Abstract
Many forms of cardiovascular disease are associated with cardiomyocyte loss via necrosis and/or apoptosis. The cumulative loss of contractile cells ultimately results in diminished cardiac function. Numerous approaches have been employed to reduce the rate of cardiomyocyte loss, or alternatively, to repopulate the heart with new cardiomyocytes. Strategies aimed at repopulating the heart include cardiomyocyte cell therapy, myogenic stem cell therapy, and cell cycle activation therapy. All three approaches are based on the assumption that the de novo cardiomyocytes will participate in a functional syncytium with the surviving myocardium. This review will discuss the current status of interventions aimed at repopulating the heart with functional cardiomyocytes.
Keywords: Myocardial Regeneration, Stem Cells, Cardiomyocyte Proliferation, Apoptosis
INTRODUCTION
During development, increases in cardiac mass are largely due to the differentiation and subsequent proliferation of cardiomyocytes. After birth, the level of cardiomyocyte cell cycle activity is dramatically reduced and subsequent increases in cardiac mass occur predominantly from hypertrophic cardiomyocyte growth. The intrinsic proliferative capacity of terminally differentiated adult cardiomyocytes appears to be rather limited, as evidenced by the very low rates of DNA synthesis using radio-isotope incorporation assays.1–3 Although a number of studies have suggested that cardiomyogenic stem cells are present in adult hearts, the ability of these cells to reconstitute significant amounts of myocardial tissue in the absence of exogenous inducers also appears to be quite limited.4 Consequently, myocardial injury typically resolves with scar formation as opposed to regenerative muscle growth. A number of approaches have emerged to lessen the degree of myocyte loss following injury, as well as to promote repopulation of damaged areas with functional cardiomyocytes. This review will focus on interventions aimed at repopulating damaged hearts with functional cardiomyocytes. Initial efforts aimed at the transplantation of cardiomyocytes as well as myogenic stem cells will be discussed. More recent studies suggesting that stem cell mobilization can be employed to promote cardiomyocyte repopulation in adult hearts are also discussed. This is followed by a review of interventions aimed at inducing cell cycle activity in cardiomyocytes surviving myocardial injury.
CARDIOMYOCYTE CELL THERAPY
The notion of direct transplantation of donor cardiomyocytes as a mechanism to promote repopulation in normal or injured myocardium dates to the early 1990s. Fetal or neonatal cardiomyocytes would a priori appear to be the ideal donor cell, as they express all of the molecular and physiologic attributes necessary for functional integration with the host myocardium. Proof of concept studies utilized donor fetal cardiomyocyte from transgenic mice expressing a cardiomyocyte-restricted, nuclear localized beta-galactosidase reporter transgene. After transplantation into the hearts of non-transgenic hosts mice, donor cells were readily identified based on the presence of nuclear beta-galactosidase activity.5 Subsequent ultrastructure studies confirmed the presence of gap junctions between donor and host cardiomyocytes,6 and numerous studies demonstrated that fetal cardiomyocytes could be transplanted into normal or injured hearts. Moreover, delivery of cardiomyocytes into injured hearts was frequently shown to have a positive impact on myocardial function, suggesting a potential therapeutic value (the reader is referred to the recent review by Dowell et al7). Despite these promising results, direct demonstration of physiologic coupling between donor and host cardiomyocytes required the development of imaging systems capable of monitoring intracellular calcium transients in individual cells within intact hearts;8 these studies demonstrated that the donor cardiomyocytes could participate in a functional syncytium with the host myocardium.
Given that embryonic stem (ES) cells can give rise to highly differentiated cardiomyocytes in vitro,9 additional studies were performed to assess the suitability of ES-derived cardiomyocytes as donor cells for transplantation experiments. Initial studies utilized a relatively simple antibiotic selection protocol to generate pure cultures of ES-derived cardiomyocytes; these cells were able to form stable grafts following transplantation into recipient hearts.10 Subsequent studies demonstrated that this selection protocol was easily scalable to bioreactor vessels, and yields as high as 109 cardiomyocytes per 2 liter volume were obtained.11, 12 The use of ES-derived cells for the treatment of injured hearts has recently been reviewed.13
MYOGENIC STEM CELL THERAPY
In addition to differentiated cardiomyocytes, therapies aimed at transplanting myogenic, or preferably cardiomyogenic, stem cells have been initiated. The first efforts focused on the use of skeletal myoblasts (SMBs). SMBs can be isolated from relatively small tissue biopsies, and can be amplified to large numbers in vitro. When cultured under appropriate conditions, SMBs will differentiate into nascent skeletal myotubes. The first demonstration that transplanted SMBs differentiated into myotubes and formed stable grafts following intra-cardiac delivery utilized C2C12 cell lines, and relied on differential expression of myofiber contractile protein isoforms to distinguish donor and host myocytes.14 Light and ultrastructure analyses suggested that the nascent myotubes were structurally uncoupled from the host myocardium. Subsequent studies demonstrated that SMBs could readily engraft infarcted myocardium,15 and furthermore could result in functional improvement following engraftment.16 Although several groups suggested that nascent myotubes reconstituted “functioning muscle”,16–18 subsequent studies demonstrated that the donor-derived cells were electrically isolated from the host myocardium19, 20 (with the exception of extremely rare fusion events which occurred between donor SMBs and host cardiomyocytes at the graft/myocardium border).20 The improvement in cardiac function observed following SMB transplantation more likely resulted from a beneficial impact on post-infarction ventricular remodeling.21 Nonetheless, the observation that SMB transplantation could promote some degree of functional recovery promoted several Phase I clinical trials to test the feasibility and safety of the approach;22–24 the current status of these trials was recently reviewed.25
A number of studies suggested the existence of extra-cardiac stem cells with cardiomyogenic potential. For example, transplantation of wild-type male hematopoietic stem cells (HSCs) into lethally-irradiated female mdx mice resulted in the presence of a low number of dystrophin-positive cardiomyocytes that appeared to contain a y-chromosome.26 Similarly, transplantation of HSCs from mice carrying a ubiquitously-expressed beta-galactosidase reporter gene into irradiated recipients gave rise to beta-galactosidase expressing cardiomyocytes.27 These observations suggested that marrow-derived cells could contribute to the adult myocardium, albeit at very low frequencies. This view was supported indirectly by pathologic analysis of female human hearts which had been transplanted into male recipients: cardiomyocytes harboring a y-chromosome could be detected. However the frequency of this phenomenon varied tremendously from lab to lab, ranging from as high as 14% of the cardiomyocytes in one study28 to very few or none of the cardiomyocytes in other studies.29–31 Subsequent demonstration of heterokaryon formation between HSCs and resident cardiomyocytes strongly argued that fusion events gave rise to “cardiomyocyte formation” in the basic and clinical studies described above.32, 33
The early suggestion that HSC marrow reconstitution might result in the formation of cardiomyocytes prompted several groups to directly transplant these cells into injured hearts. One study reported very high rates of “transdifferentiation” of HSCs into cardiomyocytes, resulting in a remarkable reconstitution of muscle mass and concomitant functional improvement.34 In contrast, several other studies using both lineage-restricted and cell fate reporter trangenes demonstrated that HSCs failed to differentiate into cardiomyocytes following transplantation into injured hearts.35–37 It was suggested that differences in the rigor of the assays used to monitor cardiomyogenic differentiation likely contributed to these markedly differing results.38
Other marrow-derived cells have been reported to exhibit cardiomyogenic activity, and/or can improve function following transplantation into injured hearts.39–43 Of particular interest, Dzau and colleagues suggested that mesenchymal stem cells (MSCs) differentiated into cardiomyocytes with a concomitant marked improvement in cardiac function following transplantation into infarcted hearts.44 However subsequent studies from this group revealed that the observed functional improvement resulted from an anti-apoptotic paracrine effect of the donor cells on at-risk cardiomyocytes as opposed to regenerative growth of the myocardium;45 a similar mechanism could very well account for the functional improvement seen in the initial HSC transplantation experiments.34 Indeed, marrow-derived cells have previously been implicated in neovascularization events.46 Those observations prompted a number of Phase I clinical trials, enthusiasm for which was bolstered somewhat by the initial suggestion of transdifferentiation activity.47–53. Although a slight to moderate improvement in cardiac function was noted following delivery of marrow- or peripheral blood-derived progenitor cells in most instances, the functional assays employed were unable to distinguish between an indirect effect (e.g. angiogenesis) imparted upon the surviving myocardium and a direct contribution of functional de novo cardiomyocytes. The current status of clinical trials utilizing stem cells from the marrow or the peripheral circulation was recently reviewed.25
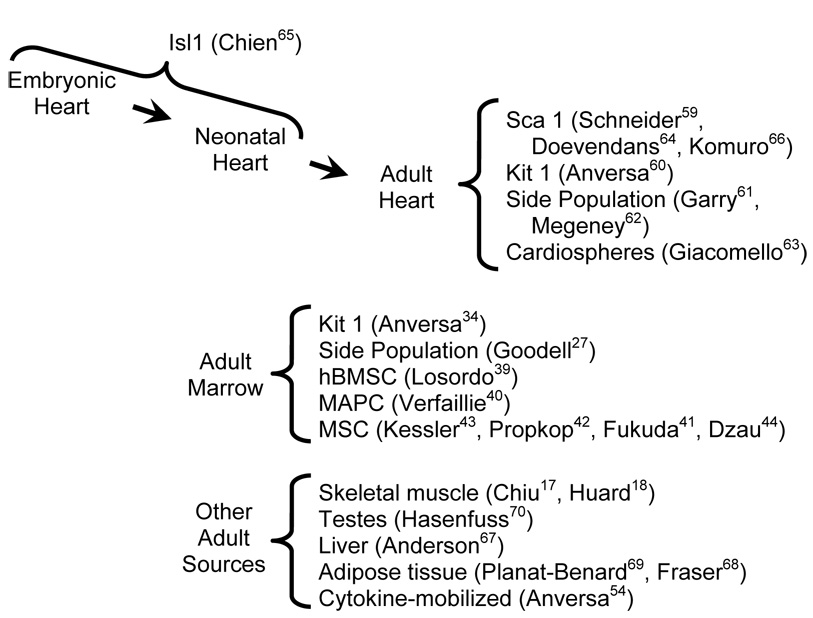
It has also been reported that treatment with cytokines which mobilize marrow-derived HSCs resulted in marked regeneration of infarcted hearts in mice.54 Given that the same cytokines are routinely and effectively used clinically for HSCs transplantation in cancer patients, several clinical trials were rapidly initiated to test their impact on post-infarction function in humans. The absence of any improvement (or deterioration) in cardiac function in a large, randomized clinical trial55 suggests that this treatment lacks the ability to induce cardiomyogenic differentiation. In support of this, subsequent reports in mice using more rigorous experimental read-outs failed to see regenerative growth in infarcted animals, despite a similar degree of HSC mobilization as reported in the earlier study.37, 56 Cytokine treatment may have beneficial albeit indirect effects on post-infarction remodelling;57 however value of this activity must be weighed against potentially deleterious vascular effects in patients with coronary artery disease, as exemplified by the perhaps predictable observation that cytokine treatment accelerated in-stent coronary artery restenosis.58 Finally, cardiac-resident stem cells have also been recently described in the neonatal and adult heart.59–64 Several studies indicated that these cells can be transplanted in or mobilized to injured myocardium, with apparent cardiomyogenic differentiation. Of particular interest are the Isl-1 cells described by Chien and co-workers65 and the Sca-1 cells described by Komuro and colleagues,66 as they appear able to undergo bona fide cardiomyogenic differentiation in vitro. Cells with apparent cardiomyogenic potential have also been reported in the liver,67 adipose tissue,68, 69 and testes.70
Figure 1 lists representative studies suggesting that neonatal- or adult-derived stem cells possess cardiomyogenic activity. While it is exciting to anticipate the potential impact such cells might have on repopulating injured myocardium, it is important to keep in mind that injured hearts typically exhibit little regenerative capacity.1, 3 Moreover, deregulation of intracellular signaling pathways typically leads to neoplastic growth in organ systems with robust stem cell activity; in contrast neoplastic growth of the myocardium is virtually nonexistent.71 These observations indicate that under normal circumstances, cardiomyogenic stem cell activity in adult mammals is quite limited. While it is possible that ex vivo growth of stem cells might enhance their ability to repopulate the heart (as was suggested for mesenchymal adult progenitor cells),40 considerable manipulation in vitro and/or in vivo might be required in order to obtain effective repopulation in injured hearts with these cells.
Figure 1.
Representative studies suggesting that neonatal or adult-derived stem cells possess cardiomyogenic activity (names in parentheses indicate the senior author of the studies, and superscript numbers indicate the citations).
CELL CYCLE ACTIVATION THERAPY
Cell cycle activation constitutes an alternative approach with which to repopulate damaged cardiac tissue with functional cardiomyocytes. As indicated above, cardiomyocytes withdraw from the cell cycle shortly after birth, and increases in cardiac mass during postnatal life are due predominantly to hypertrophic growth. Cardiac injury typically has only a modest impact on cardiomyocyte cell cycle activity. Using a lineage-restricted transgenic reporter system, only 0.008% of the cardiomyocytes at the infarct border zone exhibit cell cycle activity one week following permanent coronary artery occlusion, with even lower levels of cell cycle activity in the remote myocardium.72 While it is likely that both species and strain differences can impact on the absolute rate of cardiomyocyte proliferation (the previous experiments were performed in a DBA/2J genetic background), a survey of the literature confirms that the proliferative response following myocardial injury is limited.73
Given the potential utility of cell cycle activation as an approach to repopulate injured hearts, considerable effort has been invested to elucidate factors and gene products that regulate cardiomyocyte proliferation.74 These efforts have been greatly aided by the use of transgenic animals (which over-express a specific gene product) or gene-targeted animals (which either do not express a specific gene product, or express an altered form of a gene product). These systems effectively permit gain-of-function, loss-of-function, and change-of-function genetic modifications of virtually any gene product, and combinatorial effects can readily studied by simple interbreeding genetically modified animals.
The first animal models which exhibited altered cardiomyocyte cell cycle activity relied on targeted expression of the SV40 Large T Antigen oncoprotein to cardiomyocytes in transgenic mice.75–77 These animals developed tumors comprised of differentiated, proliferating cardiomyocytes, and thus demonstrated that cardiomyocyte cell cycle activity can be readily modulated genetically. Subsequent studies identified a multitude of gene products which, when mis-expressed in genetically modified animals, resulted in marked deregulation of cardiomyocyte cell cycle activity (the reader is referred to several recent reviews).74, 78 In most instances the effect on cardiomyocyte cell cycle activity was observed to occur in embryonic hearts; this skew in temporal distribution reflects in part the fact that many of the genetic alterations resulted in embryonic or neonatal lethality (thereby precluding the ability to ascertain the affect of the alteration at later stages of development). Nonetheless, a number of models also had altered cardiomyocyte cell cycle activity in neonatal and adult hearts.
Cell cycle progression is regulated by a number of check-points which rely on protein kinase signaling cascades.79 A key regulatory node is the restriction point, transit through which commits the cell to a new round of DNA synthesis and cell division. Restriction point transit relies on the activation of cyclin-dependent kinase (CDK) 4 and 6. CDK activity in turn is dependent upon the induction of their obligate activating partners, the D-type cyclins. Factors which positively regulate cell cycle progression (i.e., sufficient nutrients, presence of growth factors, etc.) activate signal transduction pathways which induce transcription of the D-type cyclins. Newly synthesized D-type cyclins bind to and activate CDKs, which then phoshorylate members of the retinoblastoma gene family. Phosphorylation of retinoblastoma proteins results in the release of active E2F transcription factors and the subsequent transcription of genes required for DNA synthesis. Conversely, factors which negatively regulate cell cycle progression (inhibitory cytokines, intrinsic transduction pathways, etc) act predominantly by suppressing CDK activity either directly or indirectly.
The majority of genes which have been shown to impact cardiomyocyte cell cycle activity in adult genetically modified mice are involved in some aspect of restriction point transit (Figure 2). For example, a ca. 5-fold increase in cardiac IGF-1 expression led to a 16-fold increase in the number of DNA synthesizing cardiomyocytes in adult mice as compared to their non-transgenic littermates.80 Similarly, induction of c-myc activity in conditional transgenic mice resulted in cardiac hypertrophy which was accompanied by reactivation of cardiomyocyte DNA synthesis and the formation of multi-nucleated cardiomyocytes.81 In other studies, inhibition of p38 MAP kinase activity (either pharmacologically in cultured cells or in vivo using cardiomyocyte-restricted gene targeting) resulted in cardiomyocyte DNA synthesis and proliferation.82 Thus, modulation of positive or negative signal transduction regulatory pathways which act up-stream of the restriction point is sufficient to drive cardiomyocyte cell cycle activity in adult hearts.
Figure 2.
A schematic diagram of the cell cycle highlighted with studies wherein genetic manipulation resulted in cardiomyocyte cell cycle activity in adult hearts (names in parentheses indicate the senior author of the studies, and superscript numbers indicate the citations).
Other experiments have manipulated the activities of molecules that directly regulate restriction point transit. Indeed, SV40 Large T Antigen regulates cell cycle activity by binding to and inhibiting the activity of retinoblastoma family members;83 thus cardiomyocyte cell cycle induction in transgenic mice expressing this protein75–77 resulted from direct manipulation of restriction point regulatory proteins. In agreement with this, a recent study has shown that combinatorial deletion of retinoblastoma protein and its closely related family member p130 resulted in a 135-fold increase in DNA synthesizing cells as compared to control animals.84 A similar effect was observed when D-type cyclin expression was targeted to cardiomyocytes. Adult mice expressing either cyclin D1, D2 or D3 show a remarkable 200-fold increase in DNA synthesizing cardiomyocytes as compared to their non-transgenic littermates.85, 86 Finally, over-expression of CDK-2, which acts downstream of CDKs 4 and 6, had a similar impact on cardiomyocyte DNA synthesis (approximately 100-fold increase as compared to non-transgenic littermates).87 Thus, manipulation of molecules that directly regulate the restriction point is sufficient to drive cardiomyocyte DNA synthesis in adult hearts.
The activity of regulatory proteins which act between the restriction point and the G1/S phase boundary of the cell cycle has also been modulated in cardiomyocytes of genetically modified animals. For example, expression of either dominant interfering p5372 or dominant interfering TSC288 was sufficient to promote cardiomyocyte DNA synthesis in adult hearts following myocardial injury. Both p53 and TSC2 are positive regulators of p27 (which is a negative regulator of CDK2); hence expression of dominant interfering p53 or TSC2 would interfere with p27-mediated cell cycle inhibition. Similarly, over-expression of cyclin A2, which acts at the G1/S boundary, also resulted in a slight increase in cardiomyocyte DNA synthesis levels as compared to non-transgenic littermates.89 Finally over-expression of either dominant interfering p19372 or wild-type Bcl-290 resulted in enhanced levels of cardiomyocyte DNA synthesis in adult hearts; both molecules have been shown to inhibit apoptosis, but the mechanism by which their expression modulated cardiomyocyte cell cycle activity remains elusive.
An important caveat in many of the transgenic and gene targeting experiments described above is that alteration of gene expression often occurred prior to cardiomyocyte terminal differentiation. As such, modulation of these proteins in terminally differentiated cardiomyocytes might yield a different result. In the case of the dominant interfering TSC2, p53 and p193 models, cardiomyocyte DNA synthesis was not observed under baseline conditions in adult hearts, but was only observed following myocardial injury. Thus it is likely that much, if not all, of the terminal differentiation program was enacted prior to cell cycle induction in these mice. Although transgene expression of cyclin D preceded cardiomyocyte terminal differentiation, a similar effect on cell cycle activity was observed following adenoviral delivery of cyclin D1 (containing a nuclear localization motif) and CDK4.91 In the case of conditional myc expression and pharmacologic p38 inhibition, these manipulations were also performed after cardiomyocyte terminal differentiation (although in the case of p38 inhibition, the potential impact culturing the cardiomyocytes might constitute a confounding issue). Nonetheless, these data all strongly support the notion that with appropriate manipulation adult cardiomyocytes can re-enter the cell cycle.
Although many of the experiments described above used cardiomyocyte DNA synthesis as an assay for cell cycle induction, it is clear that the cardiomyocytes must progress through the cell cycle and divide, and furthermore that the de novo formed cardiomyocytes must participate in a functional syncytium with the remainder of the heart, if the activity is to be of any therapeutic value. Modulation of IGF-1, p38 MAP kinase, retinoblastoma/p130, cyclin D and CDK2 activity was associated with increased cardiomyocyte cell number and/or immune-histologic evidence of cell cycle progression. In the case of IGF-1, transgene expression was associated with improved cardiac function following injury; however it was not clear if this resulted from cardiomyocyte cell cycle activity, or from the well-established anti-apoptotic activity of this molecule.92 In contrast, cardiomyocyte cell cycle induction in mice expressing cyclin D2 resulted in regeneration of muscle mass following permanent coronary artery occlusion in mice.86 Improvement in cardiac structure in these animals was accompanied by a marked restoration in cardiac function, as measured by intra-ventricular pressure-volume recordings.93 Paradoxically, myocardial injury in mice expressing CDK2 (which exhibited a similar increase in cardiomyocyte cell cycle activity as was seen in the cyclin D mice) resulted in a maladaptive hypertrophic response and marked deterioration in cardiac function.87 The mechanistic basis for these different responses to injury is currently not clear.
SUMMARY
The studies described above demonstrate that ability to repopulate the heart in experimental animals using cell-based approaches is now well established. In some instances cardiomyocyte repopulation was directly associated with improved cardiac function following injury. Clearly many challenges must be overcome for successful clinical application of these approaches. For example, it may prove to be impossible to modulate some of the pathways in an organ or cell type-specific manner, thus rendering them unsuitable for therapeutic use. It is also likely that some of the interventions described will be species specific (and thus not translatable to humans), or alternatively will simply not be reproducible by other groups. Nonetheless, the scope and sheer number of interventions which appear to have a positive impact on the heart is unprecedented. Thus, the potential for successful restoration of cardiac structure and function following injury is greater now than at any previous time. Concerted efforts on research in this area will hopefully turn this dream to reality.
ACKNOWLEDGMENT
We thank the National Heart, Lung and Blood Institute for support.
REFERENCES
- 1.Rumiantsev PP. Growth and hyperplasia of cardiac muscle cells. London, U.K.; New York, N.Y., U.S.A.: Harwood Academic Publishers; 1991. [Google Scholar]
- 2.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am J Physiol. 1994;266:H1439–H1445. doi: 10.1152/ajpheart.1994.266.4.H1439. [DOI] [PubMed] [Google Scholar]
- 3.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soonpaa MH, et al. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 6.Koh GY, et al. Stable fetal cardiomyocyte grafts in the hearts of dystrophic mice and dogs. J Clin Invest. 1995;96:2034–2042. doi: 10.1172/JCI118251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell JD, et al. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336–350. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 8.Rubart M, et al. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res. 2003;92:1217–1224. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- 9.Doetschman TC, et al. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 10.Klug MG, et al. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder M, et al. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 12.Zandstra PW, et al. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 13.Rubart M, Field LJ. Cardiac repair by embryonic stem-derived cells. Handb Exp Pharmacol. 2006:73–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Koh GY, et al. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993;92:1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murry CE, et al. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest. 1996;98:2209–2217. doi: 10.1172/JCI119030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor DA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 17.Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12–18. [PubMed] [Google Scholar]
- 18.Oshima H, et al. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 19.Leobon B, et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubart M, et al. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–783. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinlib L, Field L. Cell transplantation as future therapy for cardiovascular disease?: A workshop of the National Heart, Lung, and Blood Institute. Circulation. 2000;101:E182–E187. doi: 10.1161/01.cir.101.18.e182. [DOI] [PubMed] [Google Scholar]
- 22.Menasche P, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 23.Pagani FD, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 24.Siminiak T, et al. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005;26:1188–1195. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–3183. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 26.Bittner RE, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–396. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- 27.Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quaini F, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 29.Laflamme MA, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 30.Hruban RH, et al. Fluorescence in situ hybridization for the Y-chromosome can be used to detect cells of recipient origin in allografted hearts following cardiac transplantation. Am J Pathol. 1993;142:975–980. [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser R, et al. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–19. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 33.Wagers AJ, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 34.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 36.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 37.Nygren JM, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 38.Chien KR. Stem cells: lost in translation. Nature. 2004;428:607–608. doi: 10.1038/nature02500. [DOI] [PubMed] [Google Scholar]
- 39.Yoon YS, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 41.Kawada H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 42.Pochampally RR, et al. Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc Natl Acad Sci U S A. 2004;101:9282–9285. doi: 10.1073/pnas.0401558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toma C, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 44.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 45.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 46.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 47.Perin EC, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs S, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41:1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 49.Strauer BE, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 50.Tse HF, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 51.Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 52.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 53.Chen SL, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Orlic D, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 56.Deten A, et al. Hematopoietic stem cells do not repair the infarcted mouse heart. Cardiovasc Res. 2005;65:52–63. doi: 10.1016/j.cardiores.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 57.Hasegawa H, et al. Cardioprotective effects of granulocyte colony-stimulating factor in swine with chronic myocardial ischemia. J Am Coll Cardiol. 2006;47:842–849. doi: 10.1016/j.jacc.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 58.Kang HJ, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomized clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 59.Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 61.Martin CM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 62.Hierlihy AM, et al. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 63.Messina E, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 64.Goumans M, et al. Human Cardiac Progenitor Cells are Able to Differentiate into Cardiomyocytes in Vitro. American Heart Association 2005 Scientific Sessions. 2005 Abstract #337. [Google Scholar]
- 65.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuura K, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 67.Malouf NN, et al. Adult-derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol. 2001;158:1929–1935. doi: 10.1016/S0002-9440(10)64661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strem BM, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 69.Planat-Benard V, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 70.Guan K, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006 doi: 10.1038/nature04697. In Press. [DOI] [PubMed] [Google Scholar]
- 71.Butany J, et al. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima H, et al. Expression of mutant p193 and p53 permits cardiomyocyte cell cycle reentry after myocardial infarction in transgenic mice. Circ Res. 2004;94:1606–1614. doi: 10.1161/01.RES.0000132279.99249.f4. [DOI] [PubMed] [Google Scholar]
- 73.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 74.Pasumarthi KB, Field LJ. Cardiomyocyte enrichment in differentiating ES cell cultures: strategies and applications. Methods Mol Biol. 2002;185:157–168. doi: 10.1385/1-59259-241-4:157. [DOI] [PubMed] [Google Scholar]
- 75.Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988;239:1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- 76.Behringer RR, et al. Heart and bone tumors in transgenic mice. Proc Natl Acad Sci U S A. 1988;85:2648–2652. doi: 10.1073/pnas.85.8.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katz EB, et al. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol. 1992;262:H1867–H1876. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- 78.Field LJ. Modulation of the cardiomyocyte cell cycle in genetically altered animals. Ann N Y Acad Sci. 2004;1015:160–170. doi: 10.1196/annals.1302.013. [DOI] [PubMed] [Google Scholar]
- 79.Schang LM. The cell cycle, cyclin-dependent kinases, and viral infections: new horizons and unexpected connections. Prog Cell Cycle Res. 2003;5:103–124. [PubMed] [Google Scholar]
- 80.Reiss K, et al. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao G, et al. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res. 2001;89:1122–1129. doi: 10.1161/hh2401.100742. [DOI] [PubMed] [Google Scholar]
- 82.Engel FB, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005 doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeCaprio JA, et al. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 84.MacLellan WR, et al. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol Cell Biol. 2005;25:2486–2497. doi: 10.1128/MCB.25.6.2486-2497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soonpaa MH, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pasumarthi KB, et al. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 87.Liao HS, et al. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88:443–450. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 88.Pasumarthi KB, et al. Enhanced cardiomyocyte DNA synthesis during myocardial hypertrophy in mice expressing a modified TSC2 transgene. Circ Res. 2000;86:1069–1077. doi: 10.1161/01.res.86.10.1069. [DOI] [PubMed] [Google Scholar]
- 89.Chaudhry HW, et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 90.Limana F, et al. bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci U S A. 2002;99:6257–6262. doi: 10.1073/pnas.092672899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tamamori-Adachi M, et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003;92:e12–e19. doi: 10.1161/01.res.0000049105.15329.1c. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassink RJ, et al. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. doi: 10.1093/cvr/cvm101. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]