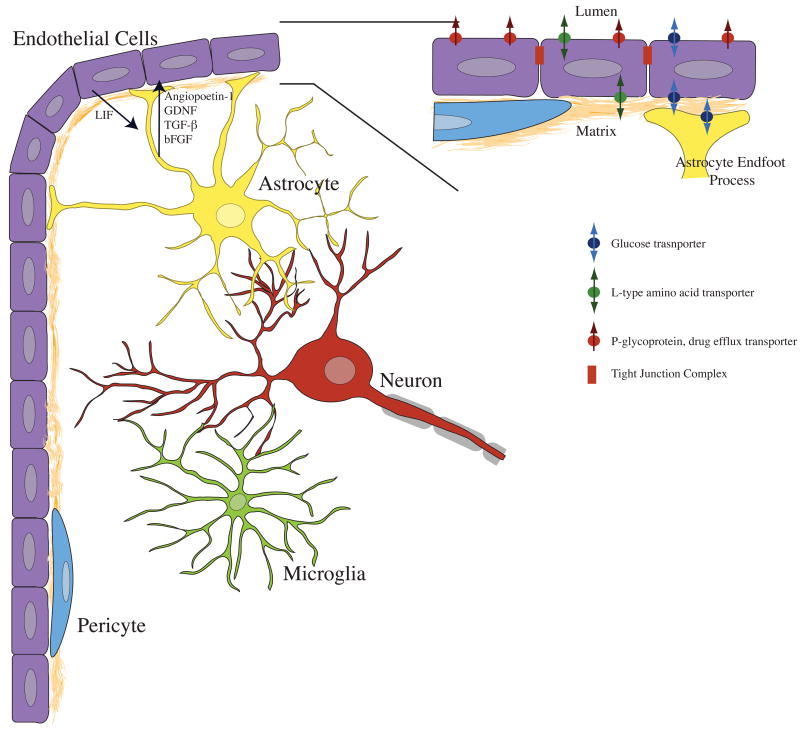
The blood-brain barrier is formed by complex interactions between several cell types (Fig. (1)). Endothelial cells form the barrier structure by tight junction interactions with other endothelial cells. Glial cells, neurons, and pericytes function to support specialization of the brain endothelial cells, and to aid in transport and metabolism. The interactions among these cell types occur by both physical and chemical mechanisms. Scanning electron microscopy of freeze fractured brain slices has shown the location of tight junctions between endothelial cells, as well as the physical basis for astrocyte/endothelial cell interactions, i.e., unique astrocytic processes known as end feet. Both co-culture and conditioned-medium experiments have provided clues about the chemical signaling that occurs between neural cells and endothelial cells [75–78]. Chemical signaling between endothelial cells and other brain cells can alter endothelial cell morphology, transporter expression, and expression of tight junction proteins [79–82].
Figure 1. Endothelial cells interact with a variety of brain cell types to produce specialized blood-brain barrier tissue.
Astrocytes produce several factors that have been shown to induce formation of tight junctions, and expression of blood-brain barrier specific protein markers. Factors including angiopoetin-1, GDNF, TGFβ and bFGF can activate endothelial cells by modulation of intracellular cAMP levels. Endothelial cells signal astrocytes via leukemia inhibitory factor (LIF). The chemical signaling among neurons, microglia, and pericytes is not as well understood. However, neurons are metabolically coupled to both astrocytes and vascular endothelial cells, and are rarely found more than 20–30 micrometers from capillary microvessels. Microglia are often associated with vascular elements in tissue slices, and pericytes occupy space within the basal lamina, directly associating with the abluminal surface of endothelial cells. Inset. Astrocytes closely associate with endothelial cells by contacting the basal lamina with specialized endfoot processes. Barrier function is determined by several factors, including tight junctions between endothelial cells, as well as cellular metabolism and transporter expression. Endogenous transporters of glucose and large neutral amino acids are present on the luminal and abluminal surfaces of endothelial cells. Xenobiotic efflux transporters such as p-glycoprotein (Pgp) are often located exclusively on the lumen-facing membrane of endothelial cells.
2. Structure
Dye transfer experiments provided the first data to support the existence of the blood-brain barrier. Experiments performed by Ehrlich, and later by others using intravenously administered Prussian blue or Evans blue dye, demonstrated that almost all tissues of the body, with the exception of the brain, were stained [83]. Prior to the use of electron microscopy, it was debated whether the endfeet of protoplasmic astrocytes or endothelial cells formed the barrier to dye diffusion into the brain [83]. Through the use of horse radish peroxidase (HRP) and electron microscopy to analyze the distribution of reaction products, it was possible to observe tight junctions between cells that had occluded the diffusion of HRP into the brain tissue [84]. The basal lamina and astrocyte endfeet ensheathing endothelial cells on their abluminal surface are clearly visible in electron micrographs [85]. Although astrocyte endfeet completely surround brain capillaries, discrete openings are present between endfeet. Granularized pericytes also surround endothelial cells, functioning to engulf many substances that cross endothelial cells paracellularly (between cells), or by transcytosis [86].
Brain capillary endothelial cells at the blood-brain barrier contain fewer vesicles than do cardiac or skeletal vessels, as can be seen in electron micrographs. HRP is not transcytosed within vesicles, indicating that vesicular transport may occur only rarely in brain endothelial cells. These observations have been confirmed by many electron microscopic studies, and endothelial cell characteristics such as tight junction formation, mitochondrial density, vesicle density, and transporter location have been quantitatively compared between tissues [87, 88]. Electron microscopy has been used to describe the localization of blood-brain barrier specific markers such as GLUT1, von Willibrand factor, and junctional proteins [89–91].
a. Basal Lamina
In brain tissue, endothelial cells exist as tube-shaped monolayers of cells bound closely together by adherens junctions and tight junctions, and surrounded by a basement membrane referred to as the basal lamina. The basement membrane is approximately 30 to 60 nanometers thick and separates endothelial cells from astrocytes, pericytes, and smooth muscle cells. It is composed primarily of connective tissue consisting of extracellular matrix proteins and proteoglycans (proteins conjugated to sugar moieties). The basement membrane provides structural support and is thought to contribute to induction of blood-brain barrier phenotype and tight junction formation, as well as cell polarity [92]. Extracellular matrix proteins present in the basal lamina include laminin, fibronectin, and collagen IV. Major proteoglycans include heparin sulfate and chondroitin sulfate. In vitro studies have shown that endothelial cells will attach to several types of collagen, laminin, and fibronectin, as well as to peptide epitopes of these proteins including YIGSR and RGD [93]. Others studies have shown that laminin can induce morphological tube formation in cultured endothelial cells, and that fibronectin enhances the proliferative capacity of endothelial cells [93]. Astrocytes have been observed to interact with endothelial cells at the basement membrane. The combined interactions among neural cells, extracellular matrix, and capillary endothelial cells are critical to the development and structure of the blood-brain barrier.
b. Tight junction proteins
Tight junctions comprise the physical barrier to transport between endothelial cells, and are formed through the interactions of several types of transmembrane and cytosolic proteins including claudins, occludins, zonal occludin (ZO) proteins, and junctional adhesion molecules (JAMs) [4, 94]. Tight junction protein complexes function to anchor cells together at cell boundaries, creating polarized cell layers and preventing paracellular transport of many neuroactive compounds. Occludins, claudins, and JAMs are transmembrane proteins that span both the inner and outer leaflets of plasma membranes [95]. ZO proteins are located in the cytoplasm in the immediate vicinity of the plasma membrane [96].
Occludin is an integral membrane protein that contains no DNA sequence homology to proteins found in adherens junctions, gap junctions, or tight junctions. Occludin is localized to the plasma membrane and contains four hydrophobic membrane spanning segments. The membrane orientation of occludin is similar to the gap junction protein connexin, forming a linear connection between cells that occludes the flow of solutes and is characterized by high electrical resistance [97]. Overexpression of occludin has been shown to induce the formation of structures resembling tight junctions [98]. Mutations in occludin disrupt the formation of tight junctions [99]. Occludin is not absolutely required for formation of tight junctions; however, transgenic mice showing decreased/zero expression of occludin develop complex phenotypes displayed as stunted growth, and brain calcification, without any apparent loss of vascular electrical resistance [100]. In vitro, tight junctions can still form in the presence of a truncated form of occludin, as well as when only trace amounts of occludin are expressed [101, 102]. Occludin is expressed in brain endothelial cells at higher levels than in other tissues; it is transcriptionally regulated, and is dynamically expressed throughout development [103].
Claudins are transmembrane proteins essential to tight junction formation both in vivo and in vitro [104]. Claudins, discovered after occludin, are part of a large family of proteins found in a variety of tissue in many isoforms. Claudins 1/3 and 5 are found on endothelial cells and contain extracellular domains that interact directly with other claudin molecules located on adjacent cells [104]. Like occludin, claudin contains four transmembrane domains. Claudin 1/3 is expressed in both barrier and non-barrier endothelial cells, and claudin 5 is found primarily in high electrical resistance tight junctions formed between barrier endothelial cells [94]. Claudin forms the physical barrier between cells and is the primary determinant of endothelial cell layer permeability, while occludin likely plays a supporting role by modulating permeability.
Inside the cell, ZO proteins interact with actin filaments to anchor claudin and occludin proteins to the cytoskeleton [96, 105]. Z0-1 located at the blood-brain barrier, and may also help localized occludin and claudin to tight junction complexes [96, 106]. Other proteins that contribute to tight junctions include JAM and endothelial cell selective adhesion molecule (ESAM). JAMs are part of the immunoglobin superfamily and bind to JAMs on adjacent cells through homophillic interaction domains. Brain endothelial cells also form adherens junctions, composed of cadherin-10, N-cadherins, VE-cadherin, and platelet endothelial cell adhesion molecule (PECAM), forming belts around cells [107, 108]. Although cadherins have been associated with the blood-brain barrier, they function primarily in communication and relay of signals between cells at adherens junctions rather than occluding paracellular transport [109].
3. Transporters
At the blood-brain barrier, most compounds are not transported paracellularly, because of tight junctions between endothelial cells. In order for compounds to cross into the brain tissue, they must therefore pass through cells. Transcellular transport occurs through a variety of mechanisms including lipophillic transcytosis, specialized transporters, receptor mediated transcytosis, and adsorbtive transcytosis [5, 110]. The primary function of these pathways is to supply the brain with nutrients. However, many drug delivery methods have targeted these transport systems to allow passage of exogenous compounds into the brain [110].
Endothelial cells as well as the glia proximal to them express the glucose transporters GLUT 1 and GLUT 5 [111–113]. The glucose transporters function to sustain the central nervous tissue with high amounts of glucose, and have been localized immunohistochemically to the cells that compose the blood-brain barrier. Another important specialized transport system involves the transport of neutral amino acids. Two systems have been described in vivo and in isolated capillaries. The A-type Na+ dependent system is located only on the abluminal membrane of endothelial cells and functions to transport amino acids out of the brain against concentration gradients [114]. The L-type Na+ independent system is present on both luminal and abluminal endothelial cell membranes [115]. Both GLUT-1 and L-type amino acid transport systems function by transporting molecules down their concentration gradients.
Iron is supplied to the brain by receptor mediated transcytosis of transferrin. The transferrin receptor is located at the blood-brain barrier on the plasma membrane of endothelial cells and on vesicles within cells [116]. Expression of transferrin receptor has been characterized in vivo, as well as in endothelial cell lines [117]. Insulin transport also occurs by receptor mediated transcytosis. Transcytosis can also occur by adsorption of positively charged peptides or plasma proteins. Electrostatically bound compounds can be packaged within vesicles and transported across the cell.
There are also many efflux transporters that localize to the blood brain barrier on the abluminal plasma membrane of endothelial cells [118]. Neurotransmitters such as GABA, serotonin, norepinephrine, and dopamine, as well as metabolites such as homovanillic acid (HVA) are also transported out of the brain by specific endothelial cell efflux transporters. Pgp, located on the luminal surface of endothelial cells is responsible for the efflux of many xenobiotics and therapeutics. Pgp is primarily responsible for transport of lipophilic compounds, moving compounds against concentration gradients, and obtaining energy through ATP consumption. The organic anion transporter (OAT3), located on the abluminal membrane of endothelial cells, is responsible for efflux of negatively charged compounds from brain tissue. Multidrug resistance associated protein (MRP2) is also expressed in brain capillary endothelial cells and contributes to the efflux of estradiol and glucuronide. The ATP-binding cassette transporter family G2, located on the luminal membrane, is responsible for the efflux of the therapeutics prazosin and mitoxantron [119].
Astrocytes present at the blood-brain barrier also express a variety of transporters. Astrocytes express GLUT1, as well as high concentrations of aquaporin-4 water channels and Kir 4.1 K+ channels that are used in the maintenance of ionic homeostasis [5]. These channels are localized and anchored to the endfeet by the heparin sulfate proteoglycan agrin. Agrin is located within the basal lamina and may also play a role in barrier tightening [5, 94].
4. Metabolism
Besides establishing a physical barrier, the blood-brain barrier represents a metabolic barrier. Extracellular peptidases and nucleotidases break down proteins and nucleotides as these traverse the blood-brain barrier. Intracellular enzymes such as monoamine oxidase and cytochrome P450 process a wide range of bioactive and toxic molecules, and can alter the chemistry of drugs as the drugs pass through endothelial cells and astrocytes [120]. GGTP, a membrane enzyme involved in amino acid transport and glutathione metabolism, is found at the blood-brain barrier in both endothelial cells and pericytes, further highlighting the metabolic importance of the cells that make up the blood-brain barrier [121–123].
5. Development
The specialization of the blood-brain barrier results from signaling between neural cells and endothelial cells. The endothelial cells at the blood-brain barrier express a variety of enzymes, markers, and transporters that either do not occur or else occur in lesser amounts in the endothelial cells of systemic blood vessels. Cultures of endothelial cells with neural cells or in the presence of cell-conditioned medium show increases in blood-brain barrier phenotypes characterized as decreases in permeability and expression of blood-brain barrier specific proteins. The latter include alkaline phosphatase, angiotensin converting enzyme, aromatic amino acid decarboxylase, EBA, GGTP, GLUT1, nonspecific cholinesterase, and von Willibrand factor [124, 125]. The morphology of brain endothelial cells is also different from that of systemic endothelial cells. Brain endothelial cells contain more mitochondria and fewer vesicles, and they display tight junctions between cells. The blood vessels of the brain tissue are surrounded by the basal lamina as well as by a number of cell types including astrocytes, microglia, pericytes, smooth muscle cells, and neurons.
Astrocytes have been shown to induce the formation of blood-brain barrier phenotypes in endothelial cells in vivo and in vitro. Astrocytes contact the basal lamina via endfoot processes and secrete a number of factors that cause phenotypic changes in endothelial cells. These factors include angiopoietin 1, glial derived neurotrophic factor (GDNF), transforming growth factor β (TGF-β), and basic fibroblast growth factor (bFGF) [79, 80]. Factors secreted by astrocytes bind receptors expressed on the surface of endothelial cells, modulating the levels of cytosolic second messengers like cAMP and cGMP, causing increased expression of tight junction proteins and transporters [126, 127]. Endothelial cells in turn secrete factors such as leukemia inhibitory factor (LIF) that induce differentiation of astrocytes.
Transplantation studies, beginning with a quail-chick chimera system, have shown that induction of blood-brain barrier phenotypes in endothelial cells is dependent upon the tissue environment in which the endothelial cells grow. Endothelial cells only acquire the full complement of barrier properties when in contact with neural tissue [128]. Other in vivo studies demonstrated that transplanted astrocytes alone are capable of inducing blood-brain barrier phenotypes in the non-barrier endothelial cells located in the eye anterior chamber [129]. These in vivo observations are supported by many in vitro studies.
The addition of glial cells, glial cell membranes, or neuronal cell membranes in culture medium increases the blood-brain barrier enzyme activity in cultured brain capillary endothelial cells [75]. However, in these experiments the increased enzyme expression was dependent upon a number of other factors including endothelial cell proliferative state [130]. A similar effect was observed when endothelial cells were cultured in the presence of astrocyte-conditioned medium without astrocytes present in culture [77]. Formation of tight junction complexes and reduced permeability to polar molecules have been observed when endothelial cells are cultured in the presence of soluble factors released from co-cultured astrocytes [76]. Direct contact between astrocytes and endothelial cells may be important in blood-brain barrier development. Close apposition of astrocytes with endothelial cells may increase signaling, causing induction of the barrier phenotype in endothelial cell layers [131]. Barrier properties can also be induced in systemic endothelial cells through close apposition with astrocytes [132].
Neurons, pericytes, and microglia are also though to play important roles in barrier formation and function, by inducing barrier specific phenotypes in endothelial cells, and by modulating astrocyte activity. There is some evidence that neurons contribute directly to the induction of tight junction formation during development. Proper targeting of occludin, one of the proteins that constitute tight junctions in endothelial cells, to the cell periphery has been shown to occur only after co-culture with neurons [133]. There is evidence for a synergistic effect on tight junction formation when astrocytes and neurons are cultured with endothelial cells [134]. Neurons in combination with extracellular matrix components can also play a role in regulation of occludin expression [133]. Neuronal activity is closely coupled with astrocyte and blood vessel functions in the brain. Astrocytes increase Ca2+ signaling and secrete the vasodilator cox1, in order to increase blood flow through brain capillaries and support the metabolic demands of neurons [135]. Neurons can also influence the contraction of smooth muscle cells lining larger blood vessels, so as to alter blood flow. Pericytes can influence barrier formation through TGF-β signaling [71]. Microglia are often juxtaposed with brain microvessels, and migrate along microvessels during development [136]. Microglia have been observed to surround brain microvessels in tissue slices from adult rats [81].
III. Cell culture models of the blood-brain barrier and cell culture analog systems
Relatively pure cell preparations can be routinely prepared from the brain, thus permitting the culture of a variety of cell types including astrocytes, microglia, oligodendrocytes, neurons and brain vasculature [137–139]. In addition, these cell types can be isolated from various brain regions and at various stages during development [140]. By use of well established protocols, usually involving enzymatic dissociation of tissue and subsequent purification steps, the cells can be cultured with high viability, on a variety of surfaces treated with cell adhesion molecules or charge alteration so as to promote cell attachment and process outgrowth [141, 142]. This type of culture system has been traditionally employed in toxicology and drug screening to assess the efficacy, dose, and mechanism of action for neuroactive compounds because of its relative ease of use and reproducibility of results. In such culture systems, drug kinetics can be estimated and the action of the test compound can be assayed through induction of changes in cell morphologies, alterations in gene or protein expression, or changes in the electrical activity of neurons and astrocytes.
Many two-dimensional cell culture systems have been used to study neurotransmitters, receptor agonists and antagonists, neurotrophins, and anticancer therapeutics. Cell culture has been used for both high-throughput screening as well as small scale laboratory studies of neurotherapeutics. Application of cell culture models is therefore one of the first steps in the development of novel therapeutics. However, many compounds that show desirable characteristic in cell culture systems fail as soon as they are moved into animal trials. In vitro cell culture systems, while valuable research tools, do not recapitulate the cell-substrate interactions that occur within tissue in vivo. The diffusion of compounds within a given tissue is typically limited by high concentrations of soluble and insoluble matrix proteins and connective tissue. Cell density in tissue is typically orders of magnitude higher when compared to traditional two-dimensional cell culture systems. Due to the cellular heterogeneity in tissue, interactions between cell types can produce differences in protein expression between in vivo and in vitro systems.
Many attempts have been made to recapitulate the cell interactions found in vivo at the blood-brain barrier by using co-cultures of neural cells and endothelial cells. Much of the current knowledge regarding the formation and structure of the blood-brain barrier development and physiology was derived from in vitro models. A number of simple and complex models exist but, to date, none have been completely successful in recapitulating transport properties and barrier function.
A. Techniques for monitoring blood-brain barrier properties
1. Permeability
Blood-brain barrier permeability has been assayed in brain capillaries in vivo as well as in vitro [143–146]. In vivo permeability is determined by intravenous administration of a compound, followed by attempts to determine the fraction that passes into the brain tissue. In vitro permeability screens can be accomplished by assay of the transport of molecules across endothelial cells grown as a monolayer on a membrane or other permeable surface [147,148]. Compounds commonly used in permeability studies include dyes such as Evans blue or Prussian blue. Sucrose is commonly used in vitro, as are caffeine, dextran, poly(ethylene) glycol, urea, aminoisobutyrate, and bovine serum albumin. For in vitro permeability testing, it is desirable to test many nontoxic compounds that vary in molecular weight and polarity. Other compounds that have been used to validate permeability screening models include amino acids, retinoids, mannitol, inulin, and the antipsychotic haloperidol.
One difficulty with the current in vitro transport screening systems is that a relatively large area must be uniformly covered with an endothelial cell monolayer if accurate measurement of transport or electrical resistance is to be made. Other complications in screening include the formation of uniform tight junctions between cells, and expression of proteins responsible for transcellular transport. Test compounds cross at any breach in the barrier, even if most sections are intact and expressing blood-brain barrier phenotypes. Furthermore, kinetic measurements can be complicated by the adsorbtion of compounds to the artificial cell substrate, or by diffusion limitations caused by the artificial membrane pore size.
2. Transendothelial electrical resistance
Transendothelial electrical resistance (TEER) systems are a powerful method for testing many blood-brain barrier models [149–151]. One of the first applications of TEER was conducted in vivo in order to measure the resistance of muscle capillaries in frog [152]. It was also initially used to measure the resistance of epithelial cell layers in vivo and in vitro. Instead of measuring the transport rate of chemicals, TEER systems measure the transport of electrical current. By positioning electrodes on opposite sides of endothelial cell layers, barrier permeability can be monitored indirectly in real time. During TEER measurement, current must flow through the tissue or cell culture systems between electrodes. Along the path between the electrodes, the current will be modified by resistors, capacitors, and other circuit elements in the environment. In an electrolyte lacking cells, the electrical properties are determined by the conductance of the solution in which the electrodes are placed, giving the resistance of the solution Rsol. In most culture systems and in vivo, the environment is filled with matrix proteins and cells that provide additional resistance and capacitance (Fig. (2)).
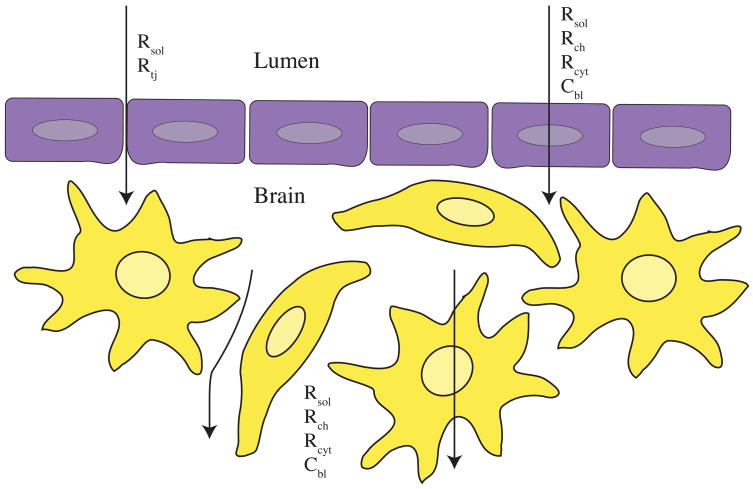
Figure 2. TEER measures the resistance of current flowing through a blood vessel or culture system.
Endothelial cell (purple) monolayers contribute several circuit elements that impede the flow of current (black arrows). Intracellular elements include the resistance of membrane channels (Rch) and cytoplasm Rcyt as well as the capacitance of endothelial cell lipid bilayers (Cbl). The formation of tight junction complexes between endothelial cells contributes to the extracellular resistance of the system (Rtj). Astrocytes (yellow) also impede the flow of current in TEER systems, by contributing to Rch, Rcyt and Cbl. The resistance of the extracellular solution (Rsol) also affects current impedance, as blood or luminal buffer can differ in conductance from the protein- and lipid-rich extracellular environment.
A mammalian cell contributes several electrical circuit elements to the system including a capacitor in the form of a lipid bilayer (Cbl), resistors in the form of membrane channels (Rch) and cytoplasm (Rcyt), and also reduces the amount of extracellular space through which current can flow [153]. It can be assumed that current will follow the path of least resistance to ground, so that rather than passing through cells, it will pass between cells giving the resistance of cell junctions (Rtj). Values of resistance can then be correlated to integrity of blood-brain barrier or endothelial monolayer (i.e., claudin 1/3, 5 or other tight junction protein expression). As endothelial cells form a contiguous monolayer, the resistance of the system will increase, and as junctions form between cells, the resistance will increase dramatically.
TEER measurement requires that at least one electrode be placed on each side of the cell monolayer or co-culture system. Most TEER systems are either impedance based or simplified two-electrode systems. Several commercial two-electrode systems are available for use with voltmeters. However, more sensitive systems under the control of a potentiostat allow data to be obtained through electrochemical impedance spectroscopy. The spectral data can be fit to equivalent circuit models for more detailed electrical characterization [154, 155]. Barrier models can be screened using this method for subsequent use in more specific compound transport experiments. TEER has been used as a screening or characterization method in most transwell permeability models and transport experiments (see text below). TEER measurements from rat brain capillaries typically range between 1100 and 1500 Ωcm−2 [94]. It is rare however, for in vitro systems to display TEER values larger than 1000 Ωcm−2.
B. Blood-brain barrier models
1. Endothelial cell isolation
Most in vitro models start with the isolation of endothelial cells from brain capillaries. Preparations of rat brain capillary endothelial cells are obtained by enzymatic or mechanical dissociation of cortical grey matter, followed by purification from white matter and cell debris using dextran, nylon mesh, glass beads or bovine serum albumin. In a second round of enzymatic digestion, the basal lamina and pericytes are removed from capillaries, and capillary fragments isolated by centrifugation through a gradient of percoll [138, 139, 156]. Cell preparations, typically grown on a surface treated with collagen, are examined by electron microscopy to examine cell morphology; by immunohistochemistry to assess expression of cell type specific markers [138], or by enzymatic activity of GGTP, alkaline phosphatase [156] or monamine oxidase [120]. Similar procedures have been used to isolate brain capillaries from bovine, porcine, and murine tissue [147, 157]. Immortalized brain endothelial cells have been produced by transfection of primary brain capillary endothelial cells with plasmids containing the SV40 large T antigen [150, 158]. Once isolated, however, endothelial cells do not typically maintain blood-brain barrier phenotypes or even endothelial cell phenotypes unless co-cultured with neural cells. Endothelial cells cultured alone often fail to express von Willibrand factor, EBA, and tight junction markers such as claudin.
Endothelial cells can also be isolated from systemic sources including aorta or umbilical vein. However, in systemic blood vessels, tight junctions are normally interrupted by gap junctions, and fenestrations are present between endothelial cells that allow most compounds to pass through.
2. Two-dimensional cell culture
Some endothelial cell culture models are achieved by the culture of cells on glass to enable observation of biochemical or immunohistochemical changes in the cell populations. Typically, brain endothelial cells must be cultured on a surface treated with matrix proteins such as collagen, laminin, fibronectin, or matrigel to promote attachment and simulate the basal lamina. Co-cultures of cells can be produced on glass or other two-dimensional substrates that allow direct contact between cells. The advantages of this type of model are that endothelial cells can interact directly with other cell types, cultures are easy to produce, and substrates can be easily viewed by light microscopy. However, this type of system lacks cellular organization, so it is not amenable to measurement of transport or TEER.
3. Transwell cultures
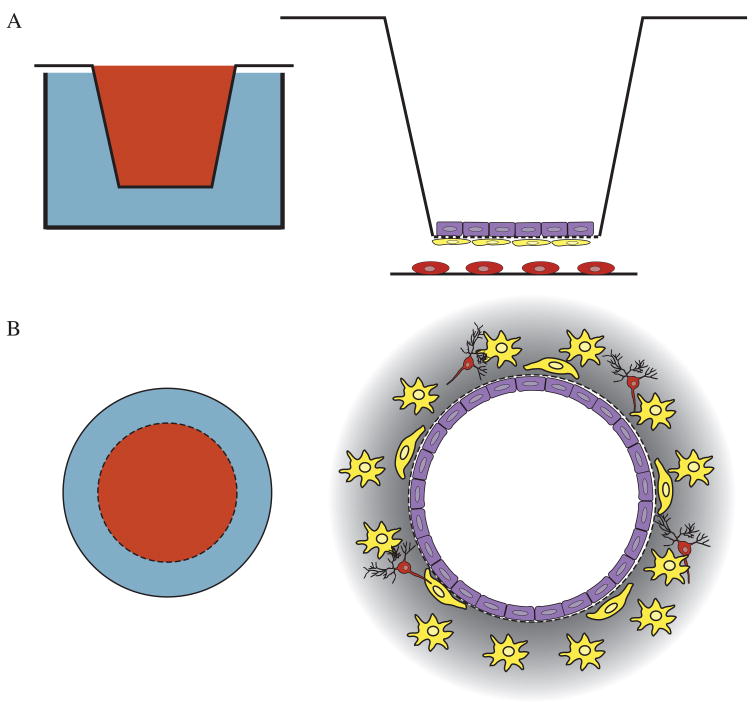
Most permeability studies involve growth of endothelial cells in the compartments of transwell tissue plate inserts (Fig. (3a)). Following collagen treatment of the transwell membrane, endothelial cells can be grown in the upper compartment to confluence and assayed for resistance and for permeability to test compounds [127, 147). With this type of culture setup it is possible to assay the effects of cell-conditioned medium; to co-culture with glia or other cell types; to measure levels of second messengers; to monitor endocytosis; and to characterize the cell preparations by immunohistochemistry [77, 127, 147]. Although enzymatic activity of endothelial cells and barrier specific markers can be assayed in co-cultures grown on glass, the transwell system provides the advantage of physical separation of the cells and allows the growth of uninterrupted endothelial cell monolayers for transport analysis. Attempts have been made to validate this model by correlative comparison to the brain uptake indices of selected compounds [146]. The permeability profile for transwell culture systems is in agreement with in vivo data, although overall permeability is generally greater for most compounds than is the case in vivo [144].
Figure 3. Transwell and dynamic in vitro blood-brain barrier (DIV-BBB) systems have been used to model blood-brain barrier development, permeability, and transport.
A. Transwell systems utilize tissue culture plate inserts containing permeable membranes that can be functionalized with proteins or peptides to permit cell attachment. The transwell inserts can be positioned within tissue culture plates for the culture of up to three cell types on separate surfaces. B. The DIV-BBB is composed of a permeable capillary tube on which cells are grown within and around. This culture setup creates a three-dimensional culture system in which multiple cell types can be cultured, and also allows endothelial cells to grow in tube-like formation. The DIV-BBB permits both the flow of medium and sampling from the cultures through ports. In the diagrams on the left, red represents luminal space and blue represents tissue space. In the diagrams on the right, purple corresponds to endothelial cells, yellow to astrocyte and red to neurons.
The transwell system allows growth of cells not only on both sides of the filter membrane, but also on the culture dish below, where cultured primary glia or glioma cells can release factors to induce barrier formation [76]. This culture setup allows the simultaneous culture of up to three cell types on three separate surfaces [159]. Bilayer-type cultures show lower permeability and full expression of blood-brain barrier markers, including tight junction formation, indicating that separation between astrocytes and endothelial cells may affect blood-brain barrier formation [132, 160].
The transwell system has been used to characterize passive diffusion of polar molecules across endothelial cell monolayers, as well as carrier-mediated and lipophilic transcytosis of lipophilic compounds [161]. This model has been tested in both serum-containing and defined serum-free conditions, and has been shown to be impermeable to sucrose [145]. In relation to xenobiotic efflux transporters, the transwell model has been characterized for Pgp expression in endothelial cells [78].
The advantages of the transwell model are its simplicity and potential to be used in transport and TEER analysis. The transwell system is the best-characterized model with respect to immunohistochemical markers, enzyme activity, compound permeability, electrode resistance, transporter expression, junction formation, cell morphology, and actin distribution. It has been characterized using a number of different cell sources including rat, porcine, bovine, and immortalized cells [162]. Some shortcomings include the inability to accurately screen multiple compounds simultaneously, complications in TEER measurement and permeability studies due to the pore size of the transwell membrane, and barrier to direct contact between co-cultured cell types [163]. Some membranes also do not permit transmission of light for microscopic analysis. Attempts have been made to produce lower resistance membranes of thinner material containing larger pores [148]. However, these culture systems have not been well characterized in terms of reproducible fabrication, cell transport properties, and tight junction formation.
4. The dynamic in vitro blood-brain barrier
An alternative to the transwell system is the hollow-fiber apparatus, also known as the dynamic in vitro blood-brain barrier (DIV-BBB) (Fig. (3b)). The DIV-BBB model attempts to recapitulate the blood-brain barrier through culture of endothelial cells within hollow-fiber capillary tubes mounted within a sealed chamber [164]. The capillary tube represents the luminal space of blood vessels and the sealed chamber represents the extraluminal space. Ports make the extraluminal space accessible, and a pump drives flow through the inner capillary tube. This culture setup can be monitored by TEER, and shows a permeability profile similar to the profile in vivo for a variety of polar substances, including sucrose, mannitol, and morphine [165].
The K+ flux, glucose consumption, and lactate production of endothelial cells and endothelial cell/glial cell co-cultures have been characterized under flow conditions [164, 165]. The system has also been used to model blood-brain barrier nucleoside efflux [167]. One of the key features of this model is the ability to generate controlled flow, allowing continuous real-time monitoring of permeability and metabolite production. The flow can be modified to allow modulation of barrier properties as well as the introduction of test compounds under pseudo-physiological conditions [166]. Also important is the three-dimensionality of this system; endothelial cells can thereby grow within a tube -like structure surrounded by co-cultured glia and neurons. This model has been characterized for the transport of serotonin, for nucleoside efflux and for a variety of model compounds. The drawbacks to the DIV-BBB model are that it does not permit microscopic evaluation of tight junction formation, and it does not allow direct contact between neural cells and endothelial cells.
5. Biomimetic hydrogel models
Tissue engineering approaches can be used to construct novel blood-brain barrier models. Multiple cell types can be cultured within and on the surface of hydrogel matrices (Fig. (4a,b)). The hydrogel matrix containing the cells can be constructed above arrays of microelectrodes for continuous real-time TEER monitoring with electrochemical impedance spectroscopy (Fig. (4b), Fig. (5)). Alginate hydrogels are used to reconstruct the three-dimensional tissue environment found in vivo. Alginate can be covalently modified by attachment of peptides or whole proteins in order to promote cell attachment and growth. The culture setup currently used by our lab consists of glial cell encapsulated in three dimensions within a matrix of alginate containing an RGD peptide epitope and laminin, for integrin receptor mediated attachment of cells [153]. Following glial cell encapsulation within the matrix, endothelial cells can be cultured on the surface of the alginate matrix. This system allows the cell types to be spatially separated yet able to interact directly at the alginate surface.
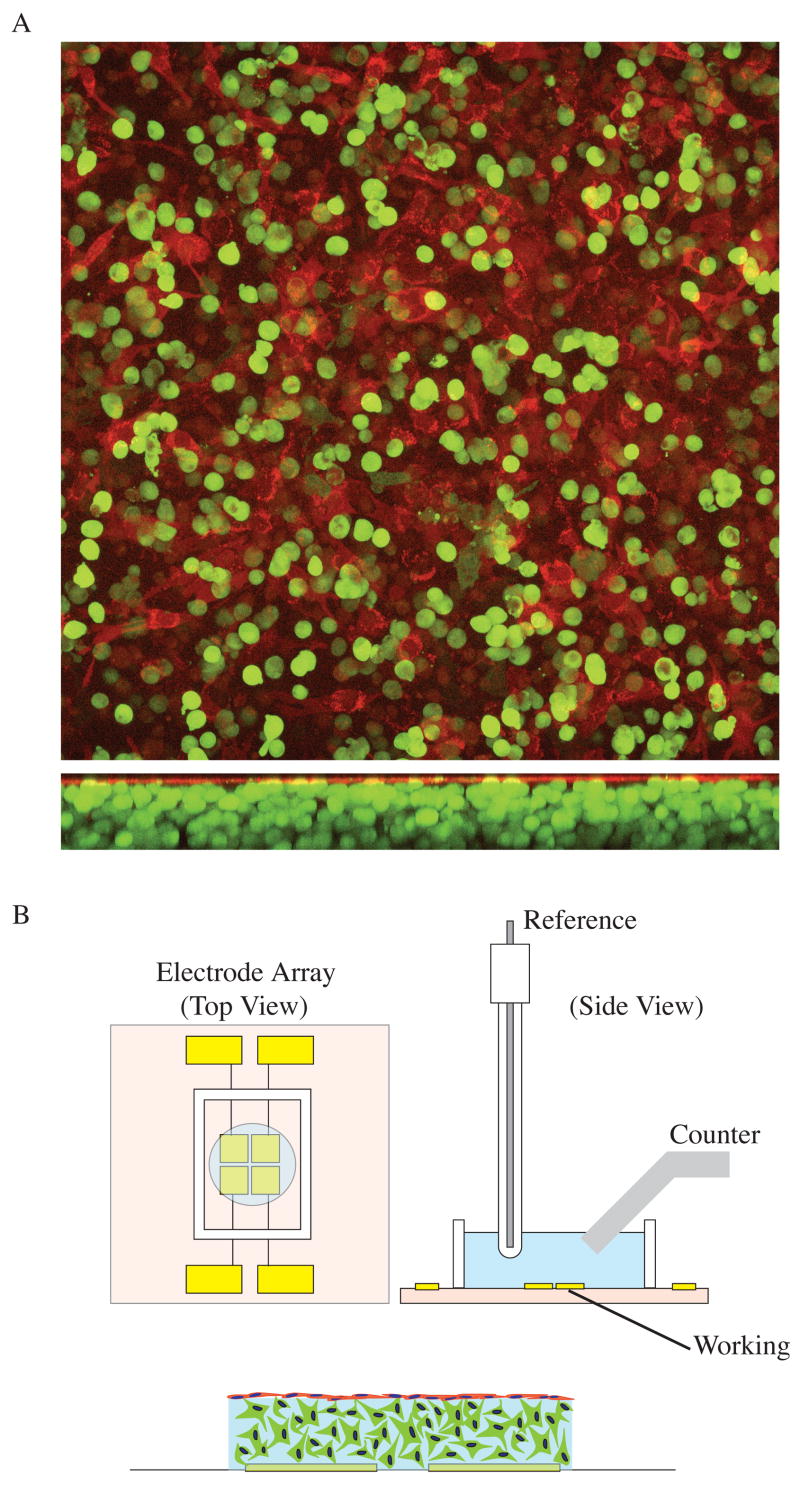
Figure 4. Hydrogel constructs permit the co-culture of glia and endothelial cells, thereby enabling direct interactions between cell types.
A. LRM 55 astroglial cells (green) and bovine aortic endothelial cells (BAEC; red) were transfected using an Amaxa Nucleofector (Amaxa, Gaithersburg, MD). Cells were transfected as separate populations, with eGFP for LRM55 and mCherry for BAEC. A Leica SP5 confocal microscope (Leica, Bannockburn, IL) was used to image live samples after 7 days in culture. This type of culture system permits direct interactions between cells at the alginate hydrogel surface. B. Cell constructs were created above arrays of microfabricated electrodes, to enable TEER measurements. Each electrode array consisted of four 3x3 mm low-impedance Au electrodes. An Ag/AgCl reference electrode and a Pt foil counter electrode were positioned in the buffer above the tissue construct and Au working electrodes to enable TEER measurement using electrochemical impedance spectroscopy. Impedance could be monitored for up to 2 weeks using a Gamry potentiostat (Gamry, Warminster, PA).
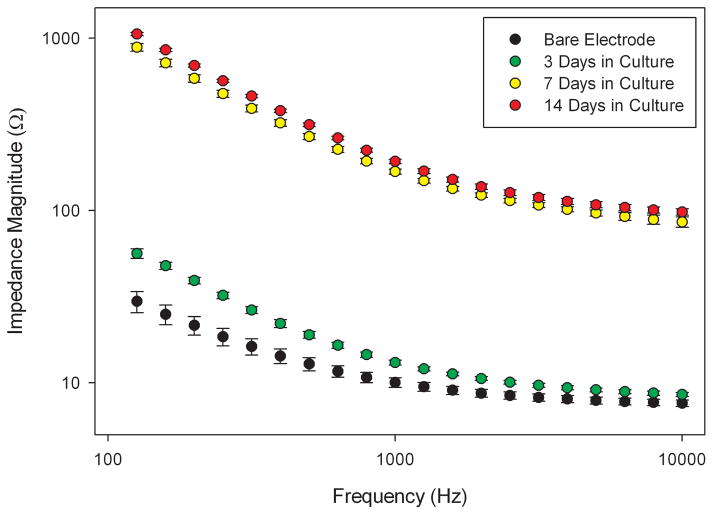
Figure 5. TEER can be used to evaluate culture systems for endothelial monolayer and barrier formation.
Endothelial cells were cultured on astrocyte-seeded hydrogels as shown in Fig. (4). Electrical impedance was measured on bare electrodes, both before a contiguous monolayer of cells had formed (3 days), and after formation of an endothelial cell monolayer (7 and 14 days). Error bars represent SEM. Impedance was measured over a range of frequencies so that the effects of cell seeding, as well as the intrinsic electrode impedance properties, could be evaluated [153]. TEER has been well established as a method for assessing endothelial cell cultures in blood-brain barrier models. Electrochemical impedance spectroscopy provides an accurate and sensitive method for evaluating TEER.
The astrocyte-endothelial tissue constructs can be cultured above microelectrodes patterned on glass, for correlation of immunohistochemical analysis with impedance-based TEER measurement. While rudimentary flow can be applied to this system the ultimate goal is to adapt this technology for use in CCA systems containing cell culture models of other organ systems involved in drug metabolism.
Alternatively, microvascular network growth can be induced within channels or within macroporous hydrogels. Polymers such as poly(ethylene)glycol-poly-L-lysine can be used to created extensive networks of endothelial tubules [168].
C. Cell culture analog systems
CCA systems are the physical representation of pharmacokinetic models. Experiments in live animals are often not well extrapolated from in vitro data, are time consuming, costly, and subject to ethical issues. The CCA attempts to compartmentalize the key organs involved in drug metabolism and transport in an on-chip setup using cells derived from those organs. Unlike pharmacokinetic models, which rely on the anticipation of all possible mechanisms of drug action and metabolism to yield accurate information, CCAs incorporate biological compartments composed of living cells. This allows study of mechanisms that may not be anticipated or predicted from mathematical models. CCAs can be fabricated to incorporate several separate compartments corresponding to various organs including lung, liver, and adipose tissue [169]. Each compartment is connected to the next by fluidics, providing a continuous flow of medium for perfusion of the cell system, which is usually contained within a hydrogel matrix.
1. Fluidics
The use of fluidics provides several advantages over static cell culture systems. Macrochannels on the millimeter scale or microchannels ranging from several micrometers in diameter to several hundreds of micrometers can be incorporated so as to supply medium and to remove waste products, but they can also be used to apply shear forces to cultured cells. In vivo, endothelial cells are subjected to continuous pulsatile flow of blood along their luminal surface. The flow rate has been monitored and characterized by blood oxygen level dependent magnetic resonance imaging (BOLD-MRI), and the shear force on the luminal wall has been calculated based on diameter of the blood vessel, velocity of flow, and viscosity of the solution. Pulsatile flow has been shown to induce angiogenesis in vitro, and hemodynamic force has been shown to cause vascular cell differentiation, as well as differentiation of embryonic stem cells into vascular wall cells [170–172]. Endothelial cells have been observed to align themselves parallel to the direction of flow in vivo and in vitro. Flow along the luminal surface can also play roles in the polarity of transporters, and in other phenotypic characteristics. The incorporation of flow into blood-brain barrier models, as well as CCAs, is critical to the simulation of transport dynamics that occur in vivo.
Fabrication of microfluidic channels is usually achieved by replica molding of polydimethyl siloxane silicone elastomer, polystyrene, or other biocompatible polymers from silicon substrates etched with a relief pattern corresponding to the channel design [173, 174]. By photolithographic techniques, it is possible to create networks of uniform channels in series or in parallel. This technology allows fluid to be dispensed in smaller volumes for small sample or high throughput analysis. Depending on the size, orientation, and velocity of flow, it is possible to achieve laminar flow and controlled mixing within channels. Higher shear values can be applied using lower fluid velocities because of the smaller channel diameter. Microfluidics also hold the potential for reducing channel size to within the physiological range of capillary diameters, with sizes on the order of 5 um to several mm. The potential for fabrication of microfluidics within polymer matrices capable of holding cells, makes this technology an even more powerful and promising avenue in cell culture modeling. Microfluidic bioreactors have been fabricated to create cell culture models capable of maintaining cardiac myocytes and other cells types in three-dimensional microenvironments, provide a structural matrix for neurite migration, and allow continuous small scale perfusion of culture compartments [175–177].
2. Hydrogel technology
Cell culture analog systems provide a physiologically relevant scaffold for cells to grow in three-dimensions. One method for controlling cell growth that has been used by our lab, as well as in a number of tissue engineering applications, is cell immobilization within hydrogel matrices. Hydrogels are polymers that have high water content, and exhibit low immunogenicity and low cytotoxicity. Poly(ethelene) glycol diacrylate (PEGDA), chitosan, collagen, agarose, and alginate are examples of hydrogels commonly used for tissue engineering applications. Cells are seeded within hydrogels by mixing cell suspensions with hydrogel monomers, which are subsequently polymerized or crosslinked. Hydrogel solutions can be gelated by a variety of mechanisms, including UV photopolymerization (for PEGDA), ionic crosslinking (for alginate), temperature change, or pH change. Once the gel has been formed, its porous nature allows most proteins, molecules, and nutrients to enter, as well as metabolites and waste to diffuse out. It is also possible to fabricate channels within hydrogel matrices. The latter has been achieved in collagen gels for creation of in vitro capillary networks as well as with high fidelity in PEGDA gels [178–180].
Many cell types, such as chondrocytes, osteoblasts, smooth muscle cells, follicle, islet cells, and dopaminergic PC12 cells have been cultured within hydrogels and have been demonstrated to remain viable [181]. Hydrogels can be functionalized to promote cell survival and process growth by covalent crosslinking of peptide sequences or proteins. Peptide sequences such as RGD, IKVAV, and YIGSR may be incorporated into hydrogels [182–184]. Incorporation of peptides or proteins is particularly important when culturing anchorage dependent cells within a nonadherent matrix.
Alginate, in particular, has been useful for the encapsulation of mammalian cells, because its mechanical properties are very similar to tissue [185–187]. Through the variation of concentration, chain type, porosity [188] and attached ligands [182, 184], it is possible to tailor alginate matrices for specific tissue culture applications.
Through a combination of tissue engineering approaches, and microfluidics it is possible to construct culture setups for toxicological experiments and for continuous cell culture monitoring [189]. In one example, bioconversion of naphthalene and GST activity were measured in real time [169]. Blood-brain barrier models may eventually be incorporated into these multi-compartment CCA systems or other bioreactor systems, for assessment of the bioconversion and permeability of neuroactive compounds.
IV. Targeted delivery of compounds to the central nervous system
In vitro modeling of blood-brain barrier tissue can provide valuable information about the mechanisms of barrier development and transport, and is a potentially useful tool for pharmaceutical screening. To date, no model has been developed that fully recapitulates the properties of the blood-brain barrier as found in vivo. However, a number of strategies have been development for delivery of nonpermeable compounds across the blood-brain barrier. Some of these strategies utilize biological mechanisms to enhance compound delivery, while others entail a physical approach to delivery [110, 190].
A. Blood-brain barrier disruption
Osmotic disruption has been used to transiently disrupt the blood-brain barrier through administration of an arterial infusion of hyperosmotic solution that temporarily shrinks endothelial cells and creates gaps between cells for paracellular diffusion of drugs into the brain tissue [191]. Osmotic barrier disruption has been used in the delivery of chemotherapeutic agents, which are often co-administered with lipid solubilizing agents [191–193]. Unfortunately, this technique also allows the diffusion of many other endogenous chemicals that affect brain function, affects large areas, and is difficult to optimize from subject to subject.
A similar technique utilizes high frequency ultrasound to induce a transient opening of the barrier at discrete locations [194, 195]. Although it is more spatially restricted, this technique also allows entry of endogenous chemicals, and the side effects are poorly characterized. Bradykinin agonists, and other molecules that affect vascular permeability, can be co-administered with central nervous system drugs to increase diffusion between endothelial cells [196]. Because these chemicals also disrupt systemic vessels, their use is not ideal.
B. Use of endogenous transport mechanisms
Less disruptive methods entail carrier meditated entry of drugs into the brain. While the majority of CNS drugs are water soluble, modern biochemical and molecular biology techniques allow the large-scale production of more lipophilic forms of these compounds that have an improved chance of entry via lipophilic trancytosis. Alternatively, drugs can be encapsulated within liposomes for transport and uptake into the brain. Recent advances in nanotechnology should permit further exploitation of the lipophilic pathway, via the use of nanoparticle or self assembling-monolayers to coat drugs and enhance the lipophilic or transport properties [197–199]. Tethering of drugs to plasma proteins can also be used to transport chemicals via plasma adsorbed trancytosis.
Another effective strategy for delivery is to use endogenous transporters that are located on the luminal surface of endothelial cells. Several therapeutics including, L-DOPA, melphalan, alpha methyl DOPA, and gabapentin, are known to cross into the brain via the L-type amino acid transport system. Other possible targets are the glucose transporter, lactate transporter, cationic amino acid transporter, and CNT2 adenosine receptor [110, 200]. Transport by these receptors can be made possible either through alteration of the chemical structure of the drug, so as that it mimics the endogenous substrate, or through linkage of the drug to the endogenous substrate. Receptor mediated trancytosis can also be exploited through similar strategies. Many of the receptors of this pathway belong to the immunoglobin superfamily and recognize monoclonal antibodies. Through tethering of drugs to antibodies, it may be possible to transport the drugs into the brain by trancytosis via the insulin or transferrin receptor.
While useful for transport of drugs across endothelial cells, these techniques do not solve the problems of metabolic processing and efflux. Many drugs that find pathways across the endothelial cells are transported back out by Pgp and other xenobiotic efflux transporters. Additionally, the endothelial cells, as well as the endfeet of astrocyte and pericytes, contain many metabolic enzymes that alter the properties of the drugs, rendering them inactive or targeting them for transport out of cells, and elimination.
C. Implantation technology
Implantation techniques allow the most localized and reliable treatment and delivery of central nervous system drugs. Implantation techniques can be used to deliver drugs or cells to damaged or diseased areas of the brain. Cell encapsulation can be used to produce tissue constructs capable of holding neurons or other cells that can supply neurotransmitters, repair lost connections, or supply trophic support following implantation [201]. Hydrogels provide an ideal substrate for holding the cells that are used for this purpose. As described previously, the hydrogel matrix can be tuned to accommodate almost any cell type and implantation strategy. Many synthetic hydrogels exhibit low immunoreactivity and cause little inflammation following implantation in tissue. Animal studies have demonstrated that dopamine neurons and neural stem cells can be delivered to diseased areas for long term treatment and restoration of function [202, 203]. Providing the brain with living cells may be a viable alternative to pharmacological intervention for some neurodegenerative diseases.
Hydrogels have also been used in experiments that involve controlled release of drugs [201, 204]. Hydrogels allow substances to diffuse at rates comparable to rates occurring in the extracellular matrix. Small hydrogel particles can be implanted that permit prolonged diffusion of compounds into the tissue [15]. Matrices can be produced that undergo biodegradation following delivery. The kinetics of drug or neurotrophin release can be studied and monitored in vitro, to produce the desired release profile, and drug capsules can be delivered locally using surgical methods that produce minimal damage and may not require craniotomy. Such spatially restricted delivery is desirable strategy for treatment of malignant brain tumors and other localized central nervous system disease. This local delivery strategy also has the potential to reduce side effects.
Finally, microfabrication can be used to produce probes or other implantable devices that contain on-board circuitry and fluidics capable of delivering compounds to targeted areas [205, 206]. Microfluidic channels can provide sustained delivery of compounds to targeted areas while creating minimal damage in the surrounding tissue during the implantation procedure. Probe dimensions, compositions, and insertion strategies can be defined and engineered so as to minimize damage and tissue response, maximize delivery, and produce desirable kinetic profiles for almost any compound. While these strategies are invasive and not yet characterized in humans, they enable direct and immediate delivery of compounds.
V. Concluding Remarks
There are a number of challenges that complicate therapeutic treatment of central nervous system disease. While many aspects of drug delivery and metabolism can be studied under simplified conditions in vitro, the high degrees of complexity and heterogeneity in the central nervous system necessitate the development of more biologically relevant culture systems. Through the integration of the fields of engineering, pharmacology, and cell biology, it may soon be possible to develop in vitro systems that exhibit the full complement of blood-brain barrier properties. Furthermore, incorporation of blood-brain barrier models into multi-compartmental CCAs will allow accurate pharmacokinetic predictions on the level of organ systems.
Current blood-brain barrier models hold the potential for the screening and rational development of many neuroactive compounds. Rational drug design can be used to produce new classes of compounds capable of either bypassing the barrier mechanisms of the central nervous system, or else exploiting endogenous biological pathways to enter the brain. Novel drug delivery methods, including the use of hydrogels, nanoparticles, and microfluidic technologies, hold the potential to improve the delivery of many classes of neuropharmaceuticals currently in clinical use.
Acknowledgments
The authors would like to acknowledge the Wadsworth Center Advanced Light Microscopy core for use of imaging facilities and Shirley Madewell and Adriana Verschoor for their help in editing. This work was supported in part by the Nanobiotechnology Center (NBTC) an STC program of the NSF under Agreement Number ECS-9876771 and by the National Institute of Biomedical Imaging and Bioengineering under Agreement Number R21EB007782 (M.R.H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Biomedical Imaging And Bioengineering or the National Institutes of Health.
VI. References
- 1.Levin VA. J Med Chem. 1980;23:682–694. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. J Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 3.Habgood MD, Begley DJ, Abbott NJ. Cell Mol Neurobiol. 2000;20:231–253. doi: 10.1023/A:1007001923498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballabh P, Braun A, Nedergaard M. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ, Rönnbäck L, Hansson E. Nature Reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 6.Ogiso T, Paku T, Iwaki M, Tanino T, Nishioka S. Biol Pharm Bull. 1994;17:1094–1100. doi: 10.1248/bpb.17.1094. [DOI] [PubMed] [Google Scholar]
- 7.Golovenko NY, Kravchenko IA, Zinkovskii VG, Andronati SA, Aleksandrova AI, Ovcharenko NV, Larionov VB. Bull Exp Biol Med. 2000;130:1153–1155. [PubMed] [Google Scholar]
- 8.Degim IT, Acarturk F, Erdogan D, Demirez-Lortlar N. Biol Pharm Bull. 2003;26:501–505. doi: 10.1248/bpb.26.501. [DOI] [PubMed] [Google Scholar]
- 9.Koshkina NV, Knight V, Gilbert BE, Golunski E, Roberts L, Waldrep JC. Cancer Chemother Pharmacol. 2001;47:451–456. doi: 10.1007/s002800000230. [DOI] [PubMed] [Google Scholar]
- 10.Tronde A, Nordén B, Marchner H, Wendel AK, Lennernas H, Bengtsson UH. Pharm Sci. 2003;92:1216–1233. doi: 10.1002/jps.10386. [DOI] [PubMed] [Google Scholar]
- 11.Bartus RT, Emerich D, Snodgrass-Belt P, Fu K, Salzberg-Brenhouse H, Lafreniere D, Novak L, Lo ES, Cooper T, Basile AS. J Pharmacol Exp Ther. 2004;310:828–835. doi: 10.1124/jpet.103.064121. [DOI] [PubMed] [Google Scholar]
- 12.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Nature. 1997;386:410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 13.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Curr Drug Deliv. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 14.Manjunath K, Venkateswarlu V. J Controlled Release. 2005;107:215–228. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Soni S, Babbar AK, Sharma RK, Maitra A. J Drug Targeting. 2006;14:87–95. doi: 10.1080/10611860600635608. [DOI] [PubMed] [Google Scholar]
- 16.Dash AK, Cudworth GC. J Pharm Toxicol Methods. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 17.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. J Controlled Release. 2006;111:252–262. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Tucker GT. Drug Concentration in Neuropsychiatry, Ciba Foundation Symposium 74. Excerpta Medica; New York, NY: 1980. pp. 13–33. [Google Scholar]
- 19.Gibson GG, Skett P. Introduction to Drug Metabolism. Chapmann and Hall; New York, NY: 1986. pp. 199–238. [Google Scholar]
- 20.Holz M, Fahr A. Advanced Drug Delivery Reviews. 2001;48:249–264. doi: 10.1016/s0169-409x(01)00118-1. [DOI] [PubMed] [Google Scholar]
- 21.Poulin P, Theil FP. J Pharm Sci. 2000;89:16–35. doi: 10.1002/(SICI)1520-6017(200001)89:1<16::AID-JPS3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Poulin P, Schoenlein K, Theil FP. J Pharm Sci. 2001;90:436–447. doi: 10.1002/1520-6017(200104)90:4<436::aid-jps1002>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Houston JB. Pharm Res. 2005;22:103–112. doi: 10.1007/s11095-004-9015-1. [DOI] [PubMed] [Google Scholar]
- 24.Vegiopoulos A, Herzig S. Mol Cell Endocrinology. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Fitch MT, van de Beek D. Nature Clinical Practice Neurology. 2008;4:97–104. doi: 10.1038/ncpneuro0713. [DOI] [PubMed] [Google Scholar]
- 26.Schacke H, Docke WD, Asadullah K. Pharmacology & Therapeutics. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 27.Tirona RG, Schwab AJ, Geng W, Pang KS. Drug Metab Disp. 1997;26:465–475. [PubMed] [Google Scholar]
- 28.Baker M, Parton T. Xenobiotica. 2008;37:1110–1134. doi: 10.1080/00498250701658296. [DOI] [PubMed] [Google Scholar]
- 29.Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KLR. Eur J Pharm Sci. 2006;27:447–486. doi: 10.1016/j.ejps.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Haining RL, Nichols-Haining M. Pharmacol Ther. 2007;113:537–545. doi: 10.1016/j.pharmthera.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Hewitt NJ, Gómez-Lechón MJ, Houston BJ, Hallifax D, Brown HS, de Maurel P, Kenna JD, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl T, Li AP, LeCluyse E, Groothuis GMM, Hengstler JG. Drug Metabolism Reviews. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- 32.Omniecinski CJ, Remmel RP, Hosagrahara VP. Toxicological Sciences. 1999;48:151–156. doi: 10.1093/toxsci/48.2.151. [DOI] [PubMed] [Google Scholar]
- 33.Omura T. Chemico-Biological Interactions. 2006;163:86–93. doi: 10.1016/j.cbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Seliskar M, Rozman D. Biochim Biophys Acta. 2007;1770:458–466. doi: 10.1016/j.bbagen.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich PF. J Pharm Exp Therapeutics. 1994;270:414–423. [PubMed] [Google Scholar]
- 36.Ryu HM, Shin SW, Yun CH, Lee SC, Ji YM, You KH. Biochem And Biophys Res Communications. 1997;231:254–256. doi: 10.1006/bbrc.1997.6084. [DOI] [PubMed] [Google Scholar]
- 37.Rieder RM, Ramsden DB, Williams ACJ. Clin Pathol: Mol Pathol. 1998;51:138–142. doi: 10.1136/mp.51.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki H, Sugiyama Y. Eur J Pharm Sci. 2000;12:3–12. doi: 10.1016/s0928-0987(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 39.Chinta SJ, Pai HV, Upadhya SC, Boyd MR, Ravindranath V. Mol Brain Res. 2002;103:49–61. doi: 10.1016/s0169-328x(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 40.Granberg L, Stergren AO, Brandt I, Brittebo EA. Drug Metab Disp. 2003;31:259–265. doi: 10.1124/dmd.31.3.259. [DOI] [PubMed] [Google Scholar]
- 41.Zuber R, Anzenbacherova E, Anzenbacher P. J Cell Mol Med. 2002;6:189–198. doi: 10.1111/j.1582-4934.2002.tb00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang KS. Drug Metab Disp. 2003;31:1507–1519. doi: 10.1124/dmd.31.12.1507. [DOI] [PubMed] [Google Scholar]
- 43.Benet LZ, Cummins CL, Wu CY. International Journal of Pharmaceutics. 2004;277:3–9. doi: 10.1016/j.ijpharm.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Castella JV, Donatoa MT, Gomez-Lecho MJ. Exp Tox Path. 2005;57:189–204. [Google Scholar]
- 45.Poch MT, Cutler NS, Yost GS, Hines RN. Drug Metab Disp. 2005;33:1174–1184. doi: 10.1124/dmd.105.004523. [DOI] [PubMed] [Google Scholar]
- 46.Galetin A, Houston JB. J Pharm Exp Therapeutics. 2006;318:1220–1229. doi: 10.1124/jpet.106.106013. [DOI] [PubMed] [Google Scholar]
- 47.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. Drug Metab Disp. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oesch F, Fabian E, Oesch-Bartlomowicz B, Werner C, Landsiedel R. Drug Metabolism Reviews. 2007;39:659–698. doi: 10.1080/03602530701690366. [DOI] [PubMed] [Google Scholar]
- 49.Lash LH, Putt DA, Cai H. Toxicology. 2008;244:56–65. doi: 10.1016/j.tox.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura S, Sugihara K, Ohta S. Drug Metab Pharmacokinet. 2006;21:83–98. doi: 10.2133/dmpk.21.83. [DOI] [PubMed] [Google Scholar]
- 51.King CD, Rios GR, Green MD, Tephly TR. Current Drug Metabolism. 2000;1:143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 52.Guo J, Zimniak L, Zimniak P, Orchard J, Singh SV. Biochem J. 2002a;366:817–824. doi: 10.1042/BJ20020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo J, Pal A, Srivastava SK, Orchard JL, Singh SV. Comp Biochem and Phys Part B. 2002b;131:443–452. doi: 10.1016/s1096-4959(01)00515-2. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Gonzalez V, Xu MJ. Journal of Dermatological Science. 2002;30:205–214. doi: 10.1016/s0923-1811(02)00107-x. [DOI] [PubMed] [Google Scholar]
- 55.Friedberg T, Bentley P, Stasiecki P, Glatt HR, Raphael D, Oesch F. J Biol Chem. 1979;254:12028–12033. [PubMed] [Google Scholar]
- 56.Ryle CM, Mantle TJ. Biochem J. 1984;222:553–556. doi: 10.1042/bj2220553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgenstern R, Lundqvist G, Hancock V, DePierre JW. J Biol Chem. 1988;263:6671–6675. [PubMed] [Google Scholar]
- 58.Bora PS, Spilburg CA, Lange LG. PNAS. 1989;86:4470–4473. doi: 10.1073/pnas.86.12.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali-Osman F, Stein DE, Renwick A. Cancer Research. 1990;50:6976–6980. [PubMed] [Google Scholar]
- 60.Sagara JI, Sugita Y. Brain Research. 2001;902:190–197. doi: 10.1016/s0006-8993(01)02384-8. [DOI] [PubMed] [Google Scholar]
- 61.Sheweita SA, Tilmisany AK. Current Drug Metabolism. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- 62.Fruehauf JP, Brem H, Brem S, Sloan A, Barger G, Huang W, Parker R. Clin Cancer Res. 2006;12:4523–4532. doi: 10.1158/1078-0432.CCR-05-1830. [DOI] [PubMed] [Google Scholar]
- 63.Pompella A, Tata VD, Paolicchi A, Zunino F. Biochem Pharm. 2006;71:231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Hanigan MH, Gallagher BC. Carcinogenesis. 1999;20:553–559. doi: 10.1093/carcin/20.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schafer C, Fe C, Brucke M, Holzhausen HJ, Bahn H, Wellma M, Visvikis A, Fischer P, Rainov NG. Acta Oncologica. 2001;40:529–535. doi: 10.1080/028418601750288271. [DOI] [PubMed] [Google Scholar]
- 66.Lee W, Kim RB. Ann Rev Pharm Tox. 2004;44:137–166. doi: 10.1146/annurev.pharmtox.44.101802.121856. [DOI] [PubMed] [Google Scholar]
- 67.Sun H, Frassetto L, Benet LZ. Pharmac Ther. 2006;109:1–11. doi: 10.1016/j.pharmthera.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Eagleson KL, Levitt P. Cerebral Cortex. 1999;9:562–568. doi: 10.1093/cercor/9.6.562. [DOI] [PubMed] [Google Scholar]
- 69.Levitt P. Science. 2004;303:48–49. doi: 10.1126/science.1093398. [DOI] [PubMed] [Google Scholar]
- 70.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. J Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjornsson CS, Lin G, Al-Kofahi Y, Narayanaswamy A, Smith KL, Shain W, Roysam B. J Neurosci Methods. 2008 in press. [Google Scholar]
- 72.Magistretti PJ, Pellerin L. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell PB. Br J Clin Pharmacol. 2000;49:303–312. doi: 10.1046/j.1365-2125.2000.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Craig LE, Spelman JP, Strandberg JD, Zink MC. Microvascular Research. 1998;55:65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 75.Tontsch U, Bauer HC. Brain Research. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 76.Raub TJ, Kuentzel SL, Sawada GA. Exp Cell Res. 1992;199:330–340. doi: 10.1016/0014-4827(92)90442-b. [DOI] [PubMed] [Google Scholar]
- 77.Mizuguchi H, Fuji A, Utoguchi N, Nakagawa S, Mayumi T. Microvascular Research. 1995;50:129–132. doi: 10.1006/mvre.1995.1045. [DOI] [PubMed] [Google Scholar]
- 78.Gaillard PJ, van der Sandt ICJ, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Pharmaceutical Res. 2000;17:1198–1205. doi: 10.1023/a:1026406528530. [DOI] [PubMed] [Google Scholar]
- 79.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowiczn D. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 80.Igarashi Y, Hiroyuki U, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N. Biochem Biophys Res Comm. 1999;261:108–112. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- 81.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain Research. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 82.Haseloff RF, Blasig IE, Bauer HC, Bauer H. Cell Mol Neurobiology. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davson H. In: Implications of the blood-brain barrier and its manipulation. Neuwelt EA, editor. Vol. 1. Plenum Publishing Corp; New York, NY: 1989. pp. 27–52. [Google Scholar]
- 84.Karnovsky MJ. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holash JA, Stewart PA. Brain Research. 1993;629:218–24. doi: 10.1016/0006-8993(93)91323-k. [DOI] [PubMed] [Google Scholar]
- 86.Farrell CR, Stewart PA, Farrell CL, Del Maestro RF. Anat Rec. 1987;218:466–9. doi: 10.1002/ar.1092180416. [DOI] [PubMed] [Google Scholar]
- 87.Stewart PA, Hayakawa EM. Brain Research. 1987;429:271–81. doi: 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- 88.Stewart PA. Microscopy Research and Technique. 1994;27:516–527. doi: 10.1002/jemt.1070270606. [DOI] [PubMed] [Google Scholar]
- 89.Farrell CL. PNAS. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorovini-zis K, Huynh HK. J Histochem Cytochem. 1992;40:689–696. doi: 10.1177/40.5.1573250. [DOI] [PubMed] [Google Scholar]
- 91.Vorbrodt AW. Brain Research Reviews. 2003;42:221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 92.Tilling T, Korte D, Hoheisel D, Galla HJ. J Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 93.Grant DS, Kleinman HK, Martin GR. Ann NY Acad of Sci. 1990;588:61–72. doi: 10.1111/j.1749-6632.1990.tb13197.x. [DOI] [PubMed] [Google Scholar]
- 94.Wolburg H, Lippoldt A. Vascular Pharmacology. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 95.Tsukita S, Furuse M. Trends in Cell Biology. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 96.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S, Tsukita S. J Cell Science. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- 99.Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. J Cell Science. 1999;112:1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- 100.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. J Cell Biol. 1996;134:1031–49. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. J Cell Science. 1997;11:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 104.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukito S. J Cell Biology. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 106.Watson PM, Anderson JM, Vanltallie CM, Doctrow SR. Neuroscience Letters. 1991;129:6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- 107.Schulze C, Firth JA. J Cell Science. 1993;104:773–782. doi: 10.1242/jcs.104.3.773. [DOI] [PubMed] [Google Scholar]
- 108.Williams MJ, Lowrie MB, Bennett JP, Firth JA, Clark P. Brain Research. 2005;1058:62–72. doi: 10.1016/j.brainres.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 109.Dejana E, Bazzoni G, Lampugnani MG. Thrombosis and Haemostasis. 1999;82:755–761. [PubMed] [Google Scholar]
- 110.Pardridge WM. Molecular Interventions. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 111.Pardridge WM, Boado RJ, Farrell CR. J Biol Chem. 1990;265:18035–1840. [PubMed] [Google Scholar]
- 112.Morgello S, Uson RR, Schwartz EJ, Haber RS. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- 113.Ngarmukos C, Baur E, Kumagai AK. Brain Research. 2001;900:1–8. doi: 10.1016/s0006-8993(01)02184-9. [DOI] [PubMed] [Google Scholar]
- 114.Betz AL, Goldstein GW. Science. 1978;202:225–226. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- 115.Sanchez del Pino MM, Hawkins RA, Peterson DR. J Biol Chem. 1992;267:25951–25957. [PubMed] [Google Scholar]
- 116.Roberts RL, Fine RE, Sandra A. J Cell Science. 1993;104:521–532. doi: 10.1242/jcs.104.2.521. [DOI] [PubMed] [Google Scholar]
- 117.Huwyler J, Froidevaux S, Roux F, Eberle AN. J Receptor and Signal Transduction Research. 1999;19:729–739. doi: 10.3109/10799899909036683. [DOI] [PubMed] [Google Scholar]
- 118.Triguero D, Buciak J, Pardridge WM. J Neurochemistry. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 119.Terasaki T, Ohtsuki S. NeuroRx. 2005;2:63–72. doi: 10.1602/neurorx.2.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meresse S, Dehouck MP, Delorme P, Bensaid M, Tauber JP, Delbart C, Fruchart JC, Cecchelli R. J Neurochemistry. 1989;53:1363–1370. doi: 10.1111/j.1471-4159.1989.tb08526.x. [DOI] [PubMed] [Google Scholar]
- 121.DeBault LE, Cancilla PA. Science. 1980;207:653–655. doi: 10.1126/science.6101511. [DOI] [PubMed] [Google Scholar]
- 122.Risau W, Dingler A, Albrecht U, Dehouck MP, Cecchelli R. J Neurochemistry. 1992;58:667–672. doi: 10.1111/j.1471-4159.1992.tb09769.x. [DOI] [PubMed] [Google Scholar]
- 123.Ogawa M, Shiozawa M, Hiraoka Y, Takeuchi Y, Aiso S. Tissue and Cell. 1998;30:597–601. doi: 10.1016/s0040-8166(98)80078-5. [DOI] [PubMed] [Google Scholar]
- 124.Sternberger NH, Sternberger LA. PNAS. 1987;8:8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenstein JM, Krum JM, Sternberger LA, Pulley MT, Sternberger NH. Dev Brain Research. 1992;66:47–54. doi: 10.1016/0165-3806(92)90138-m. [DOI] [PubMed] [Google Scholar]
- 126.Wolburg H, Neuhaus J, Kniesel U, Krau B, Schmid EM, Ocalan M, Farrell C, Risau W. J Cell Science. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 127.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, Tanner LI, Tomaselli KJ, Bard F. J Cell biology. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stewart PA, Wiley MJ. Dev Biology. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 129.Janzer RC, Raff MC. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 130.Meyer J, Rauh J, Galla HJ. J Neurochemistry. 1991;57:1971–1977. doi: 10.1111/j.1471-4159.1991.tb06411.x. [DOI] [PubMed] [Google Scholar]
- 131.Bouchaud C, Le Bert M, Dupouey P. Eur Journal of Neuroscience. 1989;140:158–165. [Google Scholar]
- 132.Hayashi Y, Nomura M, Yamagishi SI, Harada SI, Yamashita J, Yamamoto H. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- 133.Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D’Agostino S, Schiera G, De Caro V, Giandalia G, Giannola LI, Cestelli A. Molecular Neuroscience. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- 134.Schiera G, Bono E, Raffa MP, Gallo A, Pitarresi GL, Di Liegro I, Savettieri G. J Cell Mol Med. 2003;7:165–170. doi: 10.1111/j.1582-4934.2003.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Nature Neuroscience. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 136.Grossmann R. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- 137.McCarthy KD, DeVellis J. J Cell Biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bowman PD, Betz AL, Ar D, Wolinsky JS, Penney JB, Shivers RR, Goldstein GW. In Vitro. 1981;17:353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- 139.Abbott NJ, Hughes CCW, Revest PA, Greenwood J. J Cell Science. 1992;103:23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- 140.Banker G, Goslin K. Culturing nerve cells. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 141.Kam L, Shain W, Turner JN, Bizios R. Biomaterials. 2001;22:1049–1054. doi: 10.1016/s0142-9612(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 142.Kam L, Shain W, Turner JN, Bizios R. Biomaterials. 2002;23:511–515. doi: 10.1016/s0142-9612(01)00133-8. [DOI] [PubMed] [Google Scholar]
- 143.Takasato Y, Rapoport SI, Smith QR. Am J Physiol. 1984;247:484–493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 144.Dehouck MP, Jolliet-Riant P, Brée F, Fruchart JC, Cecchelli R, Tillement JP. J Neurochem. 1992;58:1790–1797. doi: 10.1111/j.1471-4159.1992.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 145.Franke H, Galla HJ, Beuckmann CT. Brain Research. 1999;818:65–71. doi: 10.1016/s0006-8993(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 146.Kido Y, Tamai I, Okamoto M, Suzuki F, Tsuji A. Pharm Res. 2000;17:55–62. doi: 10.1023/a:1007518525161. [DOI] [PubMed] [Google Scholar]
- 147.Dehouck MP, Méresse S, Delorme P, Fruchart JC, Cecchelli R. J Neurochem. 1990;54:1798–801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 148.Ma SH, Lepak LA, Hussain RJ, Shain W, Shuler ML. Lab Chip. 2005;5:74–85. doi: 10.1039/b405713a. [DOI] [PubMed] [Google Scholar]
- 149.Olesen SP, Crone C. Biophys J. 1983;42:31–41. doi: 10.1016/S0006-3495(83)84366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muruganandam A, Herx LM, Monette R, Durkin JP, Stanimirovic DB. FASEB J. 1997;11:1187–97. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 151.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD. Eur J Pharm Sci. 2001;12:215–22. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 152.Crone C, Olesen SP. Brain Research. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- 153.Frampton JP, Hynd MR, Williams JC, Shuler ML, Shain W. J Neural Eng. 2007;4:399–409. doi: 10.1088/1741-2560/4/4/006. [DOI] [PubMed] [Google Scholar]
- 154.Wegener J, Zink S, Rösen P, Galla H. Pflugers Arch. 1999;437:925–934. doi: 10.1007/s004240050864. [DOI] [PubMed] [Google Scholar]
- 155.Nieber MA, Müller J, Wolf P, Schmidt M, Robitzki AA. Biotechniques. 2006;41:445–450. doi: 10.2144/000112254. [DOI] [PubMed] [Google Scholar]
- 156.Williams SK, Gillis JF, Matthews MA, Wagner RC, Bitensky MW. J Neurochem. 1980;35:374–8. doi: 10.1111/j.1471-4159.1980.tb06274.x. [DOI] [PubMed] [Google Scholar]
- 157.Franke H, Galla H, Beuckmann CT. Brain Res Brain Res Protoc. 2000;5:248–256. doi: 10.1016/s1385-299x(00)00020-9. [DOI] [PubMed] [Google Scholar]
- 158.Roux F, Couraud PO. Cell Mol Neurobiol. 2005;25:41–58. doi: 10.1007/s10571-004-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schiera G, Sala S, Gallo A, Raffa MP, Pitarresi GL, Savettieri G, Di Liegro I. J Cell Mol Med. 2005;9:373–379. doi: 10.1111/j.1582-4934.2005.tb00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ceballos G, Rubio RJ. Neurochem. 1995;64:991–999. doi: 10.1046/j.1471-4159.1995.64030991.x. [DOI] [PubMed] [Google Scholar]
- 161.Johnson MD, Anderson BD. J Pharm Sci. 1999;88:620–625. doi: 10.1021/js9803149. [DOI] [PubMed] [Google Scholar]
- 162.Jeliazkova-Mecheva VV, Bobilya DJ. Brain Res Brain Res Protoc. 2003;12:91–98. doi: 10.1016/j.brainresprot.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 163.Berezowski V, Landry C, Lundquist S, Dehouck L, Cecchelli R, Dehouck MP, Fenart L. Pharm Res. 2004;21:756–760. doi: 10.1023/b:pham.0000026424.78528.11. [DOI] [PubMed] [Google Scholar]
- 164.Stanness KA, Guatteo E, Janigro DA. Neurotoxicology. 1996;17:481–496. [PubMed] [Google Scholar]
- 165.Stanness KA, Westrum LE, Fornaciari E, Mascagni P, Nelson JA, Stenglein SG, Myers T, Janigro D. Brain Research. 1997;771:329–42. doi: 10.1016/s0006-8993(97)00829-9. [DOI] [PubMed] [Google Scholar]
- 166.Janigro D, Leaman SM, Stanness KA. Pharm Sci Technolo Today. 1999;2:7–12. doi: 10.1016/s1461-5347(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 167.Parkinson FE, Friesen J, Krizanac-Bengez L, Janigro D. Brain Research. 2003;980:233–41. doi: 10.1016/s0006-8993(03)02980-9. [DOI] [PubMed] [Google Scholar]
- 168.Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. PNAS. 2006;103:2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Viravaidya K, Sin A, Shuler ML. Biotechnol Prog. 2004;20:316–323. doi: 10.1021/bp0341996. [DOI] [PubMed] [Google Scholar]
- 170.Cullen JP, Sayeed S, Sawai RS, Theodorakis NG, Cahill PA, Sitzmann JV, Redmond EM. Arterioscler Thromb Vasc Biol. 2002;22:1610–1616. doi: 10.1161/01.atv.0000034470.37007.58. [DOI] [PubMed] [Google Scholar]
- 171.Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Ann Biomed Eng. 2005;33:772–779. doi: 10.1007/s10439-005-3310-9. [DOI] [PubMed] [Google Scholar]
- 172.Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita J, Itoh H, Kanda K, Yaku H, Okamoto Y, Nemoto Y. J Artif Organs. 2005;8:110–118. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- 173.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 174.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 175.Lichtenberg A, Dumlu G, Walles T, Maringka M, Ringes-Lichtenberg S, Ruhparwar A, Mertsching H, Haverich A. Biomaterials. 2005;26:555–562. doi: 10.1016/j.biomaterials.2004.02.063. [DOI] [PubMed] [Google Scholar]
- 176.Taylor AM, Rhee SW, Jeon NL. Methods Mol Biol. 2006;321:167–177. doi: 10.1385/1-59259-997-4:167. [DOI] [PubMed] [Google Scholar]
- 177.Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G. Lab Chip. 2007;7:710–719. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 178.Takei T, Sakai S, Ono T, Ijima H, Kawakami K. Biotechnol Bioeng. 2006;95:1–7. doi: 10.1002/bit.20903. [DOI] [PubMed] [Google Scholar]
- 179.Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo BH. Nature. 2000;404:588–90. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- 180.Paguirigan AL, Beebe DJ. Nat Protoc. 2007;2:1782–1788. doi: 10.1038/nprot.2007.256. [DOI] [PubMed] [Google Scholar]
- 181.Kreeger PK, Deck JW, Woodruff TK, Shea LD. Biomaterials. 2006;27:714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rowley JA, Madlambayan G, Mooney DJ. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 183.Dhoot NO, Tobias CA, Fischer I, Wheatley MA. J Biomed Mater Res A. 2004;71:191–200. doi: 10.1002/jbm.a.30103. [DOI] [PubMed] [Google Scholar]
- 184.Comisar WA, Hsiong SX, Kong HJ, Mooney DJ, Linderman JJ. Biomaterials. 2006;27:2322–2329. doi: 10.1016/j.biomaterials.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 185.Kong HJ, Kaigler D, Kim K, Mooney DJ. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 186.Kong HJ, Smith MK, Mooney DJ. Biomaterials. 2003;24:4023–4029. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 187.Drury JL, Dennis RG, Mooney DJ. Biomaterials. 2004;25:3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 188.Eiselt P, Yeh J, Latvala RK, Shea LD, Mooney DJ. Biomaterials. 2000;21:1921–1927. doi: 10.1016/s0142-9612(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 189.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. Biotechnol Prog. 2004;20:338–345. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 190.Jong A, Huang SH. Current Drug Targets–Infectious Disorders. 2005;5:65–72. doi: 10.2174/1568005053174672. [DOI] [PubMed] [Google Scholar]
- 191.Preston E, Slinn J, Vinokourov I, Stanimirovic D. Journal of Neuroscience Methods. 2008;168:443–449. doi: 10.1016/j.jneumeth.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 192.Hall WH, Doolittle ND, Daman M, Bruns PK, Muldoon L, Fortin D, Neuwelt EA. Journal of Neuro-Oncology. 2006;77:279–284. doi: 10.1007/s11060-005-9038-4. [DOI] [PubMed] [Google Scholar]
- 193.Erdlenbruch B, Kugler W, Schinkhof C, Neurath H, Eibl H, Lakomek M. Journal of Drug Targeting. 2005;13:143–150. doi: 10.1080/10611860400029085. [DOI] [PubMed] [Google Scholar]
- 194.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Neuroimage. 2005;24:12–22. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 195.Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Phys Med Biol. 2007;52:5509–30. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- 196.Borlongan CV, Emerich DF. Brain Res Bull. 2003;60:297–306. doi: 10.1016/s0361-9230(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 197.Kreuter J. J Anat. 1996;189:503–505. [PMC free article] [PubMed] [Google Scholar]
- 198.Vinogradov SV, Bronich TK, Kabanov AV. Advanced Drug Delivery Reviews. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 199.Soni S, Babbar AK, Sharma RK, Maitra A. Journal of Drug Targeting. 2006;14:87–95. doi: 10.1080/10611860600635608. [DOI] [PubMed] [Google Scholar]
- 200.Tsujia A, Tamaia I. Advanced Drug Delivery Reviews. 1999;36:277–290. doi: 10.1016/s0169-409x(98)00084-2. [DOI] [PubMed] [Google Scholar]
- 201.Lu S, Anseth KS. J Control Release. 1999;57:291–300. doi: 10.1016/s0168-3659(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 202.Orive G, Ponce S, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Biomaterials. 2002;23:3825–3831. doi: 10.1016/s0142-9612(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 203.Roberts T, De Boni U, Sefton MV. Biomaterials. 1986;17:267–75. doi: 10.1016/0142-9612(96)85564-5. [DOI] [PubMed] [Google Scholar]
- 204.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. J Control Release. 1999;62:81–7. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 205.Dash AK, Cudworth GC. Journal of Pharmacological and Toxicological Methods. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 206.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. J Control Release. 2006;111:252–262. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]