Abstract
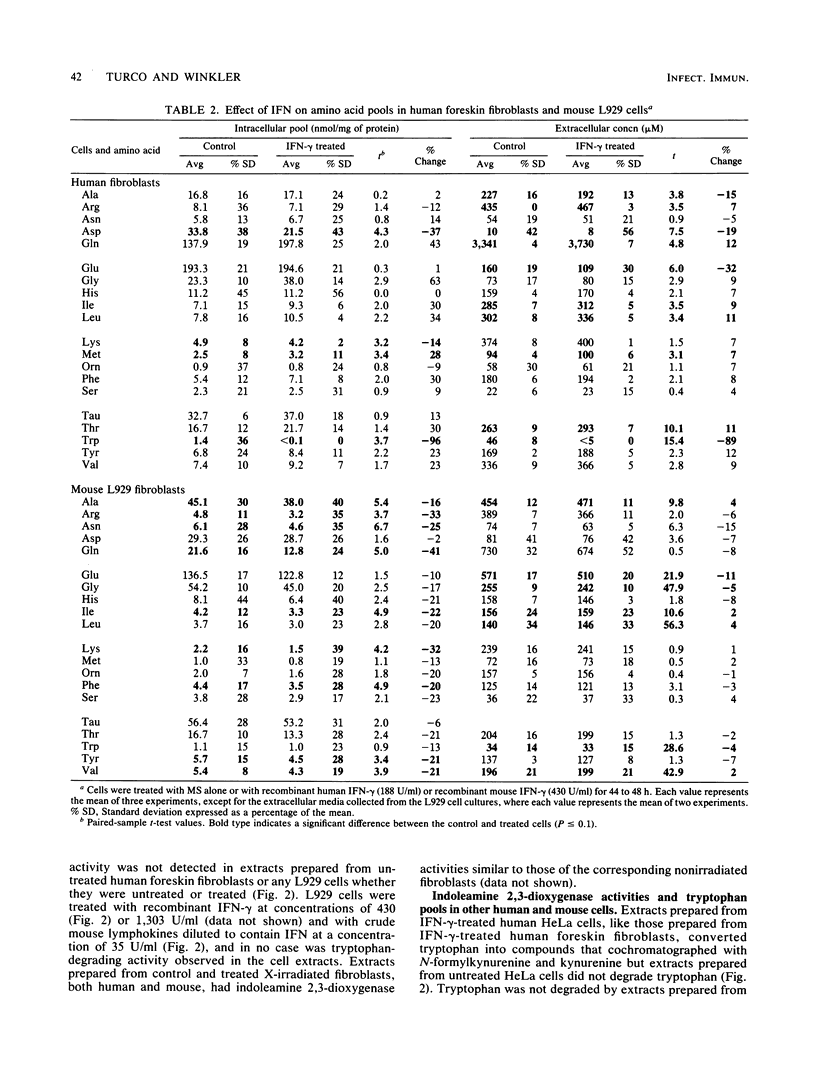
We examined the role of amino acid deprivation in gamma-interferon-induced (IFN-gamma) suppression of the growth of Rickettsia prowazekii in mouse L929 cells and human fibroblasts by measuring the amino acid pools in untreated and IFN-gamma-treated cells. In recombinant IFN-gamma-treated cultures of human fibroblasts, tryptophan was undetectable in both the intracellular pool and the extracellular medium. In contrast, tryptophan was not depleted from the intracellular pool or the extracellular medium of L929 cells treated with recombinant IFN-gamma or crude mouse lymphokines. None of the other amino acids measured was severely depleted in IFN-gamma-treated L929 cells and human fibroblasts. Extracts prepared from IFN-gamma-treated human fibroblasts exhibited indoleamine 2,3-dioxygenase activity, converting tryptophan into products that cochromatographed with N-formylkynurenine and kynurenine; however, extracts prepared from untreated human fibroblasts, untreated L929 cells, recombinant IFN-gamma-treated L929 cells, and mouse lymphokine-treated L929 cells did not degrade tryptophan. Human HeLa cells resembled the human fibroblasts in that they degraded tryptophan after IFN-gamma treatment. Similarly, mouse 3T3-A31 cells and mouse embryo fibroblasts resembled mouse L929 cells in that they did not degrade tryptophan. Supplementation of the extracellular medium with additional tryptophan reconstituted the tryptophan pool in mock-infected and R. prowazekii-infected, X-irradiated, IFN-gamma-treated human fibroblasts to values greater than those observed in untreated control cultures. However, reconstitution of the tryptophan pool did not relieve IFN-gamma-induced inhibition of rickettsial growth. Addition of kynurenine or N-formylkynurenine to rickettsia-infected human fibroblasts at concentrations four times the usual tryptophan concentration did not inhibit growth of R. prowazekii. We conclude that neither tryptophan depletion nor depletion of the other amino acids studied explains the inhibitory effect of IFN-gamma on rickettsial growth in mouse L929 cells. In IFN-gamma-treated human fibroblasts, either tryptophan depletion is not involved in the inhibition of rickettsial growth or tryptophan depletion and some other mechanism(s) together contribute to the inhibition of rickettsial growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebleu B., Content J. Mechanisms of interferon action: biochemical and genetic approaches. Interferon. 1982;4:47–94. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi G. A., Fohlin L., Bergström J. Application of high-performance liquid chromatography to the determination of free amino acids in physiological fluids. J Chromatogr. 1984 Aug 3;297:91–100. doi: 10.1016/s0021-9673(01)89032-4. [DOI] [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sayama S., Yoshida R., Oku T., Imanishi J., Kishida T., Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 2,3-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7327–7330. doi: 10.1073/pnas.78.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C. Biochemical pathways in interferon-action. Pharmacol Ther. 1984;24(2):235–257. doi: 10.1016/0163-7258(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Brandis H. Contribution of immune interferon (IFN-gamma) in lymphokine-induced anti-toxoplasma activity: studies with recombinant murine IFN-gamma. Immunobiology. 1985 Nov;170(4):270–283. doi: 10.1016/s0171-2985(85)80076-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Hirata F., Hayaishi O. Intracellular utilization of superoxide anion by indoleamine 2,3-dioxygenase of rabbit enterocytes. J Biol Chem. 1977 Apr 25;252(8):2774–2776. [PubMed] [Google Scholar]
- Turco J., Thompson H. A., Winkler H. H. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984 Sep;45(3):781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of the properties of antirickettsial activity and interferon in mouse lymphokines. Infect Immun. 1983 Oct;42(1):27–32. doi: 10.1128/iai.42.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Effect of mouse lymphokines and cloned mouse interferon-gamma on the interaction of Rickettsia prowazekii with mouse macrophage-like RAW264.7 cells. Infect Immun. 1984 Aug;45(2):303–308. doi: 10.1128/iai.45.2.303-308.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. The biology of rickettsiae. Annu Rev Microbiol. 1982;36:345–370. doi: 10.1146/annurev.mi.36.100182.002021. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]


