Abstract
In contrast to the detrimental outcomes most often associated with the resolution of co-infections, the model presented here involving a localized Pneumocystis infection of the lung, followed 2 weeks later by an influenza virus infection results in a significant beneficial outcome for the host. In the week following the influenza infection, immunocompetent co-infected animals exhibited a faster rate of virus clearance, a more rapid appearance and higher influenza-specific antibody titers in their serum and broncho-alveolar lavage fluid (BALF), significantly reduced inflammatory cytokine levels in their BALF and less evidence of morbidity relative to animals infected only with influenza virus. The beneficial outcome observed in the co-infected immunocompetent animals was dependent upon the ongoing resolution of a viable Pneumocystis infection. No differences in viral clearance were detected between co-infected and influenza-only infected uMT mice or likewise for SCID mice. These results indicate that innate responses elicited by the preceding Pneumocystis infection were not involved in the increased rate of viral clearance in immunocompetent co-infected animals. Rather, the increased rate of viral clearance was due to the enhancement of the influenza-specific antibody response which in turn was transiently dependent upon the resolution of the ongoing Pneumocystis infection.
Keywords: lung, viral, co-infection
Introduction
The progression of concurrent immune responses in the lung, elicited in response to consecutive immunological challenges, more often evolve in a deleterious rather than a beneficial manner for a host. Concurrent immune responses in the lung can occur following simultaneous and/or consecutive exposures to multiple pathogens and/or immunogens (1), upon exposure of asthmatic patients to pulmonary pathogens resulting in exacerbations of asthmatic reactions (2) and in cases in which pathogen-induced immune responses interact with responses to underlying chronic pulmonary diseases involving infections or in cases of chronic obstructive pulmonary disease (3) or emphysema. The progression of concurrent immune responses towards a beneficial or detrimental outcome should be influenced by the interaction of elicited inflammatory mediators and by the effectiveness of the adaptive immune response to clear the pathogens or immunogens involved.
Beneficial outcomes in the resolution of concurrent immune responses have been described in co-infection models and in airway inflammation models. In a co-infection model involving disparate organ sites, an amelioration of influenza-induced lung damage was associated with the establishment of a Th2-induced immune response to a Trichinella spiralis infection of the intestinal mucosa (4). The introduction of ovalbumin-specific Th1 cells in an allergic airway inflammation model resulted in a reduction of the subsequent ovalbumin-specific Th2-induced eosinophilia and mucus production in an IFNγ-dependent manner (5). These and other concurrent immune regulation models (6–8) demonstrate that Th1 or Th2-associated cytokines are capable of down-regulating the production of inflammatory-associated cytokines elicited by an opposing response in order to achieve a beneficial outcome.
Although the mutual exclusion of concurrent opposing immune responses may result in a moderation of excessive inflammatory sequelae, there is also the known risk that their interaction may elicit more deleterious inflammatory outcomes for the host. Such deleterious outcomes, resulting in an enhanced severity of disease (9, 10) suggest that preexisting type 1 or 2 immune environments generated in the lung following a recent immune challenge are also capable of amplifying rather than downregulating a subsequent opposing immune response. Additional co-infection models have attributed deleterious outcomes to pathogen-mediated alterations to the immune mechanisms elicited by one of the co-infecting pathogens (11). These various co-infection models suggest that the sequence and interval between exposures, the immunogens or pathogens involved and the tissue location of the co-infections influence the inflammatory outcome associated with the resolution of concurrent immune responses.
The duration of pathogen exposure and of the subsequent inflammatory response elicited as a result of a co-infection will be associated with the ability of the host's adaptive immune response to effectively clear the co-infecting pathogens. In turn, the clearance of the co-infecting pathogens from a local co-infection site will be dependent upon the generation and delivery of a protective pathogen-specific immune response to the tissue site in question. In co-infections models involving acute pulmonary viral infections, viral burdens did not increase as a result of decreases in the recruitment of viral-specific CD8 T cells (12) or in the total CD8 T cell accumulation (4). The lack of any corresponding increase in the viral burdens in the lungs of these co-infected animals would suggest that additional protective immune mechanisms, possibly antibody, may be contributing to the control of virus proliferation.
Using an animal model, we determined whether a beneficial immune outcome would be generated in the lungs following co-infection with two different, yet regularly encountered pulmonary pathogens that elicit opposing immune responses. The pathogens, Pneumocystis murina which typically elicits a type-2 immune response (13) and influenza type A virus which typically elicits a type-1 immune response (14), were used. Pneumocystis is a ubiquitous, opportunistic pathogen that colonizes the alveolar spaces of the lung. Immunocompetent individuals generally develop asymptomatic subclinical infections following exposure to this pathogen. Approximately 20% of healthy immunocompetent individuals have detectable Pneumocystis DNA in their oropharyngeal cavity (15). Health-care workers in regular contact with immunocompromised patients are known to be at an increased risk for becoming Pneumocystis carriers. Although immunocompetent individuals effectively resist this pathogen, their potential to act as transient reservoirs for the transmission and propagation of Pneumocystis has been demonstrated (16) and subsequently modelled in murine studies (17, 18). Due to the ubiquitous nature of Pneumocystis and that intermittent infections in immunocompetent hosts are asymptomatic, individual carriers going about their daily lives would continue to be exposed to other common pulmonary pathogens. Thus, these individuals could easily be subjected to co-infections of the lung triggering multiple immune responses simultaneously or in rapid succession.
The second pathogen used in our model is type A influenza virus. This virus is a seasonal pathogen with its peak incidence occurring during the winter months. At this time, the confinement of susceptible individuals into closer proximity with others enhances the potential for transmission throughout the general population and thus to others who have already been exposed to and are responding to other pulmonary pathogens. Unlike Pneumocystis, influenza virus is a virulent pathogen that induces a symptomatic clinical infection of the lung by infecting the respiratory and alveolar epithelium. The effective resolution of influenza infections is dependent upon the recruitment of both humoral and type-1 cell-mediated immune cells (14). Excessive inflammatory responses associated with influenza infections (19) elicit further complications and often enhance the susceptibility of the host to secondary bacterial infections. These sequelae are contributing factors to the increased mortality rates associated with influenza-induced pneumonias.
This pulmonary co-infection model examines the local outcome of opposing immune responses elicited by two disparate yet commonly encountered lung pathogens. Our model contrasts with the recent T. spiralis/influenza co-infection model which involved two distinct anatomical infection sites (4). Various other co-infection models involve two virulent pathogens in which infection by the first pathogen leads to a predisposition to infection by the second (20, 21). Pneumocystis is an avirulent pathogen in an immunocompetent host and it is not a predisposing factor to influenza infections. Rather, a co-infection of immunocompetent individuals by these two pathogens is premised upon their simultaneous existence in a common environment.
In the Pneumocystis/influenza co-infected animals, we observed a significantly reduced recovery of virus, a more rapid increase in influenza-specific antibody (Ab) titres, a reduction in lung damage, significantly altered cytokine levels in the lung and evidence of reduced morbidity 1 week after the influenza infection. If the sequence of the pathogens was reversed, no comparable enhancement of the anti-Pneumocystis response was detected. The perceived ability of the Pneumocystis-induced immune response in an immunocompetent host to augment the protective immunity of a subsequent anti-influenza response suggests that under controlled conditions, localized concurrent immune responses in the lung may be exploited for the benefit to the host.
Materials and Methods
Mice
BALB/c and C57BL/6 male and female mice at 6 to 8 weeks of age were either purchased from The Jackson Laboratory (Bar Harbor, ME) or obtained from the breeding colonies maintained at Montana State University, Bozeman, MT. The male and female BALB/c SCID mice were obtained from Montana State University breeding colonies and C57BL/6 SCID mice and uMT mice at 6 to 8 weeks of age were obtained from The Jackson Laboratory. All of the animals were housed at the Animal Resource Center at Montana State University for the duration of these experiments. The animal facilities and the experimental procedures used throughout these experiments complied with the approved institutional animal care and use committee protocols established at Montana State University.
Pathogens and infection procedures
The stock of Pneumocystis murina used for these experiments was maintained in a Pneumocystis-infected colony of BALB/c SCID mice at Montana State University. The influenza virus used was the A/PR8/8/34 (PR8; H1N1) strain. This virus was prepared at the Trudeau Institute (Saranac Lake, New York, USA). The virus stock was grown in the allantoic fluid of 10-day old chicken embryos that had been infected for 48 hours at 35°C. The harvested allantoic fluid was then stored at −80°C.
Lung homogenates from Pneumocystis-infected SCID mice were used to infect the mice in these studies. In order to administer intratracheal inoculations of Pneumocystis-infected lung homogenates, experimental mice were lightly anesthetized with 5% isoflurane in oxygen. A 100 ul inoculum of the Pneumocystis-infected lung homogenate containing 107 Pneumocystis organisms was then directly injected into the lungs of the mice. Control groups in the co-infection experiments were given lung homogenates from SCID mice that had not been infected with Pneumocystis. Aliquots of 100 ul of the uninfected lung homogenates were used. This group will be referred to as the influenza-only control group. UV-inactivated Pneumocystis-infected lung homogenates were initially prepared in the same manner as the viable Pneumocystis-infected lung homogenates. The UV inactivation was carried out according to the procedure described by Maher et al (22). Following the 24 h UV inactivation, the absence of any Pneumocystis-specific heat-shock protein 70 mRNA was confirmed by RT-PCR in order to verify the inactivation of the Pneumocystis. The β-glucan preparation was made from a β-glucan stock (Sigma Chemical Co., St. Louis MO).
PR8 influenza inoculations were also done while the mice were under a 5% isoflurane anesthesia. Mice were given a 50 ul intranasal inoculation containing 1000 PFU of PR8 influenza virus. The mice were taken at designated time points up to 10 days after the influenza infection. In those experiments where body weight was monitored, the animals were weighed on the day of the influenza infection and each day afterward until the end of the experiment. The change in body weight was determined by the difference between the body weight at the time of influenza infection and the body weight on the given day after infection.
Assessment of viral recovery
Following influenza infection, the mice were sacrificed as previously described (23). Viral recovery from the lungs of the mice was determined by plaque assay. Lungs recovered from the mice were snap-frozen in liquid nitrogen and then stored at − 80°C until analyzed. At this time the lungs were homogenized and 10-fold serial dilutions of the homogenates were used to inoculate monolayers of Madin-Darby canine kidney (MDCK) cells. Our previously described plaque assay procedure was used in the experiments reported here (24).
Recovery of broncho-alveolar lavage fluid.
Broncho-alveolar lavage fluids (BALF) were obtained using a 1.5 ml aliquot of 3 mM EDTA in HBSS. The lungs were flushed with this solution 3 times. The final recovery of BALF was 1.2 ml for each mouse. The cells recovered in the BALF were counted to obtain total cell counts and an aliquot of the recovered BALF was used to make a Diff-Quik-stained cytospin to examine the differential cell recovery from each animal. The remaining cellular content in the recovered BALF was removed by centrifugation and the fluid was then stored at − 80°C for use in determining the Ab, cytokine, albumin and lactate-dehydrogenase (LDH) content.
Albumin and lactate-dehydrogenase content in the BALF
The BALF albumin was determined by use of an albumin colorimetric assay (Sigma Diagnostics, St. Louis, MO). Color absorbency was read at 630 nm and reported in mg/ml in the BALF. The level of LDH detected in the lavage fluid was determined by colorimetric assay (CytoTox 96, Promega, Madison WI). The assay was read at an absorbance of 490 nm and reported as units/ml in the BALF. Each of the samples was tested in duplicate for the albumin and LDH assays.
Analysis of cytokine and PR8-specific Ab in BALF and serum
Cytokine levels in the BALF were determined by use of an inflammatory cytokine bead array kit (BD Biosciences PharMingen, San Diego, CA). The assays were carried out according to the manufacturer’s instruction. The detection of the cytokines was done using a FACScan cytometer and then analyzed according to the cytometric bead array software (BD Biosciences PharMingen). The amount of IL-13 recovered in the BALF was determined using a murine IL-13 Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The levels of PR8-specific Ab recovered in the BALF and serum were determined by ELISA using our prepared PR8 viral membrane preparation as previously described (23). All samples were tested in duplicate.
FACS staining of recovered cells
Cells recovered from selected tissues were stained with the following monoclonal Ab preparations in order to characterize the expression of selected surface markers. Single cell suspensions from the tracheal-bronchial lymph nodes (TBLN) and spleen were stained with FITC-conjugated peanut agglutinin (PNA) (Sigma Chemical Co., St. Louis MO), PE-conjugated anti-mouse CD38, biotin-conjugated anti-mouse CD19 and streptavidin- conjugated allophycocyanin (BD Biosciences PharMingen). Analysis of the stained cells was carried out on a FACS-Caliber and then analyzed using CellQuest software (BD PharMingen).
Statistical analysis of group data
The data is expressed as the means ± standard deviation. The results reported here are from one experiment that was representative of at least 3 independent experiments unless stated otherwise. The sample size of each group is 5–8 mice. Statistical differences between designated groups were determined using non-parametric one way ANOVA tests with bonferroni corrections to account for multiple comparisons within given experiments or by t-tests when only two experimental groups were involved.
Results
Pathogen Recovery from Lungs
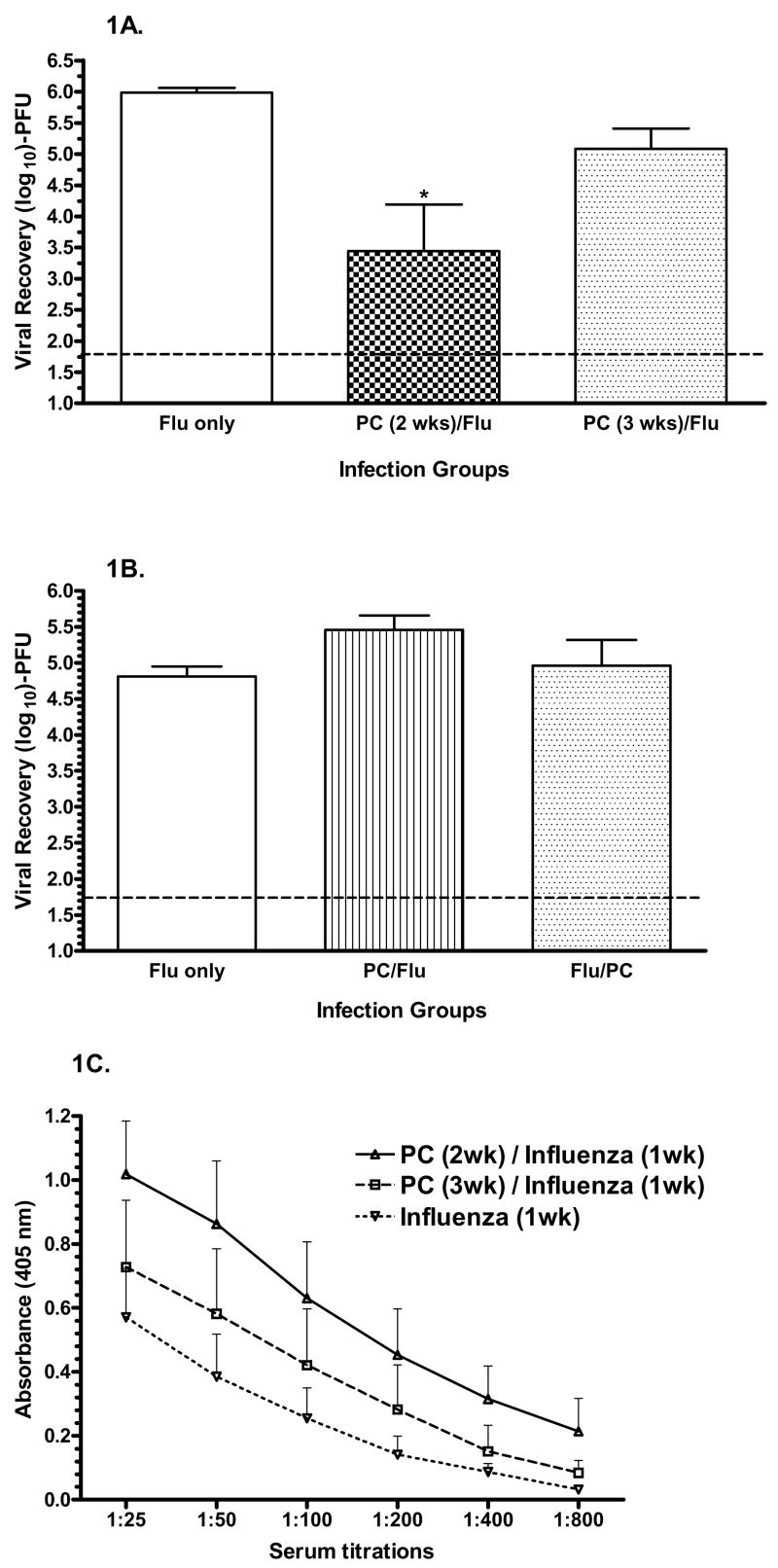
We have previously used the rate of pathogen clearance from an infection site to assess the efficacy of a local adaptive immune response (23). In our present co-infection model we found that virus remaining in the lungs 1 week after an influenza infection was reduced by more than 100-fold if a Pneumocystis infection occurred 2 weeks earlier relative to the absence of a prior Pneumocystis infection as seen in the influenza-only control group (Figure 1A). If a Pneumocystis infection was initiated 3 weeks earlier, then the recovery of virus was only 10-fold less than in the influenza-only control group. If the Pneumocystis and influenza infections were given 6 hours apart in either order, it was found that the viral recovery from the lungs was equivalent between the co-infected groups and that both co-infection groups had equivalent or slightly greater viral recoveries than seen in the influenza-only group (Figure 1B). The recovery of Pneumocystis from the lungs was not altered by an influenza infection given 6 hours before or after the Pneumocystis infection (data not shown). As a result of this finding, further co-infection experiments were based on a 2 week interval between a prior Pneumocystis infection and the influenza infection.
Figure 1.
Clearance of influenza virus from the lung following co-infection. A) Viral recovery levels from the lungs of co-infected mice. Mice were infected with a Pneumocystis-infected lung homogenate 2 (
 ) or 3 (
) or 3 (
 ) weeks prior to an influenza infection. The control mice were given the uninfected lung homogenate at 2 weeks prior to the influenza infection (▭). The lungs were assessed for influenza virus recovery by plaque assay 1 week after influenza infection. B) Mice were inoculated with the co-infecting pathogens in both sequences 6 hours apart. The lungs were assessed for viral recovery by plaque assay 1 week later. *, P<0.05 relative to the influenza-only group; n=5 or 6 mice per group; ----, limit of assay detection.
) weeks prior to an influenza infection. The control mice were given the uninfected lung homogenate at 2 weeks prior to the influenza infection (▭). The lungs were assessed for influenza virus recovery by plaque assay 1 week after influenza infection. B) Mice were inoculated with the co-infecting pathogens in both sequences 6 hours apart. The lungs were assessed for viral recovery by plaque assay 1 week later. *, P<0.05 relative to the influenza-only group; n=5 or 6 mice per group; ----, limit of assay detection.
The rate of clearance of influenza virus in the co-infection group and in the influenza-only control group was examined over a 10 day period following the virus challenge (Figure 2A). During the week following the influenza infection, viral recoveries were significantly less in the co-infected group relative to the influenza-only control group. One week after the influenza infection the greatest difference in viral recoveries was observed. At 10 days post-influenza infection, both groups had equivalent viral recoveries close to or below the limit of detection by plaque assay. The wild-type animals used in these experiments easily cleared the Pneumocystis infections. At the time of the influenza infection (day 0), the Pneumocystis burden in the co-infected animals was more than 10-fold less than what these animals had been inoculated with 2 weeks earlier (Figure 2B). At 3 and 5 days after the influenza infection, the Pneumocystis burden was further diminished and was in fact undetectable in 3 of 5 and 4 of 5 animals respectively at these times. Pneumocystis burdens were below the limit of detection in the co-infected animals at 7 and 10 days post-influenza infection. If an influenza infection was given 2 weeks prior to a Pneumocystis infection, no alteration in the recovery of the Pneumocystis from the lungs was detected 2 weeks later (data not shown).
Figure 2.
Kinetics of Influenza and Pneumocystis recovery from co-infected animals. A) Kinetic analysis of the viral recovery was done on mice that were infected with Pneumocystis 2 weeks prior to the influenza infection. Mice from the co-infected group (
 ) and influenza-only control group (▭) were taken at designated time-points following the influenza infection. The recovery of influenza virus in their lungs was assessed by plaque assay. B) Pneumocystis levels recovered in the lungs of co-infected mice at the time of and at designated time-points following the influenza infection. Lung homogenates of the lungs were made and Pneumocystis counts were determined from Diff-Quik stained slides made from the lung homogenates. The limit of detection in this assay is 104.15 Pneumocystis nuclei. N=5 mice per group; ----, limit of assay detection; *, P<0.05, ***, P<0.005 relative to influenza-only control group.
) and influenza-only control group (▭) were taken at designated time-points following the influenza infection. The recovery of influenza virus in their lungs was assessed by plaque assay. B) Pneumocystis levels recovered in the lungs of co-infected mice at the time of and at designated time-points following the influenza infection. Lung homogenates of the lungs were made and Pneumocystis counts were determined from Diff-Quik stained slides made from the lung homogenates. The limit of detection in this assay is 104.15 Pneumocystis nuclei. N=5 mice per group; ----, limit of assay detection; *, P<0.05, ***, P<0.005 relative to influenza-only control group.
To ascertain the necessity for a viable Pneumocystis infection in order to achieve this reduction in viral recovery, groups of mice were dosed with UV-inactivated Pneumocystis-infected lung homogenates or with a β-glucan preparation. The absence of any viable Pneumocystis in the UV-inactivated Pneumocystis-infected lung homogenates was determined by the lack of the Pneumocystis-specific heat-shock protein 70 mRNA (data not shown) as described by Maher et al (22). β-glucan is a sub-component of the Pneumocystis cell wall and a well known non-specific immunostimulant (25–27). This group was treated twice with 250ng of the β-glucan preparation (1 dose per week) during the 2 weeks prior to the influenza infection. A prior single (not shown) or dual administration of the UV-inactivated Pneumocystis-infected lung homogenate was not associated with any significant reduction in the recovery of virus from the lung 1 week after the influenza infection (Figure 3). No reduction in the level of virus recovery was observed in the group that received 2 prior administrations of β-glucan. In contrast, the establishment of a viable Pneumocystis infection 2 weeks prior to the influenza challenge was associated with a greater than 100-fold reduction in the recovery of influenza virus from the lung.
Figure 3.
Viral Recovery following Alternative Treatments. Influenza virus recovery was determined by plaque assay in the different infection groups 1 week after the administration of the influenza infection. Treatment of individual groups with the β-glucan (
 ), or the Pneumocystis-infected (
), or the Pneumocystis-infected (
 ), or the UV-inactivated (
), or the UV-inactivated (
 ) or the uninfected (▭) lung homogenate preparations were performed 2 weeks prior to the influenza infection. The group treated with the β-glucan preparation received a second treatment 1 week prior to the influenza virus infection. This is one of three independent experiments. ***, P<0.001 relative to viable Pneumocystis co-infection group; ---, limit of assay detection.
) or the uninfected (▭) lung homogenate preparations were performed 2 weeks prior to the influenza infection. The group treated with the β-glucan preparation received a second treatment 1 week prior to the influenza virus infection. This is one of three independent experiments. ***, P<0.001 relative to viable Pneumocystis co-infection group; ---, limit of assay detection.
Analysis of Cell Recovery in BALF
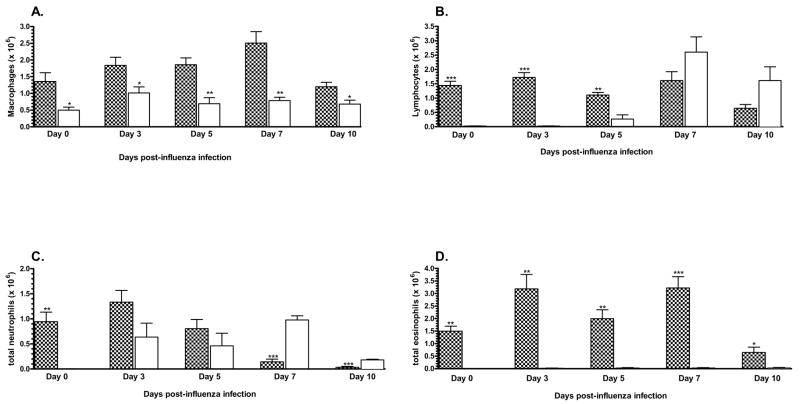
The recovery of lymphocytes and macrophages from the airways was equivalent between the two infection groups during the resolution of the influenza infection (Figure 4A and 4B). However, the recoveries of neutrophils and eosinophils from both infection groups were significantly different during this time (Figure 4C and 4D). One week after the influenza infection a significant accumulation of neutrophils was detected in the airways of the influenza-only control animals. In contrast, the accumulation of neutrophils in the airways of the co-infection group was significantly less. In fact, the neutrophil accumulation in the airways of these co-infected animals had not changed from the levels attributable to the Pneumocystis infection as detected on day 0 of the influenza infection. By the time that viral recovery was undetectable in these two groups (day 10 post-influenza infection), neutrophil accumulation in their airways had become equivalent. The recovery of eosinophils remained at negligible levels in the influenza-only control animals. In the co-infected animals an equivalent high number of eosinophils were recovered in their BALF at the time of and throughout the resolution of the subsequent influenza infection.
Figure 4.
Recovery of cells from the broncho-alveolar lavage fluid following co-infection. The lungs of each animal were lavaged 1 week after co-infection with the influenza virus. The BALF cells, lymphocytes (Figure 4A), macrophages (Figure 4B), neutrophils (Figure 4C) and eosinophils (Figure 4D) were stained by Diff-Quik stain and differential cell counts were determined by microscopy. The cell populations recovered from the BALF of the co-infected (
 ) and influenza-only infected (▭) animals were determined by multiplying the total cell recovery by the percentage of each cell subset observed in the differential cell counts. *, P<0.05. This is one of three independent experiments.
) and influenza-only infected (▭) animals were determined by multiplying the total cell recovery by the percentage of each cell subset observed in the differential cell counts. *, P<0.05. This is one of three independent experiments.
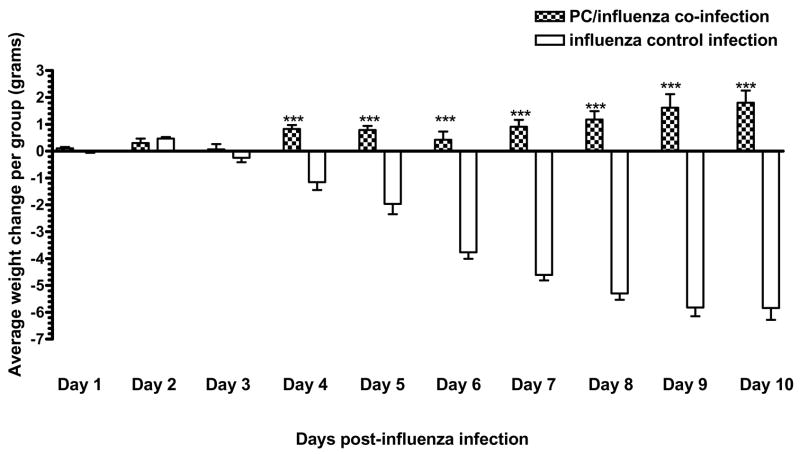
Change in weight
In order to obtain an indication of the general health of the animals as they responded to the pathogens, we monitored the daily changes in their body weights following the influenza infection. Over the 10 days following the influenza infection, the co-infected animals lost the least amount of weight (Figure 5). In fact, they had recovered the weight that they had initially lost early in their response to the influenza infection and had averaged an additional 1 gram weight gain by the end of the experiment. The influenza-only control animals as well as those treated with the UV-inactivated Pneumocystis lung homogenate (data not shown), or the 2 doses of 250 ng of β-glucan (data not shown) prior to the influenza infection continued to lose weight up to the end of the experiment.
Figure 5.
Weight changes over the course of the influenza infection. Co-infected animals (
 ) and influenza-only infected animals (▭) were weighed on the day of the influenza infection and each day thereafter until the end of the experiment at day 10 post-influenza. The change in weight for each animal was determined by the difference between the starting weight and the weight at each day following the influenza infection. The weight change for each animal in a group was then averaged with the cohort to determine the average weight change for that cohort on the day of interest. This is one of three independent experiments. n=5 or 6 mice per group; *, P<0.05, **, P<0.001, ***, P<0.0001.
) and influenza-only infected animals (▭) were weighed on the day of the influenza infection and each day thereafter until the end of the experiment at day 10 post-influenza. The change in weight for each animal was determined by the difference between the starting weight and the weight at each day following the influenza infection. The weight change for each animal in a group was then averaged with the cohort to determine the average weight change for that cohort on the day of interest. This is one of three independent experiments. n=5 or 6 mice per group; *, P<0.05, **, P<0.001, ***, P<0.0001.
Recovery of serum albumin and lactate dehydrogenase in BALF
Increased levels of serum albumin and LDH in the BALF have been used as indicators of lung damage following infection (4, 28–30). Serum albumin and LDH levels in the BALF of the co-infected animals were higher at the time of the influenza infection (day 0) (Figure 6A and 6B). Following the influenza infection, serum albumin levels in the BALF of the co-infected animals remained equivalent to the day 0 values whereas significant increases were detected in the influenza-only infection group. LDH levels in the co-infected animals also remained unchanged following the influenza infection whereas levels in the influenza-only infection group rose by more than 3 and 6 fold at 7 and 10 days respectively, following the influenza infection. Neither the single nor dual administration of the UV-inactivated Pneumocystis-infected lung homogenate (data not shown) nor the administration of β-glucan (data not shown) were associated with any reduction in the level of serum albumin detected in the BALF following the influenza infection.
Figure 6.
Determination of serum albumin and lactate dehydrogenase levels in the BALF 1 week after infection with the influenza virus. Aliquots of 100 ul of the BALF from each co-infected (
 ) and influenza-only infected (▭) animal were assessed for serum albumin (Figure 6A) and LDH (Figure 6B) levels according to the instructions enclosed with the manufacturer’s assay instructions. This is one of three independent experiments. N=5 or 6 mice per group; ***, P<0.001; **, P<0.01; *, P<0.05.
) and influenza-only infected (▭) animal were assessed for serum albumin (Figure 6A) and LDH (Figure 6B) levels according to the instructions enclosed with the manufacturer’s assay instructions. This is one of three independent experiments. N=5 or 6 mice per group; ***, P<0.001; **, P<0.01; *, P<0.05.
Recovery of inflammatory cytokines in BALF
One week after the influenza infection the levels of TNFα, IL-10, IFNγ, macrophage chemotactic protein-1 (MCP-1) and IL-6 recovered in the BALF of the co-infected animals were significantly reduced relative to those detected in the influenza-only control animals (Figure 7) as well as the animals given the UV-inactivated PC-lung homogenate (data not shown) or the 2 × 250 ng B-glucan doses (data not shown). In contrast to this trend, the levels of IL-13 recovered in the BALF of the co-infected animals remained higher as the influenza infection resolved. IL-5 levels in the BALF were not significantly altered by any treatment or infection prior to the influenza infection (data not shown). By 10 days post-influenza infection the inflammatory cytokine levels in the BALF of the influenza-only control group were still higher than in the co-infected animals although the levels were greatly diminished in both groups relative to that detected on day 7. IL-13 levels were still higher in the co-infected group at this time.
Figure 7.
Determination of cytokine levels in the BALF following influenza infection. Cytokine levels in the BALF of the co-infected (
 ) and influenza-only infected mice (▭) were determined 1 week after influenza infection. The lung lavage fluids were assessed for the presence of the selected cytokines using cytokine bead array kits or by ELISA for the IL-13 levels. Cytokine bead array samples were screened using the FACScan and the data was analyzed by the bead array software. Il-13 ELISA results were calculated from the standard curve as per the manufacturer's instructions. N=5 or 6 animals per group; ***, P<0.0001; **, P<0.005; *, P<0.05.
) and influenza-only infected mice (▭) were determined 1 week after influenza infection. The lung lavage fluids were assessed for the presence of the selected cytokines using cytokine bead array kits or by ELISA for the IL-13 levels. Cytokine bead array samples were screened using the FACScan and the data was analyzed by the bead array software. Il-13 ELISA results were calculated from the standard curve as per the manufacturer's instructions. N=5 or 6 animals per group; ***, P<0.0001; **, P<0.005; *, P<0.05.
Germinal Center B cell analysis
The expansion of germinal centers (GC) within secondary lymphatic tissues is indicative of the induction of a humoral immune response. Within expanding GC, B cell proliferation, affinity maturation and differentiation into Ab secreting plasma cells is a continual process. FACS analysis of GC B cell proliferation was carried out on both the spleen (Figure 8A) and the local tracheal-bronchial lymph node (TBLN) (Figure 8B) which drains the lung. In the spleen and the TBLN of the co-infected animals, GC B cell proliferation was significantly greater than seen for the influenza-only control group 1 week after the influenza infection. By 10 days post-influenza infection GC B-cell proliferation within the spleen had diminished in the co-infected animals to a level equivalent to that observed in the influenza-only control group. In contrast, the extent of GC B-cell proliferation in the TBLN at this time was still greater in the co-infected animals although not significantly due to variability within this group.
Figure 8.
Analysis of germinal center B cell proliferation. Single cell suspensions were made from the spleen and TBLN of each animal in the co-infected (
 ) and influenza-only infected (▭) groups at 7 and 10 days after co-infection with the influenza virus. The cells were stained with anti-mouse CD19, anti-mouse CD38 and PNA in order to determine the percentage of B cells that were proliferating within the germinal centers. Analysis was completed by gating on the CD19 positive B cells and then determining the percentage of CD38neg/PNApos B cells. The total number of germinal center B cells was calculated multiplying the total cells recovered by the percentage of CD38neg/PNApos B cells. This is one of three independent experiments. N=5 or 6 animals per group; ***, P<0.001; *, P<0.05.
) and influenza-only infected (▭) groups at 7 and 10 days after co-infection with the influenza virus. The cells were stained with anti-mouse CD19, anti-mouse CD38 and PNA in order to determine the percentage of B cells that were proliferating within the germinal centers. Analysis was completed by gating on the CD19 positive B cells and then determining the percentage of CD38neg/PNApos B cells. The total number of germinal center B cells was calculated multiplying the total cells recovered by the percentage of CD38neg/PNApos B cells. This is one of three independent experiments. N=5 or 6 animals per group; ***, P<0.001; *, P<0.05.
Influenza Ab titers detected in the BALF and serum
Serum and BALF influenza-specific antibodies elicited following an infection are significant factors in the rate of viral clearance and in the provision of protective humoral immunity against re-infection. These antibodies were measured in order to determine whether the immune response to the Pneumocystis infection altered the local and/or systemic influenza-specific antibodies levels. In the lungs of the co-infected animals influenza-specific IgA (Figure 9A), IgG (Figure 9B) and IgM (Figure 9C) antibodies appeared earlier and remained at significantly higher levels following the influenza infection. In the serum, influenza-specific IgG (Figure 9D) and IgM (Figure 9E) levels were detectable in both infection groups by day 7 but significantly higher levels were observed in the co-infected animals at this time and out to day 10.
Figure 9.
Detection of influenza-specific Ab titers in the BALF and serum. ELISA tests were performed on the BALF and serum from the co-infected (
 ) and the influenza-only infected (▭) animals 7 and 10 days after infection with influenza virus. Influenza-specific IgG (Figure 9A) and IgM (Figure 9B) were measured in the serum. Influenza-specific IgG (Figure 9C), IgA (Figure 9D) and IgM (Figure 9E) were also measured in the BALF. Absorbance readings were taken at 405nm. This is one of three independent experiments, n=5 or 6 animals. *, P<0.05; **, P<0.01; *** P<0.005.
) and the influenza-only infected (▭) animals 7 and 10 days after infection with influenza virus. Influenza-specific IgG (Figure 9A) and IgM (Figure 9B) were measured in the serum. Influenza-specific IgG (Figure 9C), IgA (Figure 9D) and IgM (Figure 9E) were also measured in the BALF. Absorbance readings were taken at 405nm. This is one of three independent experiments, n=5 or 6 animals. *, P<0.05; **, P<0.01; *** P<0.005.
The levels of influenza-specific IgA detected in the serum of both infection groups were equivalently low following the influenza infection (data not shown). Neither the administration of the UV-inactivated Pneumocystis-infected lung homogenate or the β-glucan had any effect upon the level of influenza-specific antibodies detected in the serum or BALF during the resolution of the influenza infection (data not shown).
Absence of antibody negates enhanced viral clearance in co-infected mice
Our evidence suggested that the more rapid appearance of influenza-specific antibody and the higher serum and BALF levels of these antibodies may be responsible for the enhanced rate of viral clearance and be associated with the reduced levels of morbidity observed in the co-infected animals. However, IL-13 which is elicited in response to Pneumocystis infections is known to induce goblet cell metaplasia and mucus secretion at the level of the respiratory epithelium. If the levels of IL-13-induced mucus production inhibited viral attachment to susceptible respiratory epithelial cells, then this could account for a reduction in viral recovery and the diminished indications of morbidity observed in the co-infected animals. In order to determine whether the enhanced rate of viral clearance in the co-infected animals was antibody-mediated, we co-infected uMT, SCID, and immunocompetent mice. Although the uMT mice lack B cells and thus the ability to produce antibody, they were still capable of equivalent levels of goblet cell metaplasia and mucus production at the level of the respiratory epithelium in response to the Pneumocystis infection as seen in the wild-type co-infected animals (29), data not shown).
In the absence of only the humoral immune response, viral recovery 7 days after influenza infection in the co-infected and influenza-only infected uMT mice was equivalent (Figure 10A). In the absence of an adaptive immune response, the recovery of virus from co-infected and influenza-only infected SCID mice was equivalent. In fact, the levels of viral recovery were equivalent between all of the SCID and uMT infection groups at this time. In addition, the level of viral recovery in the wild-type influenza-only group was equivalent to that found each of the SCID and uMT infection groups. Significant differences in viral recovery were found only relative to the co-infected wild-type group. At this time, both uMT infection groups and the SCID influenza-only group exhibited more than 3 times the weight lost of the wild-type co-infection group (Figure 10B). The wild-type influenza-only infection group had lost more than twice the weight lost by the co-infected wild-type group. The minimal weight losses observed in the co-infected SCID group were unexpected considering the level of viral recovery and will require further analysis.
Figure 10.
Viral recovery and weight changes in co-infected wild-type, uMT and SCID mice. A). Lungs were harvested from co-infected wild type (
 ), SCID (
), SCID (
 ) and uMT (
) and uMT (
 ) and from the influenza-only infected wild type (▭), SCID (
) and from the influenza-only infected wild type (▭), SCID (
 ) and uMT (
) and uMT (
 ) mice for analysis of viral recovery by plaque assay 1 week after influenza infection. B). Body weights were monitored daily following influenza infection and weight changes were calculated relative to the weight of each animal on the day of influenza infection. N = 6 animals for analysis of viral recovery, N = 8 animals for body weight changes; **, P<0.001 relative to co-infected wild type animals.
) mice for analysis of viral recovery by plaque assay 1 week after influenza infection. B). Body weights were monitored daily following influenza infection and weight changes were calculated relative to the weight of each animal on the day of influenza infection. N = 6 animals for analysis of viral recovery, N = 8 animals for body weight changes; **, P<0.001 relative to co-infected wild type animals.
Discussion
In numerous circumstances, the process of resolving infections involving multiple pathogens can have deleterious effects on the host. The acquisition of secondary infections and the deleterious outcomes that follow are often related to pathogen-associated or immune-associated damage that is incurred during the response to the first pathogen. In contrast, the resolution of the co-infection model presented here results in a beneficial outcome for the host. In this model an opportunistic ubiquitous pathogen of the lung, Pneumocystis, is followed by a virulent pulmonary pathogen, influenza A virus. Although both of these pathogens infect the lung, neither is associated with a predisposition to cause a subsequent infection by the other. Yet a co-infection involving these pathogens is plausible in health care, day care and home settings.
The enhanced resistance to the influenza co-infection in Pneumocystis-infected mice was characterized by significant reductions in the level of influenza virus and pro-inflammatory cytokines recovered from the lung, an accelerated appearance of influenza-specific Ab in the airways, significantly greater levels of these Ab in the serum and BALF during the resolution of the influenza infection, and less evidence of morbidity. These findings were not observed in the mice infected only with influenza virus or in mice that had been treated orally with either an UV-inactivated Pneumocystis preparation or with a B-glucan preparation prior to the influenza infection. Our results indicated that the increased rate of viral clearance observed in the immunocompetent co-infected animals was dependent upon an accelerated and enhanced influenza-specific antibody response which in turn was dependent upon a temporal association with the resolution of an ongoing Pneumocystis infection.
In the present experiments, the exposure sequence and the time between the pathogen exposures were found to be critical. If exposure to both pathogens occurred within 6 hours of one another in either order, no alteration in the recovery of either pathogen was detected. In these experiments, the greatest Pneumocystis burden occurred within 6 hours of the influenza infection and yet no affect on the resolution of an influenza infection was detectable. This suggested that the Pneumocystis itself or some antigenic component derived from it was likely not responsible for the enhancement of the anti-influenza response. The Pneumocystis-induced response had to be well established in order to enhance the ensuing anti-influenza antibody response. In addition the Pneumocystis-induced response had to be elicited in response to a viable Pneumocystis infection and not in response to an inoculation with an inactivated Pneumocystis preparation or in response to a subunit preparation. This implied that a feature of the Pneumocystis-induced response may be responsible for the detectable alterations in the influenza response. The transient nature of these alterations was apparent if changes were made to the interval between the pathogen exposures. If the interval between the Pneumocystis and then the influenza infection was extended to 3 weeks, the enhanced effect on viral clearance was diminished relative to that observed for a 2 week interval. This implied that the potency of the enhancement was associated with the progression of the Pneumocystis-induced response. The transient nature of the enhancement observed in our co-infection model was analogous to the transient suppression in a previously described local co-infection model in which a type-1 Toxoplasma gondii-induced response effectively suppressed the development or inhibited an already established type-2 Nippostrongylus brasiliensis-induced response in the small intestine (31). In this model the local type-1 response was dominant and no benefit to the host was detected. In our co-infection model the local type-2 Pneumocystis response dominated and a benefit to the host was realized. Despite the hierarchy of the responses in these co-infection models being reversed, the results suggest that the time frame, within which sequential pathogen exposures must occur in order that the elicited in vivo immune responses may influence each other, is limited.
A significant alteration in the recruitment of some cell subsets into the lungs following the influenza infection was observed in the co-infected animals. Macrophage and lymphocyte accumulations were not significantly different between the infection groups following the influenza infection. The equivalent lymphocyte recovery levels in both groups following the influenza infection contrasted with the increased lymphocyte recoveries observed in other co-infection models in which increased inflammation and decreased survival of the host were observed (20, 31, 32). Neutrophil recruitment in the co-infected animals was not enhanced by the influenza infection. Influenza infection of lung epithelial cells is known to elicit the release of neutrophil chemoattractants such as CXCL8/IL-8 from these cells (14, 33). The diminished viral recoveries, in conjunction with the reduced indications of lung damage and unchanged neutrophil recruitment levels suggest that the production of neutrophil chemoattractants may be diminished. In our co-infection model the enhanced rate of viral clearance occurred without any increase in the recruitment of lymphocytes or neutrophils. This absence of any increased recruitment supports a previous contention that anti-viral immune responses operate in an inflammatory environment that is several times greater than is necessary to achieve viral clearance (4) and that a moderation of the elicited inflammatory response may be affordable and of a benefit to the host without compromising viral clearance (19). The absence of an increase in lymphocyte and neutrophil recovery from the co-infected mice suggested that regulating the recruitment of these inflammatory cells may have been critical in controlling the level of host morbidity in the co-infected animals.
The co-infected animals lost the least amount of body weight following the influenza infection and only this group was able to return to their starting body weights by the end of the experiment. The reduced viral titers in the lungs of the co-infected animals comply with the diminished morbidity levels in this group and with the reduced albumin and LDH levels in their BALF. These observations also comply with the reduced levels of pro-inflammatory cytokines (TNFα, IFNγ, MCP-1, IL-6) detected in the BALF of the co-infected animals. Although the reduced viral burden may be responsible for this, the level of anti-inflammatory Th-2-associated cytokines detected in the BALF of these co-infected animals may also have contributed to the reduced pro-inflammatory cytokine production and thus the reduced morbidity and lung damage. One such anti-inflammatory cytokine in the lung is IL-10. As a regulatory cytokine, IL-10 inhibits the production of a variety of CC (including MCP-1) and CXC chemokines, IL-6, IL-12 and subsequently IFNγ, IL-18, TNFα as well as auto-regulating itself (7, 34). Following Pneumocystis infections, IL-10 has been shown to remain at effective inhibitory levels in the lungs of immunocompetent mice up to 14 days after infection (35). The diminished levels of influenza-induced TNFα, IFNγ, IL-6, MCP-1 and IL-10 in a previously Pneumocystis-infected mouse may well be the result of the Pneumocystis-induced IL-10 levels at the time of the influenza infection. A second anti-inflammatory cytokine detected at significant levels at the time of the influenza infection was IL-13. This cytokine is associated with type-2 immune responses and has been considered to be equivalent to IL-10 in regards to its anti-inflammatory capabilities (36). Both IL-10 and IL-13 have been shown to exert their regulation of pro-inflammatory cytokine production by interference with NF-κB nuclear translocation (34, 36). The role of both of both of these cytokines in this model is under further investigation.
The detection of elevated influenza-specific Ab titers in the BALF and serum of the co-infected animals implied that the preceding Pneumocystis infection had endowed the host with a means to enhance the influenza-specific Ab response. The inability of the UV-inactivated Pneumocystis preparation or the β-glucan preparation to induce an equivalent level of antibody enhancement demonstrated that neither preparation could elicit the same immune mediators or mechanisms as those associated with the response to the viable Pneumocystis infection. This observation is similar to the inability of a toxoplasma lysate preparation to elicit the equivalent suppressive effect that a viable type-1 T. gondii-induced response had upon the resolution of a type-2 N. brasiliensis-induced response (31). In both co-infection models, a viable infection rather than simply exposure to a component(s) of the pathogen is required in order to provide a stronger or more complete stimulus to effectively influence the immune response induced by the second pathogen. In the spleen and TBLN of the co-infected animals there was a significantly greater level of germinal center (GC) B cell proliferation than in the influenza-only infected animals. The co-infected animals would likely have established actively proliferating GC B cell sites within these lymphatic tissues as a result of the ongoing resolution of the preceding Pneumocystis infection. The subsequent arrival of unrelated antigens to these proliferating GC sites may have facilitated the development of the accelerated increase in influenza-specific Ab titers.
The detected enhancement effect could also be associated with the generation of a local immune environment within the lung prior to the influenza infection that would confer an ability to produce increased levels of influenza-specific antibodies. Within the lung, inducible broncho-associated lymphoid tissue (iBALT) has been shown to play an effective role in the clearance of influenza infections in mice lacking peripheral lymphoid tissues and to endow these animals with the ability to survive higher viral doses while incurring fewer pathological consequences than are associated with systemic immune responses (37). If local iBALT containing actively proliferating GC were established within the lungs during the resolution of the Pneumocystis infection, then their presence could have a significant impact on the resolution of the influenza infection. Further studies assessing the potential involvement of iBALT in the acceleration of Ab production, the appearance in the lung of enhanced anti-influenza antibody titers and the rapid viral clearance in the co-infection group are being undertaken. As a corollary to the expansion of GC in the spleen and TBLN and the possibility of an iBALT contribution to the local immune response, it is worthy to note that in the case of a prior influenza infection, no such enhanced clearance of the secondary Pneumocystis infection was detected. Thus, it would seem that a unique trait of the Pneumocystis-induced immune response in an immunocompetent host is required for the enhancement of the immune response to the subsequent influenza infection.
In order to determine whether the diminished viral recoveries detected in the co-infected animals were the result of clearance by an enhanced antibody response or due to the inability of the virus to access the respiratory epithelium following the Pneumocystis infection, we incorporated uMT mice into the co-infection model. As with immunocompetent mice, uMT mice still retain the goblet cell metaplasia and mucus production at the level of the respiratory epithelium in response to a Pneumocystis infection (29) but are unable to produce antibody in response to any immune challenges. SCID mice were included in this procedure to compare how the loss of antibody in the Pneumocystis-infected uMT mice relative to the loss of adaptive immunity in the Pneumocystis-infected SCID mice impacted the clearance of the subsequent influenza co-infection. That the viral recoveries of both uMT infection groups were equivalent suggested that the Pneumocystis-induced goblet cell metaplasia and mucus production did not limit access of the virus to susceptible respiratory epithelial cells and thus were not factors in reducing the viral recovery in this co-infection model.
Since viral recoveries in the SCID and uMT co-infection groups were not significantly different, it was unlikely that cell-mediated responses could account for the accelerated rates of viral clearance observed at 5 days after the influenza infection of the co-infected animals. Earlier studies have demonstrated that significant levels of influenza-specific CD8 cytotoxic T cells are not detectable in the lungs of mice until 7 days post-infection (38, 39). Adoptive transfer studies using Thy1.1 CD4 T cells demonstrate that detectable levels of influenza-specific CD4 T cells do not appear in the airways until 6 days post-infection (40). These rates of recruitment for both of these T cell subsets lag behind the rate of viral clearance that we observe in the immunocompetent co-infected animals. As the critical difference between the wild type and uMT animals is that the absence of B cells in the uMT mice results in their inability to produce antibody, we concluded that the increased rate of viral clearance observed in the co-infected immunocompetent animals was due to the enhancement of the influenza-specific antibody response associated with the resolution of the ongoing Pneumocystis infection.
The novelty of this localized co-infection model is that the beneficial outcome to its resolution only involved one infected tissue, the lung. This tissue is a principle mucosal site for pathogen exposure and entry into a host and thus is one that requires and gets significant immunological attention from the host. The susceptibility of the lung to immune and/or pathogen-associated damage makes the development of abbreviated yet effective local immune responses more desirable. If an opportunistic pathogen that causes an asymptomatic immune response in an immunocompetent host, such that an enhanced resistance to infection by a more virulent pathogen is achieved, then finding a means to enhance pulmonary immunity shortly before an impending immunological challenge may have significant therapeutic impact upon vaccine efficacy and/or disease outcomes.
Acknowledgments
The authors wish to acknowledge Ann Harmsen, Soo Han, Trenton Bushmaker, and Katie Shampeny for their technical support. We would also like to express our thanks to Tammy Marcotte and her colleagues at the Animal Resource Center at Montana State University for their animal care expertise.
Special abbreviations
- APC
allophycocyanin
- iBALT
inducible broncho-alveolar lung tissue
- BALF
broncho-alveolar lavage fluid
- MDCK
Madin-Darby canine kidney
- LDH
lactate dehydrogenase
- PNA
peanut agglutinin
- TBLN
tracheal-bronchial lymph node
- GC
germinal center
Footnotes
Source of support: This research was supported by National Institutes of Health grants HL55002 (A.G.H), RR020185 (A.G.H) and support from RR16455 (INBRE grant, Tim Ford; grant coordinator).
References
- 1.Foulongne V, Guyon G, Rodiere M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–359. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 2.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–26. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 4.Furze RC, Hussell T, Selkirk ME. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect Immun. 2006;74:1924–1932. doi: 10.1128/IAI.74.3.1924-1932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon γ regulates allergic airway inflammation and mucus production. J Exp Med. 1999;190:1309–1317. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 7.Conti P, Kempuraj D, Kandere K, Gioacchino MD, Barbacane RC, Castellani ML, Felaco M, Boucher W, Letourneau R, Theoharides TC. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 9.Marshall AJ, Brunet LR, Gessel Yv, Alcaraz A, Bliss SK, Pearce EJ, Denkers EY. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- 10.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi MH, Gary BA, Pomeroy C, Inayat MS, Oakley OR. A murine model of dual infection with cytomegalovirus and Pneumocystis carinii: Effects of virus-induced immunomodulation on disease progression. Virus Res. 2005;114:35–44. doi: 10.1016/j.virusres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Haque A, Rachinel N, Quddus MR, Haque S, Kasper LH, Usherwood E. Co-infection of malaria and alpha-herpesvirus: exacerbated lung inflammation or cross-protection depends on the stage of viral infection. Clin Exp Immunol. 2004;138:396–404. doi: 10.1111/j.1365-2249.2004.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner NN, Swain S, Tighe M, Harmsen A, Harmsen A. Role of type 1 IFNs in pulmonary complications of Pneumocystis murina infection. J Immunol. 2005;174:5462–5471. doi: 10.4049/jimmunol.174.9.5462. [DOI] [PubMed] [Google Scholar]
- 14.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 15.Medrano FJ, Montes-Cano M, Conde M, Horra Cdl, Respaldiza N, Gasch A, Perez-Lozano MJ, Varela JM, Calderon EJ. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005;11:245–250. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson JC, Cushion MT. Pneumocystis: not just pneumonia. Curr Opin Microbiol. 2005;8:393–398. doi: 10.1016/j.mib.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Gigliotti F, Harmsen AG, Wright TW. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun. 2003;71:3852–3856. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chabe M, Dei-Cas E, Creusy C, Fleurisse L, Respaldiza N, Camus D, Durand-Joly I. Immunocompetent hosts as a reservoir of Pneumocystis organisms: Histological and RT-PCR data demonstrate active replication. Eur J Clin Microbiol Infect Dis. 2004;23:89–97. doi: 10.1007/s10096-003-1092-2. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki M, Yanagihara K, Higashiyama Y, Fukuda Y, Kaneko Y, Ohno H, Miyazaki Y, Hirakata Y, Kadota J, Tashiro T, Kohno S. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur Respir J. 2004;24:143–149. doi: 10.1183/09031936.04.00126103. [DOI] [PubMed] [Google Scholar]
- 21.Alonso J-M, Guiyoule A, Zarantonelli ML, Ramisse F, Pires R, Antignac A, Deghmane AE, Huerre M, Werf Svd, Taha M-K. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol Lett. 2003;222:99–106. doi: 10.1016/S0378-1097(03)00252-0. [DOI] [PubMed] [Google Scholar]
- 22.Maher N, Vermund S, Lasbury M, Lee CH, Bartlett M, Unnasch TR. Development and evaluation of molecular viability assay for Pneumocystis carinii. J Clin Microbiol. 2000;38:1947–1952. doi: 10.1128/jcm.38.5.1947-1952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiley JA, Tighe MP, Harmsen AG. Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J Immunol. 2005;175:3186–3196. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
- 24.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of IFNγ by influenza HA-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown GD, Herre J, Williams DL, Willment JA, Marshall ASJ, Gordon S. Dectin-1 Mediates the Biological Effects of B-Glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol. 2000;164:3755–3763. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 27.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall B-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem. 2003;278:2043–2050. doi: 10.1074/jbc.M209715200. [DOI] [PubMed] [Google Scholar]
- 28.Wiley JA, Harmsen AG. Bone marrow-derived cells are required for the induction of a pulmonary inflammatory response mediated by CD40 ligation. Am J Pathol. 1999;154:919–924. doi: 10.1016/S0002-9440(10)65339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swain SD, Meissner NN, Harmsen AG. CD8 T cells modulate CD4 T-cell and eosinophil-mediated pulmonary pathology in Pneumocystis pneumonia in B-cell deficient mice. Am J Pathol. 2006;168:466–475. doi: 10.2353/ajpath.2006.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drent M, Cobben NAM, Henderson RF, Wouters EFM, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 31.Liesenfeld O, I, Dunay R, Erb KJ. Infection with Toxoplasma gondii reduces established and developing Th2 responses induced by Nippostrongylus brasiliensis infection. Infect Immun. 2004;72:3812–3822. doi: 10.1128/IAI.72.7.3812-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoicov C, Whary M, Rogers AB, Lee FS, Klucevsek K, Li H, Cai X, Saffari R, Ge Z, Khan IA, Combe C, Luster A, Fox JG, Houghton J. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J Immunol. 2004;173:3329–3336. doi: 10.4049/jimmunol.173.5.3329. [DOI] [PubMed] [Google Scholar]
- 33.Arndt U, Wennemuth G, Barth P, Nain M, Al-Abed Y, Meinhardt A, Gemsa D, Bacher M. Release of macrophage migration inhibitory factor and CXCL8/Interleukin-8 from lung epithelial cells rendered necrotic by influenza A virus infection. J Virol. 2002;76:9298–9306. doi: 10.1128/JVI.76.18.9298-9306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KW, Malefyt RdW, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 35.Qureshi MH, Harmsen AG, Garvy BA. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J Immunol. 2003;170:1002–1009. doi: 10.4049/jimmunol.170.2.1002. [DOI] [PubMed] [Google Scholar]
- 36.Lentsch AB, Czermak BJ, Jordan JA, Ward PA. Regulation of acute lung inflammatory injury by endogenous IL-13. J Immunol. 1999;162:1071–1076. [PubMed] [Google Scholar]
- 37.Moyron-Qiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of Inducible Bronchus Associated Lymphoid Tissue (iBALT) in Respiratory Immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 38.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 39.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig−/− mice. J Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roman E, Miller E, Harmsen A, Wiley J, Andrian UHv, Huston G, Swain S. Primary CD4 T cell response generates heterogeneous CD4 effector cells differing in phenotype, migration and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]