Abstract
Background
Few comparative studies exist of metabolic brain changes among neurodegenerative illnesses. We compared brain metabolic abnormalities in Alzheimer’s disease (AD) and in Parkinson’s disease with dementia (PDD) as measured by proton magnetic resonance spectroscopy (MRS).
Methods
Twelve patients with idiopathic PDD, 22 patients with probable mild AD, and 61 healthy older controls underwent posterior cingulate MRS.
Results
Patients with AD showed reduced N-acetylaspartate (NAA)/creatine (Cr) (p <0.05) and increased choline (Cho)/Cr (p <0.05) and myo-Inositol (mI)/Cr (p <0.01) compared to controls. Patients with PDD showed reduced NAA/Cr (p <0.05) and glutamate (Glu)/Cr (p <0.01) compared to controls. There was reduced Glu/Cr in PDD compared to AD (p <0.01).
Conclusion
Patients with AD and patients with PDD showed distinct brain metabolic MRS profiles. Findings suggest that comparison of brain MRS profiles across dementias provides useful direction for future study.
Keywords: Parkinson Disease, Alzheimer Disease, MR Spectroscopy, N-acetylaspartate, Gyrus Cinguli
1. Introduction
The aging demographic of our society creates the prospect for a future major healthcare crisis from providing care to patients with dementia. As of 2007 there is now more than five million persons in the United States diagnosed with Alzheimer’s disease (AD)1. Medicare and Medicaid expenditures for patients with AD are projected to more than double over the next eight years1. Other neurodegenerative diseases also challenge the U.S. healthcare system. Parkinson disease (PD) is one of the most frequently diagnosed neurological disorders in older adults, with prevalence between 80 to 200 per 100,000. Prevalence increases with age, with the disease affecting 1% of persons over the age of 802. Furthermore, persons with idiopathic PD run up to a six-fold risk of developing dementia compared to age-matched older controls3. Researchers have distinguished a dementia in PD, or PD dementia (PDD), that is clinically4, cognitively5, neuropsychologically6, and neuropathologically7 distinct from that of patients with dementia with Lewy Bodies (DLB)4 or AD with late motor complications8. However, more information comparing PDD to other dementias is needed to better define the disease process and elucidate possible treatment mechanisms.
The current study compares patients with AD to patients with PDD using proton MR spectroscopy (MRS). A wealth of MRS studies has been performed in patients with AD. Reduction in N-acetylaspartate (NAA) is the most frequent MRS finding in AD9, and is thought to represent the loss of functional integrity of neurons9, as both cortical lobar abnormalities10 and subcortical white matter abnormalities11 are observed in association with reduced NAA. Studies have also documented abnormalities of myo-Inositol (mI), a sugar alcohol similar to glucose, in AD patients12. Our group recently also documented abnormal elevations of a related inositol, scyllo-Inositol (sI)13. Fewer studies in AD patients have detected abnormal measurements of choline-containing compounds (Cho)10. Reduction of glutamate has been documented in AD patients through measuring the GLX peak (glutamate + glutamine)14, although it is likely that GLX represents the larger metabolic abnormalities in the brains of AD patients rather than direct reduction in the glutamate neurotransmitter pool. Thus, most MRS studies in AD have detected reduced NAA and increased mI, with less consistent findings for other metabolites.
The few studies to examine MRS in PDD have shown preliminary findings of interest for comparison with other patient groups. One such study investigated MRS of the basal ganglia and occipital cortex, reporting that reduced occipital NAA/creatine (Cr) ratios were detected in patients with PDD when compared to non-demented PD controls, but the comparison of PDD patients to healthy normal controls did not reach significance15. Our group recently reported that NAA/Cr was reduced in the posterior cingulate of PDD patients compared to non-demented PD patients and healthy controls16. Another study observed elevated lactate in the occipital lobe of four PDD patients compared to 10 non-demented PD patients and controls17, although no other studies of PD patients have detected elevated lactate18. We are unaware of MRS studies conducted to date that have compared patients with AD to patients with PDD. This represents a key knowledge gap, as other neuroimaging studies have reported differences between patients with PDD and patients with AD in MRI volumes of the hippocampus19, cerebral atrophy on MRI voxel-based morphometry20, white matter hyperintensity MRI volumes21, and cerebral blood flow on SPECT22.
A comparative MRS study could provide further insights regarding the metabolic effects of two common dementing illnesses, which share similarities in terms of neuroanatomical distribution of neuropathology23 and can have similar neurocognitive symptom profiles24. The current study was performed to investigate MRS findings in the posterior cingulate in patients with PDD in comparison to patients with AD and healthy older adults at 3 T field strength. Prior studies have demonstrated that the posterior cingulate often demonstrates metabolic abnormalities in both AD10 and cognitively impaired persons with PD25. This region also suffers very early neuropathology in AD23 and may be involved in PDD8.
2. Methods
2.1. Participants
All patients and healthy controls were recruited from the University of Alabama at Birmingham (UAB) Alzheimer's Disease Research Center (ADRC). Patients with AD were primarily referred to the ADRC from the UAB Memory Disorders Clinic, a tertiary care outpatient clinic. Patients with PDD were referred from the UAB Movement Disorders Clinic, which is also a tertiary care outpatient clinic. Both patient groups are referred to the ADRC from these respective clinics on the basis of clinical evidence of dementia on neurological examination, with all pertinent medical records from the prior evaluation made available to the ADRC diagnostic team. Control participants were community-dwelling individuals who volunteered for participation in the ADRC. Sources of contact with healthy older adults included community health fairs, ADRC-sponsored lectures and workshops, and media advertisement.
All participants in the ADRC undergo neurological and neuropsychological evaluation. Diagnosis is made in the course of a consensus conference comprised of neurologists, neuropsychologists, and allied health professionals from the ADRC. General exclusion criteria for the ADRC included metabolic diseases, head injuries, large vessel strokes, severe psychiatric illness and neuro-developmental conditions. Participants completed the Dementia Rating Scale (DRS)26 and the Mini-Mental State Examination (MMSE)27 as part of their evaluations. Participants also completed the Geriatric Depression Scale (GDS)28. Dementia was staged in all patients using the Clinical Dementia Rating Scale (CDR)29.
Twelve movement disordered patients presented with no known cause for their movement disorder and were considered to have idiopathic PD. These patients were determined to have a dementia secondary to their PD based upon a clinical history of movement disorder for at least one year prior to dementia, report of a family member or caregiver of declines from premorbid function, neurological findings, and neuropsychological test results. Such criteria are generally consistent with DSM IV criteria for dementia and the current clinical standards for PDD diagnosis30. Persons with clinical features consistent with DLB, atypical PD, or Alzheimer's disease and with secondary parkinsonism were excluded. All PDD patients were treated with anti-parkinsonian medications at the time of the study (carbidopa/levodopa n = 8, carbidopa/levodopa/entacapone n = 5, pramipexole n = 3, ropinirole n = 2, pergolide n = 2, carbidopa n = 1, amantadine n = 1). All PDD patients were clinically determined to be within the effective therapeutic range of their movement disorder medications at the time of MRS data collection. Half of the PDD patients were also taking a cholinesterase inhibitor for treatment of memory loss (donepezil n = 3, galantamine n = 2, rivastigmine n =1 ). The PDD patients had been diagnosed with a movement disorder for an average of 7.33 (± 6.02) years and obtained a mean score of 22.67 (± 5.29) on the motor section of the Uniform Parkinson’s Disease Rating Scale (UPDRS)31. These PDD patients were considered to have mild dementia, with CDR staging of either 0.5 or 1 in all but one participant (Table 1).
Table 1.
Demographics, Staging, and Clinical Data for Study Participants
| Variable | Controls N = 61 |
Mild AD N = 22 |
PDD N = 12 |
p value |
|---|---|---|---|---|
| Age mean | 66.46 | 74.09 | 71.00 | 0.002 |
| (SD) | (8.76) | (10.04) | (3.33) | |
| Sex (males / females) | 19 / 42 | 9 / 13 | 12 / 0 | 0.001 |
| Race (Caucasian / African-American) | 52 / 9 | 21 / 1 | 12 / 0 | 0.182 |
| Education mean | 15.21 | 14.52 | 16.08 | 0.218 |
| (SD) | (2.25) | (2.78) | (2.94) | |
| Dementia Rating Scale mean | 139.19 | 118.52 | 120.50 | 0.001 |
| (SD) | (2.88) | (11.20) | (13.32) | |
| MMSE mean | 29.49 | 24.77 | 25.83 | 0.001 |
| (SD) | (0.95) | (2.47) | (2.59) | |
| CDR* Staging (0 / 0.5 / 1 / 2) | 61 / 0 / 0 / 0 | 0 / 11 / 11 / 0 | 0 / 5 / 6 / 1 | 0.001 |
| Geriatric Depression Scale mean | 5.88 | 8.8 | 10.42 | 0.017 |
| (SD) | (5.30) | (6.45) | (4.36) | |
| Cholinesterase Inhibitor (yes/no) | ---- | 16 / 6 | 6 / 6 | 0.001 |
CDR = Clinical Dementia Rating Scale
Twenty-two patients with probable mild AD were recruited from the UAB Memory Disorders Clinic into the ADRC. Diagnosis of probable AD was determined in the ADRC diagnostic consensus conference and was based on the NINCDS-ADRDA criteria32 excluding other causes of dementia as best as possible. These AD patients were classified as mild based upon clinical consensus, and had a CDR staging of 0.5 or 1.0 (Table 1). The majority of patients were taking a cholinesterase inhibitor for treatment of memory loss at the time of the study (donepezil n = 11, galantamine n = 4, rivastigmine n = 1).
Sixty one older adults were characterized as cognitively normal following ADRC diagnostic consensus conference. All controls had CDR = 0.
All participants gave informed consent to the study as part of this Institutional Review Board-approved protocol.
2.2. MRS methods
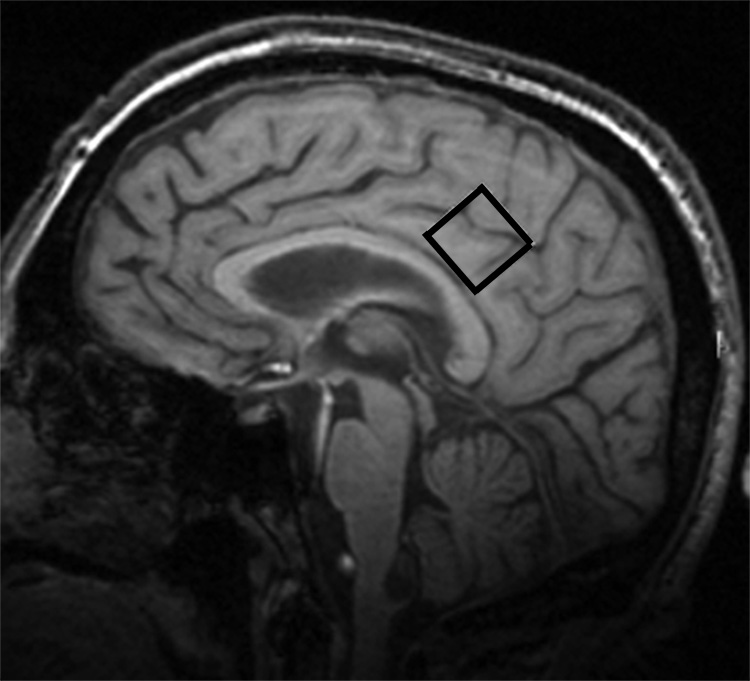
The MRS protocol was performed at 3 T using a Philips Intera MRI system. Data were collected from a 2×2×2 cm voxel of interest (VOI) located in a portion of the posterior cingulate gyrus (Figure 1). Data acquisition used PRESS sequences with 2048 samples, spectral bandwidth of 2000 Hz, and 128 acquisitions, using TR/TE = 2000/32 ms for NAA, Cho, and mI and TR/TE = 2000/80 for obtaining and quantifying measurements of glutamate (Glu)33. An automated higher-order shim procedure was applied to correct for magnetic field inhomogeneity over the VOI. Suppression of the water signal was adjusted prior to data collection using a frequency selective double inversion recovery sequence. All spectra were measured by one spectroscopist who was blinded to the clinical diagnosis of the participants. Quantification of MR spectroscopy was performed using a time domain fitting analysis that utilizes prior in-vitro knowledge34. In-vivo spectra were referenced to the methyl signal of N-acetylaspartate (2.008 ppm). Using this procedure, metabolic ratios of NAA, Cho, mI, (TE = 32 ms) and Glu (TE = 80 ms) were obtained relative to Cr, with average Cramer-Rao lower bounds derived to determine the variability in MRS measurement within each participant’s MRS data (Figure 2).
Figure 1.
T1 sagittal MRI showing voxel of interest within the posterior cingulate gyrus.
Figure 2.
Representative spectra from study participants: A) patient with Parkinson disease with dementia; B) patient with Alzheimer disease; C) healthy older adult control. See text for abbreviations.
2.3. Statistical analyses
Group differences on age, DRS and MMSE, self-reported depression, and education were compared using One-way ANOVA with Least Significant Differences (LSD) post hoc35. Sex, self-reported race, CDR staging, and the proportion of patients on cholinesterase inhibitors between the AD and PDD groups were compared with chi-square. MRS ratios of AD and PDD patients and controls were compared with pairwise comparisons. The correlation of MRS ratios with mental status scores was assessed with Pearson correlation in all participants. The ability of MRS ratios to classify group status (AD versus PDD) was determined with stepwise discriminant function analyses (DFA: F in = 3.84; F out = 2.71). All analyses were performed using SPSS 12.0 with an alpha level set at .05 (2-tailed).
3. Results
3.1. Demographics, staging, and clinical variables
The ANOVA comparing age across groups was significant: F (2, 92) = 6.78, p < 0.05. Post-hoc analyses demonstrated that controls were younger than the AD patients (p < 0.01) but not the PDD patients (p = 0.099). The AD and PDD patient groups did not differ in age (Table 1). Chi-square analysis for sex was significant, X2 = 19.52, p < 0.001. The PDD patient group was comprised of all males while the distribution of males to females was 41% in the AD patient group and 31% in the control group. There were no statistical differences in racial distribution, X2 = 3.41, p > 0.10, although all PDD patients were Caucasian. Education did not differ among the study groups, F (2, 91) = 1.55, p > 0.10. The ANOVAS for DRS, F (2, 82) = 67.73, p < 0.001, and MMSE, F (2, 90) = 73.60, p < 0.001, were both significant. Post-hoc analyses showed that both AD (p < 0.001) and PDD patients (p < 0.001) had lower DRS scores compared to controls, but did not differ from each other (p > 0.10). This same pattern was observed for the MMSE, with both AD (p < 0.001) and PDD patients (p < 0.001) having lower scores compared to controls, although there was no difference in MMSE between the patient groups (p = 0.08). Distributions of CDR scores were similar in both dementia groups, X2 = 1.95, p > 0.10. The ANOVA for depression was significant, F (2, 77) = 4.30, p < 0.05, with depression higher in both AD (p < 0.05) and PDD (p < 0.05) compared to controls, although the comparison between AD and PDD was not significant (p > 0.10). The PDD mean score was just above a clinical cutoff of 10. There was no significant difference between the proportion of AD and PDD patients taking cholinesterase inhibitors, X2 = 1.76, p > 0.10.
3.2. MRS measures
Due to differences in age and distribution of sex among the groups, these variables were applied as covariates in the group analyses of MRS data. Compared to controls, AD patients demonstrated lower NAA/Cr (7% reduction) (p < 0.05) and higher Cho/Cr (5% increase) (p < 0.05) and mI/Cr (13% increase) (p < 0.01) (Table 2). Effect sizes (Cohen’s d) ranged from a medium effect size of 0.47 (Cho/Cr) to a strong effect size of 0.89 (mI/Cr). Compared to controls, PDD patients showed lower NAA/Cr (8% reduction) (p < 0.05) and Glu/Cr (17% reduction) (p < 0.01). Strong effect sizes were observed for both NAA/Cr and Glu/Cr group differences (d = 1.09). Significantly lower Glu/Cr ratios were observed in PDD patients versus AD patients (15% reduction) (p < 0.01). There were no other significant differences in MRS between AD and PDD patients.
Table 2.
MRS Ratios of the Posterior Cingulate Gyrus across Study Groups
| MRS | Controls | Mild AD | PDD | Controls | Controls | Mild AD |
|---|---|---|---|---|---|---|
| Ratio | N = 61 | N = 22 | N = 12 | vs. | vs. | vs. |
| Mean (SD) | Mean (SD) | Mean (SD) | Mild AD | PDD | PDD | |
| Cramer-Rao | Cramer-Rao | Cramer-Rao | p (Effect | p (Effect | p (Effect | |
| Bounds | Bounds | Bounds | Size)* | Size)* | Size)* | |
| NAA/Cr† | 1.353 (0.120) | 1.257 (0.190) | 1.243 (0.088) | 0.026 (0.67) | 0.014 (1.09) | 0.458 (0.11) |
| .069 | .075 | .070 | ||||
| Cho/Cr | 0.648 (0.051) | 0.683 (0.086) | 0.648 (0.059) | 0.016 (0.47) | 0.881 (0.00) | 0.127 (0.10) |
| .044 | .054 | .050 | ||||
| mI/Cr | 0.910 (0.099) | 1.025 (0.172) | 0.982 (0.147) | 0.001 (0.89) | 0.470 (0.63) | 0.053 (0.24) |
| .067 | .074 | .069 | ||||
| Glu/Cr | 0.540 (0.075) | 0.529 (0.098) | 0.448 (0.085) | 0.565 (0.12) | 0.006 (1.09) | 0.004 (0.81) |
| .085 | .105 | .089 |
All effects adjusted for group differences on age or gender
NAA = N-acetylaspartate; Cr = creatine; Cho = choline containing compounds; mI - myo-Inositol; Glu = glutamate
Comparison of MRS ratios in patients treated with cholinesterase inhibitors versus untreated patients within the AD group revealed a higher Cho/Cr ratio (0.709) in treated versus untreated AD patients (0.614) (t (20) = 2.58, p = 0.018). No other differences in MRS ratios were observed between treated and untreated AD patients in NAA/Cr (treated = 1.280; untreated = 1.197; t (20) = 0.91, p = 0.376), mI/Cr (treated = 1.035; untreated = 0.999; t (20) = 0.42, p = 0.678), or Glu/Cr (treated = 0.525; untreated = 0.539; t (17) = 0.29, p = 0.777) . Likewise, no differences were observed in MRS ratios between PDD patients treated and not treated with cholinesterase inhibitors in NAA/Cr (treated = 1.278; untreated = 1.208; t (10) = 1.43, p = 0.183), Cho/Cr (treated = 0.637; untreated = 0.660; t (10) = 0.65, p = 0.533), mI/Cr (treated = 0.937; untreated = 1.026; t (10) = 1.05, p = 0.317), or Glu/Cr (treated = 0.419; untreated = 0.478; t (10) = 1.25, p = 0.241) .
3.3. Correlation of MRS ratios and mental status scores
In all participants, NAA/Cr showed modest positive correlations with both the MMSE (r = 0.25, p < 0.05) and DRS total score (r = 0.27, p < 0.05), as did Glu/Cr with MMSE (r = 0.22, p < 0.05) and DRS (r = 0.29, p < 0.05). Cho/Cr was negatively correlated with DRS (r = −0.23, p < 0.05), as was mI/Cr with MMSE (r = −0.39, p < 0.001) and DRS (r = −0.34, p < 0.01).
3.4. Group classification by MRS ratios
The stepwise DFA model classifying AD patients from PDD patients was significant (p < 0.05) and retained Glu/Cr as the only predictor variable. The model correctly classified 67.7% of the participants (57.9 % AD, 83.3% PDD).
4. Discussion
This study suggests that MRS ratios from the posterior cingulate gyrus demonstrate distinct metabolic brain abnormalities in probable AD patients and probable PDD patients when compared to healthy older adults. MRS profiles in AD and PDD both demonstrated abnormality in NAA/Cr but other metabolic abnormalities were distinct to each dementia. Lastly, MRS ratios were able to discriminate between AD and PDD at rates greater than chance. These findings suggest that specific metabolic brain abnormalities exist in AD and PDD, potentially reflecting underlying neuropathology rather than the general effects of dementia. Such information is potentially of value given the difficulties of making a clinical differential between patients with AD who have Parkinsonian features versus patients with PDD.
The distinguishing MRS characteristics of each dementia provide clues regarding the mechanisms of metabolic brain abnormality in PDD and in AD. The metabolic profile in PDD showed reduced cortical NAA/Cr, consistent with prior reports15, 16, as well as reduced Glu/Cr, which has recently been demonstrated by our group in non-demented patients with PD36. To our knowledge, the current report represents the first documentation of reduced cortical glutamate in patients with PDD. The fact that reduced Glu/Cr is observed in PDD as well as non-demented patients with PD suggests that cortical metabolic abnormalities may precede the development of overt dementia in PD. Recent conceptualizations of diseases involving Lewy Bodies (i.e. DLB, PD, PDD) posit that these diseases actually represent a continuum of pathology rather than distinct clinical entities, whereby Lewy Body pathology progresses from midbrain and subcortical structures to involve cortical regions such as the cingulate37–41. In concert with these findings, a recent neuropathological study showed that a cortical glutamate marker is reduced in the autopsy brain tissue of PD patients42, suggesting along with our findings that in advanced PD there is either neuronal loss or downregulation of glutaminergic cortical efferents from the basal ganglia. The positive correlations of Glu/Cr with mental status measures, albeit modest, still suggest relationships of reduction of this metabolite with the presence of cognitive deficits in PDD, although no causal relationship can be determined from this association. As noted previously, the Glu/Cr signal represents not only changes in the neurotransmitter pool of glutamate but also the general metabolic activity of glutamate in the brain. Another distinct possibility is that Glu/Cr changes in PDD and non-demented patients with PD actually represent distinct, unrelated processes. In contrast to findings in PDD, we did not observe reduced Glu/Cr in AD, which has been suggested by prior findings14. This was possibly due to the relatively mild cases of AD that were studied in our clinical sample.
In AD, the brain metabolic pattern is one that is well documented in the literature, consisting of reduced NAA/Cr9, 10 and elevated mI/Cr9, 10. It has been suggested that increased mI/Cr in AD possibly represents gliosis9 or is a marker of inflammation10, although the mechanism of elevated mI in AD remains to be determined. Prior comparison of AD with other dementias have found that while reduced NAA was not specific to AD, mI increases were only observed in patients with AD43 (although see44). Our findings showed trends towards increased mI/Cr in AD compared to PDD, consistent with our prior report that mI/Cr is not increased in PDD patients16, and possibly suggesting that the same degree of gliosis or inflammation is not present in PDD when compared to AD.
Both PDD and AD showed reduced NAA/Cr. This finding is consistent with the interpretation of NAA as a marker of functional integrity of neurons10, 11 and emphasizes that posterior cortical pathology is likely implicated in both diseases22. MRS of other dementias in which neuronal loss is prevalent such as frontotemporal dementia44 and vascular dementia44 have also found reduced cortical NAA. Thus, reduction of NAA is a common finding in many dementias (although see44). The positive correlation of NAA/Cr with mental status measures also suggests a relationship of this metabolite to neuronal function.
An associated finding of the current study is that AD patients who were receiving cholinesterase inhibitors showed a significant increase in Cho/Cr compared to AD patients not on cholinesterase inhibitors (+1.4 SD difference), although cholinesterase inhibitor treatment did not show the same effect in patients with PDD. Prior studies investigating choline-containing compounds with MRS have been mixed, with some groups reporting increases in Cho/Cr10 and others decreases in Cho45. The variability in findings have suggested that very strict control of medication use, diet, and age would be needed to reliably measure Cho9. We suggest that Cho/Cr increases in AD could be explained by use of cholinesterase inhibitors, much as improvements in NAA have been reported in clinical trial studies of donepezil in AD46. However, the current study was not designed to assess changes in MRS related to use of cholinesterase inhibitors, and such findings should be considered strictly observational and preliminary.
One limitation of the current study is a lack of neuropathological confirmation of diagnosis in our patients. Other researchers have indicated that PDD and DLB overlap in clinical and pathological studies30, and that it is difficult to distinguish these two on the basis of clinical findings alone. Both dementias share many features in common, with the time of onset of symptoms being a major determinant in the diagnosis30. While no neuropathological data are currently available for the PDD participants in this study, these patients have been diagnosed using currently accepted criteria by dementia and movement disorders specialists, and thus the data are in accordance with other reports of PDD in the literature30. Furthermore, our MRS findings in PDD are distinct from those previously observed in studies of DLB. Of the two studies reported to date in DLB, both have observed normal NAA/Cr in midline parietal/posterior cingulate regions44, 47, and one of the studies reported increased Cho/Cr44. Notably, to our knowledge there have been no comparative MRS studies of PDD and DLB published in the scientific literature. Interpretation of findings could prove different based upon the ultimate autopsy diagnosis of our participants.
Another study limitation is not using absolute metabolic quantification, but we rather used MRS ratios in reference to Cr. While this method is relatively resistant to the effects of brain atrophy within the voxel of interest, it would be desirable to replicate these findings using tissue classification for grey and white matter within the voxel and absolute quantification of metabolites. The use of covariate analysis was employed to correct for demographic differences in our groups. A future study that attempts to match participants a priori on these variables would be desirable. Lastly, the effects of anti-Parkinson medications on the current findings cannot be ruled-out. We found MRS differences in our prior study of patients with PDD compared to non-demented PD patients, all of whom were taking anti-Parkinson medications, suggesting that MRS ratios can differ by group even when anti-Parkinson medications are held constant16.
In conclusion, patients with AD and PDD showed different patterns of brain metabolic abnormalities compared to healthy controls. Reductions in NAA/Cr were evident in both groups, while increased mI/Cr was present in the AD group and reduced Glu/Cr in the PDD group. MRS data appears to provide a useful means of observing specific brain metabolic patterns in these dementia groups. Future studies examining MRS changes in other PD patients not receiving anti-Parkinson medications, as well as comparing patients with PDD to patients with DLB, would be of interest.
Acknowledgements
This study was funded by grants from the National Institute of Aging (Alzheimer’s Disease Research Center - 1P50 AG16582-01: Harrell, PI); (1R01 AG021927-01: Marson, PI) and Alzheimer’s of Central Alabama. The funding sources had no involvement in the study design, collection, analysis, and interpretation of data or the report writing and decision regarding submission. The authors acknowledge that there were no financial or other conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of Interest - None to disclose
References
- 1.Alzheimer's Association. Alzheimer's Disease Facts and Figures. Washington, D.C.: Alzheimer's Association; 2007. [Google Scholar]
- 2.Feinberg TE, Farah MJ. Behavioral Neurology and Neuropsychology. New York: McGraw-Hill; 1997. [Google Scholar]
- 3.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 4.Benecke R. Diffuse Lewy body disease - a clinical syndrome or a disease entity? J Neurol. 2003;250 Suppl 1:I39–142. doi: 10.1007/s00415-003-1108-9. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74(9):1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvan I, Mohr E, Williams J, Gomez C, Chase TN. Differential memory and executive functions in demented patients with Parkinson's and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1991;54(1):25–29. doi: 10.1136/jnnp.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol (Berl) 2003;106(1):83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 8.Dickson D. Alzheimer-Parkinson disease overlap: Neuropathology. In: Clark C, Trojanowski J, editors. Neurodegenerative Dementias. New York City: McGraw-Hill; 2000. pp. 247–259. [Google Scholar]
- 9.Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56(5):592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herminghaus S, Frolich L, Gorriz C, Pilatus U, Dierks T, Wittsack HJ, et al. Brain metabolism in Alzheimer disease and vascular dementia assessed by in vivo proton magnetic resonance spectroscopy. Psychiatry Res. 2003;123(3):183–190. doi: 10.1016/s0925-4927(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 12.Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res. 2004;1003(1–2):26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Griffith HR, den Hollander JA, Stewart CC, Evanochko WT, Buchthal SD, Harrell LE, et al. Elevated brain scyllo-inositol concentrations in patients with Alzheimer's disease. NMR Biomed. 2007;20:709–716. doi: 10.1002/nbm.1132. [DOI] [PubMed] [Google Scholar]
- 14.Hattori N, Abe K, Sakoda S, Sawada T. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer's disease. Neuroreport. 2002;13(1):183–186. doi: 10.1097/00001756-200201210-00041. [DOI] [PubMed] [Google Scholar]
- 15.Summerfield C, Gomez-Anson B, Tolosa E, Mercader JM, Marti MJ, Pastor P, et al. Dementia in Parkinson disease: a proton magnetic resonance spectroscopy study. Arch Neurol. 2002;59(9):1415–1420. doi: 10.1001/archneur.59.9.1415. [DOI] [PubMed] [Google Scholar]
- 16.Griffith HR, den Hollander JA, Okonkwo OC, O'Brien T, Watts RL, Marson DC. Brain N-acetylaspartate is reduced in Parkinson Disease with dementia. Alzheimer Dis Assoc Disord. 2007 doi: 10.1097/WAD.0b013e3181611011. In press. [DOI] [PubMed] [Google Scholar]
- 17.Bowen BC, Block RE, Sanchez-Ramos J, Pattany PM, Lampman DA, Murdoch JB, et al. Proton MR spectroscopy of the brain in 14 patients with Parkinson disease. AJNR Am J Neuroradiol. 1995;16(1):61–68. [PMC free article] [PubMed] [Google Scholar]
- 18.Firbank MJ, Harrison RM, O'Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson's disease. Dement Geriatr Cogn Disord. 2002;14(2):64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- 19.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Mov Disord. 2003;18(7):784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 20.Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 21.Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O'Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14(10):842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- 22.Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003;20(2):1309–1319. doi: 10.1016/S1053-8119(03)00364-1. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J. Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J Neural Transm. 1996;103(4):455–490. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- 24.Janvin CC, Larsen JP, Salmon DP, Galasko D, Hugdahl K, Aarsland D. Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with lewy bodies and Alzheimer's disease. Mov Disord. 2006;21(3):337–342. doi: 10.1002/mds.20726. [DOI] [PubMed] [Google Scholar]
- 25.Camicioli RM, Korzan JR, Foster SL, Fisher NJ, Emery DJ, Bastos AC, et al. Posterior cingulate metabolic changes occur in Parkinson's disease patients without dementia. Neurosci Lett. 2004;354(3):177–180. doi: 10.1016/j.neulet.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 26.Mattis S. Dementia rating scale (DRS) Odessa, FL: Psychological Assessment; 1988. [Google Scholar]
- 27.Folstein M, Folstein S, McHugh P. Mini-Mental State: A practical guide for grading the cognitive state of the patient for the physician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Yesavage J. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 31.Fahn S, Elton R. UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 32.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 33.Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21(4):1762–1771. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Lamb HJ, Doornbos J, den Hollander JA, Luyten PR, Beyerbacht HP, van der Wall EE, et al. Reproducibility of human cardiac 31P-NMR spectroscopy. NMR Biomed. 1996;9(5):217–227. doi: 10.1002/(SICI)1099-1492(199608)9:5<217::AID-NBM419>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 35.SPSSInc. SPSS Base 10.0 Applications Guide. Chicago: SPSS Inc; 2000. [Google Scholar]
- 36.Griffith HR, Okonkwo OC, O'Brien T, den Hollander JA. Reduced brain glutamate in patients with Parkinson disease. NMR Biomed. 2007 doi: 10.1002/nbm.1203. In press. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord. 2006;21(12):2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Rub U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson's disease. J Neurol Sci. 2006;248(1–2):255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 40.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64(8):1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 42.Kashani A, Betancur C, Giros B, Hirsch E, El Mestikawy S. Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol Aging. 2007;28(4):568–578. doi: 10.1016/j.neurobiolaging.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203(3):829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- 44.Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O'Brien PC, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chantal S, Labelle M, Bouchard RW, Braun CM, Boulanger Y. Correlation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer disease. Arch Neurol. 2002;59(6):955–962. doi: 10.1001/archneur.59.6.955. [DOI] [PubMed] [Google Scholar]
- 46.Jessen F, Traeber F, Freymann K, Maier W, Schild HH, Block W. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology. 2006;67(3):528–530. doi: 10.1212/01.wnl.0000228218.68451.31. [DOI] [PubMed] [Google Scholar]
- 47.Molina JA, Garcia-Segura JM, Benito-Leon J, Gomez-Escalonilla C, del Ser T, Martinez V, et al. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. Eur Neurol. 2002;48(3):158–163. doi: 10.1159/000065520. [DOI] [PubMed] [Google Scholar]