Abstract
We investigated natural resistance in mice to Yersinia enterocolitica, an enteric bacterial pathogen of humans, with a view to determine host genetic factors that are important in resistance. Most mouse strains studied (C3H/HeN, BALB/c, BALB.B, DBA/2, A, Swiss, and SWR) were highly susceptible to infection (50% lethal dose [LD50], 2 X 10(2) to 6 X 10(2) Y. enterocolitica administered intravenously [i.v.]). In contrast, C57BL/6 mice were highly resistant (LD50, 2 X 10(5) Y. enterocolitica administered i.v.). Resistance to i.v. Yersinia infection did not appear to be related to the Ity locus (which codes for resistance to Salmonella typhimurium and other pathogens) because Ityr mice (C3H/HeN, DBA/2, A, and SWR) were more susceptible to Y. enterocolitica than were Itys (C57BL/6) mice. In addition, because BALB.B mice (congenic to C57BL/6 mice at the H-2 locus) were susceptible, resistance was probably not H-2 linked. BALB/c X C57BL/6 F1 mice were intermediate in their resistance to Y. enterocolitica infection (LD50, 3 X 10(4) organisms administered i.v.), suggesting that resistance to Y. enterocolitica depends on a gene dosage affect or a resistance gene(s) interaction between susceptible and resistant parents. Further studies with C57BL/6 and BALB/c mice as prototype resistant and susceptible strains were undertaken. A time course study of Y. enterocolitica growth in various organs following i.v. infection revealed no strain difference in bacterial growth during the first 48 h of infection. Thereafter, however, C57BL/6 mice were capable of restricting Y. enterocolitica growth in all tissues (liver, lung, spleen, kidneys), whereas extensive bacterial proliferation occurred in BALB/c mice tissues. BALB/c mice were also more susceptible to oral Y. enterocolitica infection than were C57BL/6 mice, demonstrating increased mortality and greater numbers of bacteria in the Peyer's patches. Finally, whereas thymus-bearing C57BL/6 X BALB/c F1 mice were resistant to infection, athymic (nude) C57BL/6 X BALB/c F1 mice were susceptible. These studies provide a model to investigate natural immunity to enteric pathogens at mucosal surfaces, as well as provide the basis for clarifying the role of host genotype in Y. enterocolitica resistance.
Full text
PDF

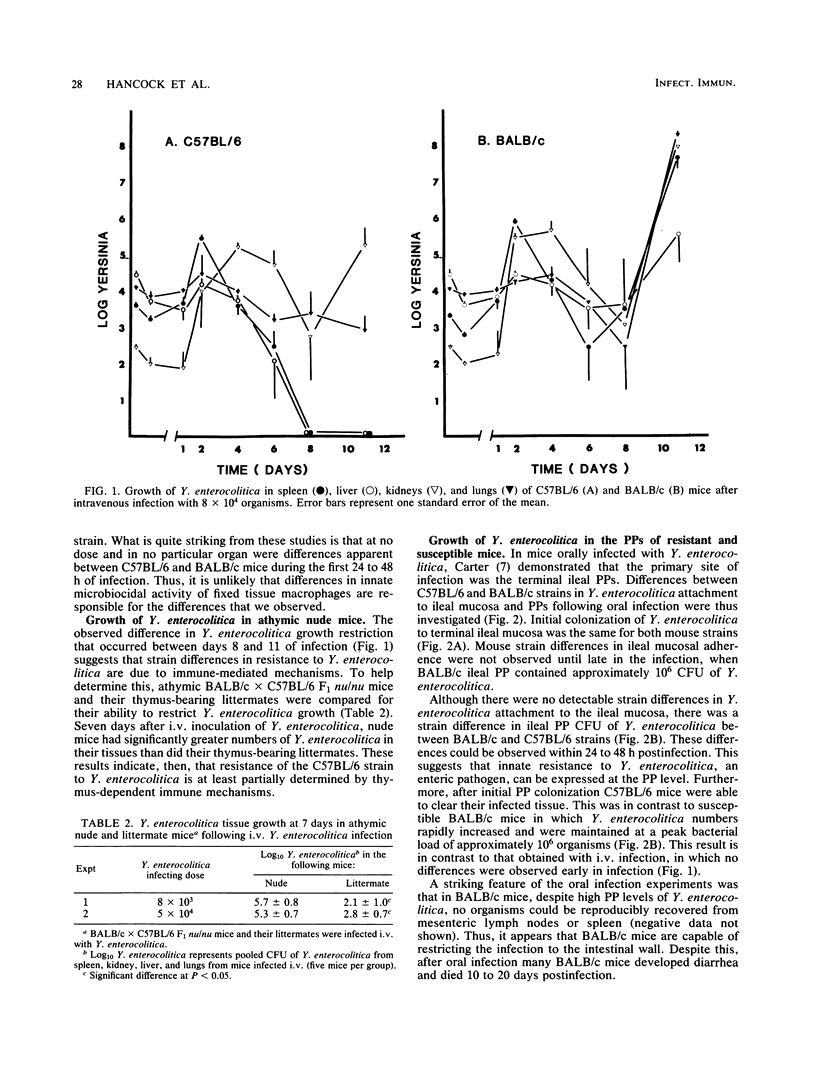
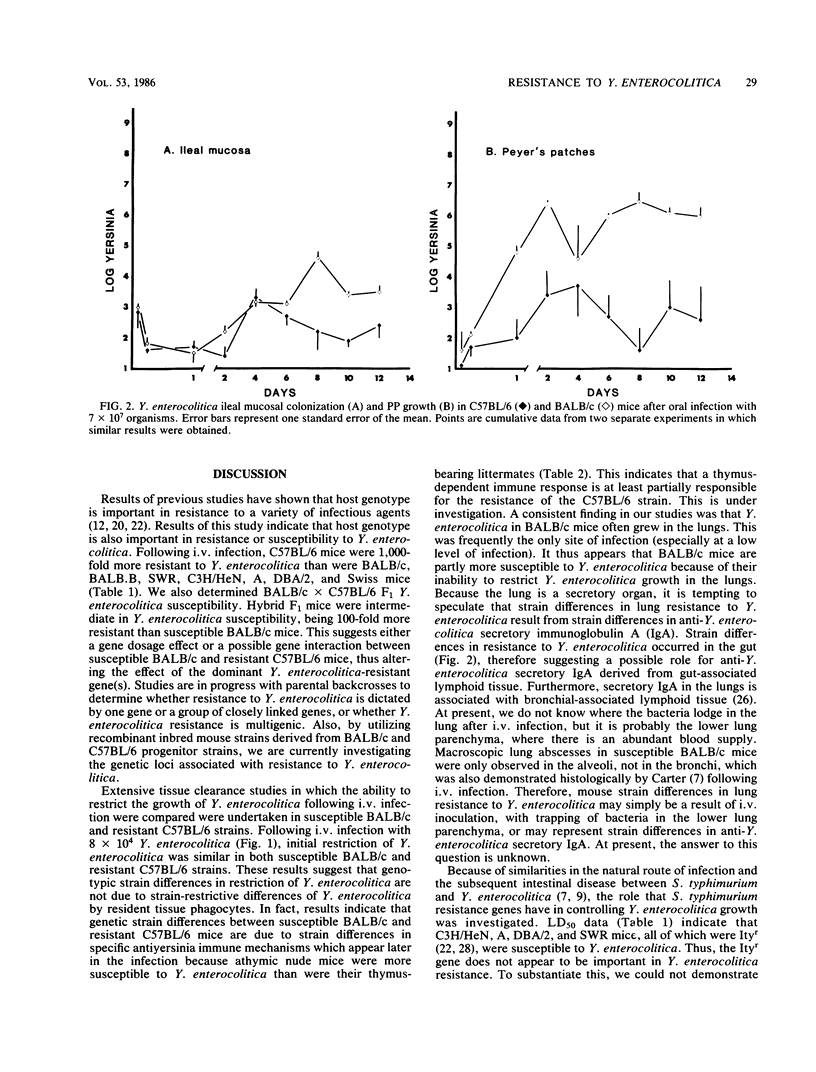


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottone E. J. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5(2):211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S748–S758. doi: 10.1093/clinids/5.supplement_4.s748. [DOI] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Inman L. R. Diarrhea due to Escherichia coli strain RDEC-1 in the rabbit: the peyer's patch as the initial site of attachment and colonization. J Infect Dis. 1981 Mar;143(3):440–446. doi: 10.1093/infdis/143.3.440. [DOI] [PubMed] [Google Scholar]
- Carter P. B. Animal model of human disease. Yersinia enteritis. Animal model: oral Yersinia enterocolitica infection of mice. Am J Pathol. 1975 Dec;81(3):703–706. [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Varga C. F., Keet E. E. New strain of Yersinia enterocolitica pathogenic for rodents. Appl Microbiol. 1973 Dec;26(6):1016–1018. doi: 10.1128/am.26.6.1016-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequeker J., Jamar R., Walravens M. HLA-B27, arthritis and Yersinia enterocolitica infection. J Rheumatol. 1980 Sep-Oct;7(5):706–710. [PubMed] [Google Scholar]
- Kohl S. Yersinia enterocolitica infections in children. Pediatr Clin North Am. 1979 May;26(2):433–443. doi: 10.1016/s0031-3955(16)33715-4. [DOI] [PubMed] [Google Scholar]
- Laitinen O., Leirisalo M., Skylv G. Relation between HLA-B27 and clinical features in patients with yersinia arthritis. Arthritis Rheum. 1977 Jun;20(5):1121–1124. doi: 10.1002/art.1780200512. [DOI] [PubMed] [Google Scholar]
- Lenz T., Schulte K. L., Meyer-Sabellek W. Yersinia enterocolitica septicemia during long-term immunosuppressive treatment. J Infect Dis. 1984 Dec;150(6):963–963. doi: 10.1093/infdis/150.6.963. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Bashore M., Carter P. B. Nonspecific resistance to infection expressed within the Peyer's patches of the small intestine. Infect Immun. 1982 Jul;37(1):390–392. doi: 10.1128/iai.37.1.390-392.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Isolation and functional characteristics of adherent phagocytic cells from mouse Peyer's patches. Immunology. 1982 Apr;45(4):769–774. [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Taylor B. A. Control of natural resistance to Salmonella typhimurium and Leishmania donovani in mice by closely linked but distinct genetic loci. Nature. 1980 Oct 2;287(5781):440–442. doi: 10.1038/287440a0. [DOI] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi I. D., Pearson A. D., Suckling W. G., Klein C. Long-term fecal excretion and resistance induced in mice infected with Yersinia enterocolitica. Infect Immun. 1978 Jul;21(1):342–344. doi: 10.1128/iai.21.1.342-344.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: Ity gene is expressed in vivo by 24 hours after infection. J Immunol. 1983 Dec;131(6):3014–3020. [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. II. Interaction with cultured cells in vitro. Microbiol Immunol. 1977;21(7):365–377. doi: 10.1111/j.1348-0421.1977.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Vantrappen G., Ponette E., Geboes K., Bertrand P. Yersinia enteritis and enterocolitis: gastroenterological aspects. Gastroenterology. 1977 Feb;72(2):220–227. [PubMed] [Google Scholar]
- Vesikari T., Nurmi T., Mäki M., Skurnik M., Sundqvist C., Granfors K., Grönroos P. Plasmids in Yersinia enterocolitica serotypes O:3 and O:9: correlation with epithelial cell adherence in vitro. Infect Immun. 1981 Sep;33(3):870–876. doi: 10.1128/iai.33.3.870-876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]


