Abstract
Results of a series of 12 ns molecular dynamics (MD) simulations of the reactant state (with and without a Mg2+ ion), early and late transition state mimics are presented based on a recently reported crystal structure of a full-length hammerhead RNA. The simulation results support a catalytically active conformation with a Mg2+ ion bridging the A9 and scissile phosphates. In the reactant state, the Mg2+ spends significant time closely associated with the 2′OH of G8, but remains fairly distant from the leaving group O5′ position. In the early TS mimic simulation, where the nucleophilic O2′ and leaving group O5′ are equidistant from the phosphorus, the Mg2+ ion remains tightly coordinated to the 2′OH of G8, but is positioned closer to the O5′ leaving group, stabilizing the accumulating charge. In the late TS mimic simulation, the coordination around the bridging Mg2+ ion undergoes a transition whereby the coordination with the 2′OH of G8 is replace by the leaving group O5′ that has developed significant charge. At the same time, the 2′OH of G8 forms a hydrogen bond with the leaving group O5′ and is positioned to act as a general acid catalyst. This work represents the first reported simulations of the full-length hammerhead structure and TS mimics, and provides direct evidence for the possible role of a bridging Mg2+ ion in catalysis that is consistent with both crystallographic and biochemical data.
The hammerhead ribozyme [1] is an archetype system to study RNA catalysis. [2,3] A detailed understanding of the hammerhead mechanism provides insight into the inner workings of more complex cellular catalytic RNA machinery such as the ribosome, and ultimately may aid the rational design of new medical therapies [4] and biotechnology. [5,6]
Despite a tremendous amount of experimental and theoretical effort, [1,2,7,8] the details of the hammerhead ribozyme mechanism have been elusive. In particular, one of the main puzzles involves the apparent inconsistency between the interpretation of thio effect experiments [9,10] and mutational data [8] with available crystallographic structural information of the minimal hammerhead sequence. [11,12,13] Results from the biochemical experiments suggest that a pH-dependent conformational change, inconsistent with crystallographic data, [11,12,13] must precede or be concomitant with the catalytic chemical step. This includes a possible metal ion bridge between the A9 and scissile phosphates that in previous crystal structures were ~20 Å apart. Moreover, the function of the 2′OH group of G8 remains unclear.
Very recently, the crystallographic structure of a full length hammerhead sequence has been determined at 2.2 Å resolution. [14] The naturally occurring full length hammerhead sequence exhibits enhanced catalytic activity and a different metal ion requirement relative to the minimal motif. [15] The crystal structure has the A9 and scissile phosphates in close proximity, consistent with the interpretation of thio effect measurements, [9] and the 2′OH of G8 and N1 of G12 poised to act as a general acid and base, respectively, consistent with photocrosslinking experiments [16] and mutational data. [8] However, the divalent metal ions required for catalysis were not resolved in this structure. This letter reports the first simulations of the full-length hammerhead ribozyme in the reactant, early and late transition states along the reaction coordinate. Results support the requirement for a bridging Mg2+ ion between the A9 and scissile phosphates in the catalytically active conformation, and provide evidence of a role of the metal ion in catalysis that is consistent with both crystallographic and biochemical data.
Simulations were performed with CHARMM [17] (version c32a1) using the all-atom nucleic acid force field [18,19] with extension to reactive intermediates (e.g., transition state mimics) [20] and TIP3P water model. [21] Simulations of the reactant state (with and without a Mg2+ ion), early and late TS mimics were each performed at 298 K and 1 atm in a rhombododecahedral cell (with PME[22] electrostatics) in the presence of ~10,000 water molecules and 0.14 M NaCl, and carried out to 12 ns following 1 ns of solvent equilibration. In three simulations, a single Mg2+ ion was positioned so as to bridge the A9 and scissile phosphates that in the crystallographic structure are around 4.3 Å, which is well suited for Mg2+-bridging coordination. [23] The Mg2+ ion is critical for stability, and adopts different coordination states along the reaction coordinate, verified by preliminary QM/MM calculations (see supporting information) that are supportive of a catalytic role consistent with experiments.
A stable Mg2+ ion bridge between the A9 and scissile phosphates is formed in the catalytically active conformation
The simulation results support a catalytic role for a Mg2+ ion bridging the A9 and scissile phosphates. In the simulations with a bridging Mg2+ ion, the average distance between the A9 and scissile phosphates remain within the crystallographic value of 4.3 Å, whereas in the absence of Mg2+ this key contact between stems I and II drifts to over 7 Å (Table 1). In the reactant state, the Mg2+ coordination between the C1.1 and A9 phosphate oxygens fluctuates between axial-axial and axial-equatorial modes, resulting in a shorter average oxygen-oxygen distance than that observed in the X-ray structure. This may suggest that in the reactant state the preferred binding mode of Mg2+ is different, e.g., between A9 and N7 of G10.1, [24,25] and that a conformational change brings Mg2+ into a bridging position between A9 and the scissile phosphate leading to the transition state. [9] The present simulation results suggest that the close proximity of the A9 and scissile phosphates observed in the new full-length hammerhead structure [14] can be stabilized by a Mg2+ ion bridge that brings together stems I and II and facilitates formation of near-attack conformations (see supporting information) in a way different from previous simulations based on the minimal sequence structures. [26,27,28,29]
Table 1.
Key distances (Å) in the hammerhead active site
| X-ray Structure | Reactant | Reactant w/o Mg2+ | Early-TS mimic | Late-TS mimic | |
|---|---|---|---|---|---|
| C1.1:OP2 ↔ A9:O P2 | 4.27 | 3.36(49) | 7.16(110) | 4.00(06) | 4.01(07) |
| Mg2+ ↔ G8:O2′ | 3.08‡ | 3.97(102) | 2.24(13) | 3.21(23) | |
| Mg2+ ↔ C1.1:O5′ | 4.04‡ | 4.22(21) | 3.68(35) | 2.09(05) | |
| G8:HO2′ ↔ C1.1:O5′ | 4.57(135) | 7.61(81) | 5.09(74) | 2.36(42) | |
| C17:O2′ ↔ C1.1:P | 3.18 | 3.61(23) | 3.83(19) | 1.88(11) | 1.75(04) |
A proposed Mg2+ site was assumed directly between the crystallographic positions of C1.1:OP2 and A9:OP2
The simulation results were calculated over the last 10 ns with data collected every 1 ps. Shown are average values and standard deviations in the parentthesis.
In the early TS, the Mg2+ ion is positioned to shift the pKa of the 2′OH of G8 to act as a general acid
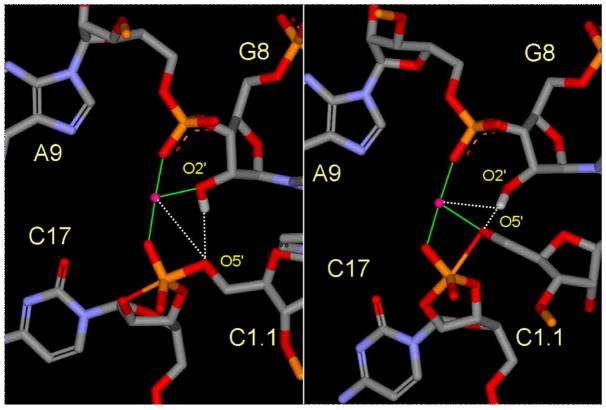
In the reactant state, the Mg2+ spends significant time closely associated with the 2′OH of G8 (Figure 1), but remains fairly distant from the leaving group O5′ position. In the early TS mimic simulation, where the nucleophilic O2′ and leaving group O5′ are equidistant from the phosphorus, the Mg2+ ion becomes directly coordinated to the 2′OH of G8, and is positioned closer to the O5′ leaving group. The coordination of the Mg2+ ion in the early TS mimic simulation is consistent with a role of shifting the pKa of the 2′OH in G8 so as to act as a general acid (Figure 2, left).
Figure 1.
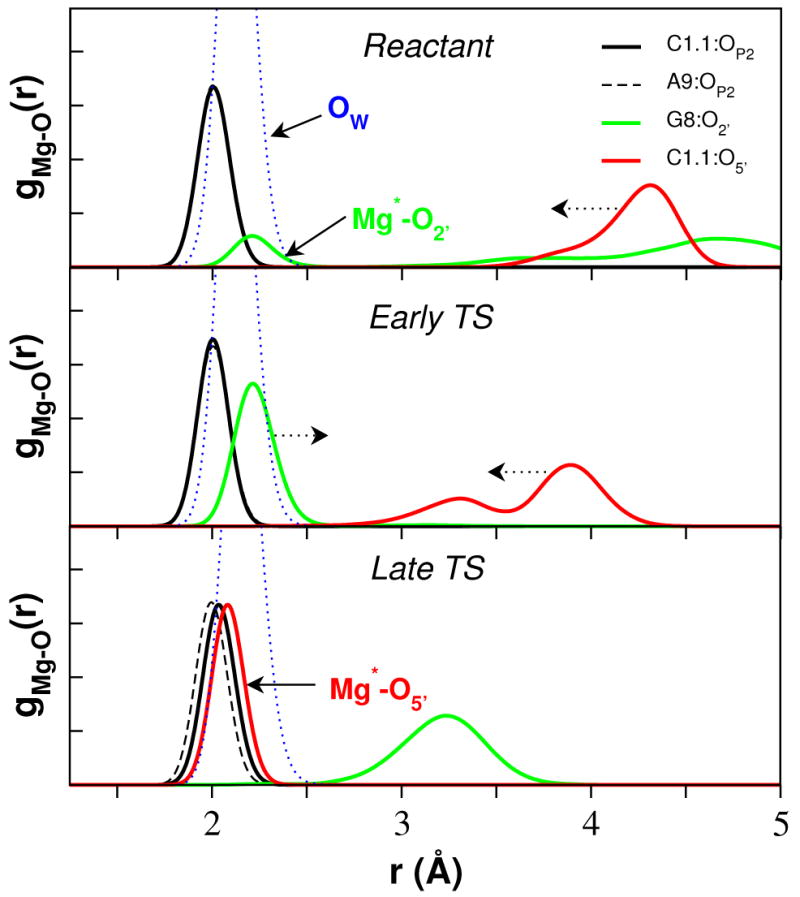
Radial distribution functions of key oxygens around Mg2+ in the active site for the reactant, early and late TS mimic simulations.
Figure 2.
Snapshots of the active site from the early TS mimic (left) and late TS mimic (right) simulations depicting the Mg2+ ion direct coordination (green lines) and key hydrogen bonds and indirect Mg2+ coordination (dotted lines). For clarity, the water molecules are not shown.
In the late TS, the Mg2+ ion can act as a Lewis acid catalyst to stabilize the leaving group and is poised to assist proton transfer from the 2′OH of G8
In the late TS mimic simulation, a transition occurs whereby the Mg2+ coordination with the 2′OH of G8 is replaced by direct coordination with the leaving group O5′ (Figure 1). In this way, the Mg2+ provides electrostatic stabilization of the accumulating charge of the leaving group (i.e., a Lewis acid catalyst). [7] At the same time, the 2′OH of G8 forms a hydrogen bond with the leaving group O5′ and is positioned to act as a general acid catalyst (Figure 2, right).
Comparison with experiment
The present simulation results, together with the crystallographic structure, tie together several key experimental results relating to the role of Mg2+ in catalysis. Thio/rescue effect experiments [9] have suggested that a single metal bound at the P9/G10.1 site (the A9 phosphate in the present work) in the ground state acquires an additional interaction with the scissile phosphate in proceeding to the transition state. Kinetic analysis [30] along with photocrosslinking experiments [16] and mutational data [8] have implicated the roles of the 2′OH of G8 and the N1 of G12 as a general acid and base, respectively, and have been interpreted to be consistent with a transition into an active conformation with appropriate architecture for acid-base catalysis. However, recent kinetic analysis indicate the pKa of the general acid is downshifted by around 4–7 pKa units in a metal-dependent manner, correlated with the metal pKa.[31] The simulation results suggest that the Mg2+ interacts strongly with the 2′OH of G8 in the early TS mimic and could contribute to a significant lowering of the pKa value, and in the late TS mimic the G8 2′OH is hydrogen bonded to the leaving group and poised to act as a general acid catalyst (Figure 2). The Mg2+ ion may additionally play a direct role in stabilizing the negative charge accumulated in the leaving group in the late TS, and if a proton from the G8 2′OH is ultimately transferred, the coordination of Mg2+ is positioned to revert back to stabilize the resulting G8 2′ alkoxide.
The simulation results presented here are consistent with the direct participation of a single bridging Mg2+ ion in hammerhead ribozyme catalysis, although the possibility of involvement of a second ion cannot be definitively precluded. [32,33,34] The Mg2+ preserves the integrity of the active site structure, and may serve as an epicenter in the transition state that coordinates the A9 and scissile phosphates, G8 2′OH general acid and O5′ leaving group. The present work underscores the need for further investigation of the chemical reaction profile using combined QM/MM models.
Supplementary Material
Computational methodology and the RMSD plots of all simulations are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
The authors are grateful for support from the National Institutes of Health, the IBM-Rochester Life Sciences Group, the the Army High Performance Computing Research Center (AHPCRC) and the Minnesota Supercomputing Institute (MSI).
References
- 1.Scott WG. Q Rev Biophys. 1999;32:241–294. doi: 10.1017/s003358350000353x. [DOI] [PubMed] [Google Scholar]
- 2.Scott WG. Curr Opin Struct Biol. 1998;8:720–726. doi: 10.1016/s0959-440x(98)80091-2. [DOI] [PubMed] [Google Scholar]
- 3.Doherty EA, Doudna JA. Annu Rev Biophys Biomol Struct. 2001;30:457–475. doi: 10.1146/annurev.biophys.30.1.457. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein M, Tsui R, Guinan P. Drugs of the Future. 2004;29:893–909. [Google Scholar]
- 5.Vaish NK, Dong F, Andrews L, Schweppe RE, Ahn NG, Blatt L, Seiwert SD. Nature Biotech. 2002;20:810–815. doi: 10.1038/nbt719. [DOI] [PubMed] [Google Scholar]
- 6.Breaker RR. Curr Opin Biotechnol. 2002;13:31–39. doi: 10.1016/s0958-1669(02)00281-1. [DOI] [PubMed] [Google Scholar]
- 7.Takagi Y, Ikeda Y, Taira K. Top Curr Chem. 2004;232:213–251. [Google Scholar]
- 8.Blount KF, Uhlenbeck OC. Annu Rev Biophys Biomol Struct. 2005;34:415–440. doi: 10.1146/annurev.biophys.34.122004.184428. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Karbstein K, Peracchi A, Beigelman L, Herschlag D. Biochemistry. 1999;38:14363–14378. doi: 10.1021/bi9913202. [DOI] [PubMed] [Google Scholar]
- 10.Suzumura K, Takagi Y, Orita M, Taira K. J Am Chem Soc. 2004;126:15504–15511. doi: 10.1021/ja0472937. [DOI] [PubMed] [Google Scholar]
- 11.Scott WG, Murray JB, Arnold JRP, Stoddard BL, Klug A. Science. 1996;274:2065–2069. doi: 10.1126/science.274.5295.2065. [DOI] [PubMed] [Google Scholar]
- 12.Murray JB, Terwey DP, Maloney L, Karpeisky A, Usman N, Beigelman L, Scott WG. Cell. 1998;92:665–673. doi: 10.1016/s0092-8674(00)81134-4. [DOI] [PubMed] [Google Scholar]
- 13.Murray JB, Szöke H, Szöke A, Scott WG. Mol Cell. 2000;5:279–287. doi: 10.1016/s1097-2765(00)80423-2. [DOI] [PubMed] [Google Scholar]
- 14.Martick M, Scott WG. Cell. 2006;126:309–320. doi: 10.1016/j.cell.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canny MD, Jucker FM, Kellogg E, Khorova A, Jayasena SD, Pardi A. J Am Chem Soc. 2004;126:10848–10849. doi: 10.1021/ja046848v. [DOI] [PubMed] [Google Scholar]
- 16.Lambert D, Heckman JE, Burke JM. Biochemistry. 2006;45:7140–7147. doi: 10.1021/bi052457x. [DOI] [PubMed] [Google Scholar]
- 17.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 18.Foloppe N, MacKerell AD., Jr J Comput Chem. 2000;21:86–104. [Google Scholar]
- 19.MacKerell AD, Jr, Banavali NK. J Comput Chem. 2000;21:105–120. [Google Scholar]
- 20.Mayaan E, Moser A, Mackerell AD, Jr, York DM. J Comput Chem. doi: 10.1002/jcc.20474. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 22.Essmann U, Perera L, Berkowitz ML, Darden T, Hsing L, Pedersen LG. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 23.Mayaan E, Range K, York DM. J Biol Inorg Chem. 2004;9:807–817. doi: 10.1007/s00775-004-0583-7. [DOI] [PubMed] [Google Scholar]
- 24.Peracchi A, Beigelman L, Scott EC, Uhlenbeck OC, Herschlag D. J Biol Chem. 1997;272:26822–26826. doi: 10.1074/jbc.272.43.26822. [DOI] [PubMed] [Google Scholar]
- 25.Peracchi A, Beigelman L, Usman N, Herschlag D. Proc Natl Acad Sci USA. 1996;93:11522–11527. doi: 10.1073/pnas.93.21.11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermann T, Auffinger P, Scott WG, Westhof E. Nucleic Acids Res. 1997;25:3421–3427. doi: 10.1093/nar/25.17.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann T, Auffinger P, Westhof E. Eur Biophys J. 1998;27:153–165. doi: 10.1007/s002490050121. [DOI] [PubMed] [Google Scholar]
- 28.Torres RA, Bruice TC. Proc Natl Acad Sci USA. 1998;95:11077–11082. doi: 10.1073/pnas.95.19.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres RA, Bruice TC. J Am Chem Soc. 2000;122:781–791. [Google Scholar]
- 30.Han J, Burke JM. Biochemistry. 2005;44:7864–7870. doi: 10.1021/bi047941z. [DOI] [PubMed] [Google Scholar]
- 31.Roychowdhury-Saha M, Burke DH. RNA. 2006;12:1846–1852. doi: 10.1261/rna.128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lott WB, Pontius BW, von Hippel PH. Proc Natl Acad Sci USA. 1998;95:542–547. doi: 10.1073/pnas.95.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue A, Takagi Y, Taira K. Nucleic Acids Res. 2004;32:4217–4223. doi: 10.1093/nar/gkh753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclerc F, Karplus M. J Phys Chem B. 2006;110:3395–3409. doi: 10.1021/jp053835a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Computational methodology and the RMSD plots of all simulations are provided. This material is available free of charge via the Internet at http://pubs.acs.org.