Abstract
Angiogenesis is dependent on the coordinated action of numerous cell types. A key adhesion molecule expressed by these cells is the αvβ3 integrin. Here, we show that although this receptor is present on most vascular and blood cells, the key regulatory function in tumor and wound angiogenesis is performed by β3 integrin on bone marrow–derived cells (BMDCs) recruited to sites of neovascularization. Using knockin mice expressing functionally stunted β3 integrin, we show that bone marrow transplantation rescues impaired angiogenesis in these mice by normalizing BMDC recruitment. We demonstrate that αvβ3 integrin enhances BMDC recruitment and retention at angiogenic sites by mediating cellular adhesion and transmigration of BMDCs through the endothelial monolayer but not their release from the bone niche. Thus, β3 integrin has the potential to control processes such as tumor growth and wound healing by regulating BMDC recruitment to sites undergoing pathological and adaptive angiogenesis.
Introduction
Angiogenesis, the formation of new blood vessels from existing vasculature, is crucial for many physiological and pathological processes including, but not limited to, fetal development, tissue repair, and tumor growth. Originally, angiogenesis was believed to primarily rely on the expansion of local vascular endothelial (VE) cells; however, the process is much more complicated and involves coordination of vascular cells with fibroblasts, immune cells of blood and tissue origin, and circulating blood components. Numerous studies have demonstrated the involvement of recruited bone marrow (BM)–derived cells (BMDCs) in neovascular development (Lyden et al., 2001; Ziegelhoeffer et al., 2004; Peters et al., 2005). Although the identity and origin of these cells remains unclear and somewhat controversial, a role for BMDCs in angiogenesis has been documented by multiple groups (Yang et al., 2004; Khakoo and Finkel, 2005; Peters et al., 2005; Grunewald et al., 2006; Jin et al., 2006). These BMDCs appear to promote angiogenesis through the release of proangiogenic factors at sites of neovascularization to stimulate expansion of local blood vessels (Ziegelhoeffer et al., 2004; Grunewald et al., 2006; Ruiz et al., 2006). Despite growing evidence depicting a key regulatory role of these cells in angiogenesis, the mechanisms underlying BMDC release, recruitment, and retention at sites of neovascularization are just now beginning to be investigated.
As in leukocyte adhesion and trafficking, specific key steps of BMDC recruitment are potentially mediated by cell adhesion molecules (Eliceiri and Cheresh, 2001; Mahabeleshwar et al., 2007). The primary class of receptors known to mediate cell adhesion to other cells and extracellular matrix are integrins. Although many integrins have been shown to be involved in various aspects of angiogenesis, one of the most intriguing players remains integrin αvβ3 (Carmeliet, 2002). The vast majority of studies have focused on the regulatory function of endothelial αvβ3 in angiogenesis (Reynolds et al., 2002, 2004; Mahabeleshwar et al., 2006); however, this receptor is also present on a variety of BMDCs. It has been suggested that β3 integrin is a common surface marker for tissue-specific stem cells and its expression was found to be correlated to the properties of quiescent hematopoietic stem cells (Umemoto et al., 2006).
One of the most intriguing aspects of β3 integrin function in angiogenesis is the reported discrepancy between the effects of αvβ3 inhibitors on pathological angiogenesis and the phenotype of the β3 integrin knockout mice (Brooks et al., 1994a,b; Eliceiri and Cheresh, 1999, 2001; Reynolds et al., 2002; Taverna et al., 2004; Mahabeleshwar et al., 2006; Weis et al., 2007). Importantly, recent studies using β3 integrin knockout mice clearly demonstrate not suppressing but the stimulatory role of αvβ3 on angiogenesis in certain tissues (Kanamori et al., 2006; Weis et al., 2007). These studies further emphasize the need to solidify the very complex role of β3 integrins in the regulation of physiological and pathological neovascularization.
Expression levels of αvβ3 on the surface of myeloid cells were shown to be regulated by cytokines and chemokines (De Nichilo and Burns, 1993). Cytokines and chemokines also play vital roles in the mobilization and homing of BMDCs (Grunewald et al., 2006; Ruiz et al., 2006). Stromal derived factor-1 (SDF-1), a CXC chemokine family member, controls numerous homeostatic, developmental, and pathological processes through interaction with its cognate receptor, CXCR4, which is highly expressed by BMDCs (Epstein, 2004; Burger and Kipps, 2006; Ruiz et al., 2006). Emerging evidence indicates that the SDF-1/CXCR4 axis plays a pivotal role in the mobilization of hematopoietic cells from BM into peripheral blood and in dictating positional engraftment of these cells at angiogenic sites (Orimo et al., 2005; Grunewald et al., 2006).
The importance of BMDCs in neovascular development (De Palma et al., 2005; Grunewald et al., 2006), the unique pattern of β3 integrin expression and cellular regulation (Chandhoke et al., 2004; Mahabeleshwar et al., 2006; Umemoto et al., 2006), and the intriguing, yet controversial, role of β3 integrin receptor in angiogenesis (Brooks et al., 1994a; Eliceiri and Cheresh, 1999; Reynolds et al., 2002; Taverna et al., 2004; Kanamori et al., 2006) has prompted us to focus on the role of this integrin in the biology of BMDCs in angiogenesis. As a basic experimental model, we used knockin mice (DiYF mice) in which β3 integrin tyrosines 747 and 759 are mutated to phenylalanine. We have previously shown that defective tyrosine phosphorylation of β3 integrin in DiYF mice resulted in abnormal β3 function denoted by impaired endothelial cell adhesion, spreading, migration, and tumor angiogenic defects (Mahabeleshwar et al., 2006). This mutation is not limited to endothelial cells but affects numerous cell types involved in the regulation of angiogenesis, including platelets, leukocytes, and, potentially, other BMDCs. Previous in vitro studies have indicated that Y747 phosphorylation is crucial for αvβ3 integrin function on myeloid cell lines (Blystone et al., 1997; Chandhoke et al., 2004). The present study is based on our surprising initial finding that transplantation of wild-type (WT) BM completely rescues angiogenic defects in tumors and wounds of DiYF mice. We have crossed DiYF mice with GFP-expressing transgenic mice to create DiYF/GFP mice as a source of BM to delineate the role of β3 integrin in the release and recruitment of BMDCs to sites of angiogenesis. Our analysis revealed that αvβ3 integrin on BMDCs plays a key role in tumor associated pathological angiogenesis as well as in wounds via regulation of BMDC adhesion and transmigration into sites of neovascularization, but not their release from the bone niche.
Results
BM transplantation (BMT) restores normal tumor growth and wound healing in DiYF mice
We have previously shown that endothelial cell function and angiogenesis are impaired in DiYF mice. In this study, we investigate the role of β3 integrin in BMDC recruitment to sites of neovascularization. To this end, we performed a series of BMT and assessed the role of BM in angiogenesis-dependent responses, i.e., tumor growth and wound healing.
In accord with our previous findings (Mahabeleshwar et al., 2006), the growth of implanted B16F10 melanoma was reduced about twofold in DiYF mice compared with WT counterparts. Transplantation of WT BM into irradiated DiYF hosts (WT→DiYF) normalized tumor growth to that of control WT mice receiving WT marrow (WT→WT; Fig. 1 A). These results were corroborated using murine RM1 prostate carcinoma cells (Fig. 1 B). Alternatively, transplantation of DiYF BM into irradiated WT hosts (DiYF→WT) resulted in stunted B16F10 and RM1 tumor growth, a difference similar to that observed in mice not undergoing BMT. These data suggest that reduced tumor growth in DiYF mice is at least partially a consequence of altered BMDC β3 integrin function.
Figure 1.
Reduced tumor growth and delayed wound healing in DiYF mice are BM dependent. (A) Weights of subcutaneous B16F10 tumors in WT and DiYF mice (11 d after implantation) or mice undergoing BMT (13 d) with WT or DiYF donor marrow. Data represent mean ± SEM (n = 10 per group). (B) Weights of subcutaneous RM1 tumors in WT and DiYF mice (9 d after implantation) or mice undergoing BMT with WT donor marrow (11 d after implantation). Representative tumors are depicted above their respective dataset. Data represent mean ± SEM (without BMT, n = 14 per group; with BMT, n = 18 per group). (C) Analysis of wound healing in WT and DiYF mice. Gross visualization of healing wounds 3 (top left) and 7 (bottom left) d after wound creation in WT and DiYF mice not undergoing (top left) and undergoing (bottom left) BMT with WT or DiYF donor marrow. Percentage of wound closure in WT and DiYF mice (top right) and WT and DiYF mice (bottom right) undergoing BMT. Data represent mean ± SEM (WT and DiYF, n = 10 per group; WT→WT, n = 16; WT→DiYF, n = 12). *, P < 0.05.
To illustrate that the observed BMT angiogenic rescue effect wasn't specific to our tumor model, we examined wound healing. By analyzing wound closure, we found diYF mice displayed an early (3–7 d) wound healing delay (extent of closure) and extended time to complete closure (Fig. 1 C, top). Whereas reconstitution of DiYF mice with WT BM restored the wound healing process, transplantation of DiYF BM into WT mice reduced the extent of wound closure 3 d after surgery compared with WT mice with WT BM (Fig. 1 C, bottom). Gross inspection of healing wounds illuminated an angiogenic defect as the potential causative factor in the delay exhibited by DiYF mice, a condition corrected by BMT with WT marrow. These data demonstrated that impaired tumor growth and wound healing in DiYF mice are a consequence of altered β3 integrin function of BMDCs and not of local remodeling tissues and that BM reconstitution may reverse such angiogenic defects.
DiYF angiogenic defects are reversed in mice transplanted with normal BM
Having previously reported angiogenic defects in implanted tumors of DiYF mice (Mahabeleshwar et al., 2006), we further investigated the phenotype of these defects and whether they were BM dependent. Histological examination of B16F10 and RM1 tumor, as well as wound sections, illuminates this angiogenic defect (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1). Double staining of B16F10 tumor sections with the endothelial cell marker CD31 and the pericyte marker neuro/glial cell 2 chondroitin proteoglycan (NG2) revealed a substantial (greater than threefold) decrease in both CD31- and NG2-positive blood vessel densities in tumors of DiYF hosts (Fig. 2 A). Total VE cell and pericytes positive areas in tumor tissues were also decreased (by 55 and 45%, respectively) in DiYF hosts (Fig. S1 B, top left) with no accompanying differences in blood vessel size (Fig. S1 B, top right). Colocalization of endothelial cells and pericytes in tumor vasculature revealed no morphological differences between B16F10 tumor sections regardless of host genotype. A reduction in the basement membrane protein laminin was also evident in tumors of DiYF hosts (Fig. S1 B, middle left). In addition, smooth muscle actin (SMA), a myofibroblast and adult smooth muscle pericyte marker, exhibited a fourfold decrease in DiYF B16F10 tumor sections compared with WT mice (Fig. 2 B). Immunohistochemical analysis revealed a decrease in von Willebrand factor vessel density (Fig. 2 C) and area (Fig. S1 B, bottom) in DiYF mice wound tissues compared with WT mice. Reduced staining for these vascular markers has previously been shown in RM1 tumor sections (Mahabeleshwar et al., 2006) and is consistent with that of healing wounds of DiYF mice (Fig. 2 C and Fig. S1 B, middle right) as well. These data illustrate the angiogenic defects of implanted tumors and wounds of DiYF mice.
Figure 2.
The angiogenic phenotype of DiYF mice is BM dependent. (A) Immunofluorescent detection of CD31 (red) and NG2 (green) in B16F10 tumor sections from WT and DiYF mice (left). Microvessel density in B16F10 tumors from WT and DiYF mice (right). Bar, 100 μm. Data represent mean ± SEM. **, P < 0.01. (B) SMA-positive microvessel density of B16F10 tumor sections from WT and DiYF mice. Data represent mean ± SEM. *, P < 0.05. (C) von Willebrand factor (vWF)–positive microvessel density of tissue sections from 10-d-old wounds of WT and DiYF mice. Data represent mean ± SEM. *, P < 0.05. (D) Immunofluorescent detection of CD31 (red) and NG2 (green) in B16F10 tumor sections from WT and DiYF mice after BMT with WT donor marrow (left). Bar, 200 μm. Insets depict similarities in vessel cellular organization. CD31 and NG2 microvessel density in B16F10 tumors from WT and DiYF mice after BMT with WT donor marrow (right). Data represent mean ± SEM. (E) SMA microvessel density in B16F10 tumors from WT and DiYF mice after BMT with WT donor marrow. Data represent mean ± SEM. (F) Immunofluorescent detection of CD31 (red) and NG2 (green) in B16F10 tumor sections from WT mice after BMT with WT or DiYF donor marrow (left). Bar, 200 μm. CD31 and NG2 microvessel area in B16F10 tumors from WT mice after BMT with WT or DiYF donor marrow (right). Data represent mean ± SEM. *, P < 0.05. (G) SMA microvessel density in B16F10 tumors from WT mice after BMT with WT and DiYF donor marrow. Data represent mean ± SEM. *; P < 0.05.
Histological examination of B16F10 and RM1 tumor sections from DiYF hosts undergoing BMT with WT donor marrow revealed a possible restoration of angiogenesis (Fig. S1 C). This was confirmed immunohistochemically by double staining B16F10 tumor sections with anti-CD31 and -NG2 antibodies (Fig. 2 D and Fig. S1 D). The density of SMA-positive vessels in tumor sections of WT marrow recipient DiYF mice was similar to that of WT mice receiving WT marrow (Fig. 2 E). Conversely, we performed BMT wherein WT BM was reconstituted with DiYF marrow. Although immunohistochemical analysis of B16F10 tumor sections in these mice revealed no statistically significant difference in blood vessel density (Fig. S1 E), transplantation of DiYF BM into WT mice caused a reduction in blood vessel and pericyte area compared with WT mice receiving WT BM (Fig. 2 F). SMA-positive vessel densities in tumor sections of DiYF→WT mice were also reduced by >40% compared with WT→WT mice (Fig. 2 G). Thus, transplantation of DiYF BM into WT mice yields tumor angiogenic defects as well. These data therefore indicate that β3 integrin function of BM cells plays a key role in angiogenesis.
Platelet defects are not causative in the DiYF angiogenic defect
Because the DiYF mutation affects αIIbβ3 signaling and platelet-dependent responses (Law et al., 1999), we next assessed to what extent, if any, that platelets contribute to the abnormal angiogenesis exhibited by DiYF mice. Using an anti-mouse GPIbα antibody, platelets were depleted in both WT and DiYF mice causing a >90% reduction in circulating platelets compared with control IgG. This reduced platelet level was maintained during the entire experiment through repeated antibody treatment. Although platelet depletion resulted in a reduction of tumor growth in both WT and DIYF mice, tumors in platelet-depleted WT hosts were ∼70% larger than those formed in DiYF counterparts (0.323 ± 0.034 vs. 0.191 ± 0.051 g, n = 10, P < 0.05), a difference paralleling that of nondepleted WT and DiYF mice (0.493 ± 0.051 vs. 0.346 ± 0.046 g, n = 10, P < 0.05). Immunohistochemical analysis of tumor sections with laminin revealed that the difference in tumor vascular density between WT and DiYF mice remained after platelet depletion (unpublished data), a difference similar to that of WT and DiYF mice. Thus, although platelets are indeed important for tumor progression and angiogenesis, impaired platelet function is not the predominant contributor to altered tumor progression in DiYF mice.
Recruitment of DiYF BMDCs to sites of neovascularization is impaired
Because BMT rescued the angiogenic phenotype of DiYF mice, we next assessed whether recruitment of BMDCs into the developing vasculature was defective in these mice. To monitor the extent of BMDC recruitment, DiYF mice were crossed with GFP-expressing transgenic WT (WT/GFP) mice. The resultant DiYF/GFP and normal WT/GFP mice were used as donors for BMT experiments. In brief, WT/GFP marrow was transplanted into WT mice (WT/GFP→WT) and DiYF mice (WT/GFP→DiYF) and WT marrow was reconstituted with donor DiYF/GFP marrow (DiYF/GFP→WT). More than 80% of nucleated cells and 95% of platelets in blood expressed GFP 8 wk after BMT.
Analysis of B16F10 tumors formed in chimeric mice revealed large numbers of GFP+ cells located within the tumor periphery and in the vicinity of tumor vasculature (Fig. 3 A). GFP+ cell numbers were elevated in WT/GFP→WT and WT/GFP→DiYF mice compared with those from DiYF/GFP→WT mice (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1). Importantly, there was no significant difference in the presence of GFP+ cells in tumors from WT/GFP→WT and WT/GFP→DiYF mice (Fig. 3 B), indicating that recruitment of BMDCs and not host vasculature deficiencies are responsible for the impaired angiogenesis exhibited by DiYF mice. These findings were confirmed by assessing infiltration of BMDCs into healing wounds 7 d after wound initiation. As shown previously (Fig. 1 C, bottom right), histological analysis of wound tissues at this time point revealed almost complete wound closure and vascular similarities in WT and DiFY mice. Similar to our tumor model, recruitment of GFP+ cells to wound tissues was impaired in DiYF/GFP→WT mice compared with mice receiving WT/GFP marrow regardless of genotype (Fig. 3 C). In fact, there was no significant difference in the presence of GFP+ cells in wound tissues of WT/GFP→WT and WT/GFP→DiYF mice. Results of our tumor and wound studies emphasize the importance of BMDCs in angiogenic processes and indicate that β3 integrin function is essential for BMDC recruitment to sites of neovascularization.
Figure 3.
Infiltration of BMDCs is β3 integrin dependent. (A) Immunofluorescent detection of SMA (red) and GFP (green) on B16F10 tumor tissues in WT mice after BMT with WT/GFP or DiYF/GFP donor marrow. Bar, 100 μm. (B) Quantification of GFP-positive cells in B16F10 tumor sections from WT and DiYF mice after BMT with WT/GFP or DiYF/GFP donor marrow. Data represent mean ± SEM. *, P < 0.05; **, P < 0.01. (C) Quantification of GFP-positive cells in wound tissue (day 7) sections from WT and DiYF mice after BMT with WT/GFP or DiYF/GFP donor marrow. Data represent mean ± SEM. *, P < 0.05; **, P < 0.01.
The majority of tumor-infiltrating BMDCs are of hematopoietic origin
The localization of BMDCs in tumor neovasculature of DiYF/GFP→WT, WT/GFP→WT, and WT/GFP→DiYF mice was examined by double staining of tumor sections for GFP (to visualize recruited BMDCs) and CD31 or VE-Cadherin (endothelium markers), laminin (blood vessel basement membrane), or SMA. BMDCs were prominent in the vicinity of blood vessels of B16F10 and RM1 tumors but were distinctly separate from the endothelial lining stained by CD31 or VE-Cadherin (Fig. 4 A and Fig. S3 B, available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1). GFP-positive cells were situated adjacent to both the basement membrane (Fig. 4 B) and SMA-positive cells (Fig. 4 C). Western analysis of B16 F10 tumor lysates (WT/GFP→WT and DiYF/GFP→WT) showed decreased expression levels of GFP in tumors with DiYF/GFP→WT compared with WT/GFP→WT (Fig. S3, C and D). Additionally, Western analysis of DiYF/GFP→WT also exhibited reduced expression levels of VE-Cadherin and CD31 compared with WT/GFP→WT (Fig. S3, C and D). Our results indicate that although BMDCs did not directly incorporate into the tumor neovasculature, they are situated in close proximity to blood vessels and might affect angiogenesis by secreting key effectors. This is further supported by several other studies emphasizing the role of promyeloid cells in tumor vasculature (Grunewald et al., 2006; Shojaei et al., 2007, 2008; Purhonen et al., 2008). These groups demonstrated that BMDCs do not incorporate into endothelium. A recent study using B16 melanoma clearly shows the lack of markers such as CD31, von Willebrand factor, and VEGFR2 on BMDCs (Purhonen et al., 2008). Moreover, several comprehensive studies conclusively show that it is the population of myeloid cells from BM that makes all the difference in tumor growth (Shojaei et al., 2007, 2008).
Figure 4.
Localization of BMDCs in the area of blood vessels in tumor tissues. (A) Immunofluorescent detection of CD31 (red) and GFP (green) in B16F10 (top) and RM1 (bottom) tumor sections from WT and DiYF mice after BMT with WT/GFP or DiYF/GFP donor marrow. Bar, 50 μm. (B) Immunofluorescent detection of laminin (red) and GFP (green) in B16F10 tumor sections from WT and DiYF mice after BMT with WT/GFP donor marrow. Bar, 100 μm. (C) Immunofluorescent detection of SMA (red) and GFP (green) in B16F10 tumor sections from WT and DiYF mice after BMT with WT/GFP or DiYF/GFP donor marrow. Bar, 100 μm.
Studies using models of ischemia have shown that the majority of BMDCs recruited to sites of insult are leukocytes, macrophages, T lymphocytes, and fibroblasts (Ziegelhoeffer et al., 2004). By costaining B16F10 tumor sections for GFP and CD45, a marker for leukocytes or hematopoietic cells, including hematopoietic progenitor/stem cells except erythrocytes and platelets, we found that BM-derived GFP+ cells were frequently CD45 positive (Fig. 5 A) with the extent of GFP+CD45+ double positive cells in DiYF/GFP→WT mice reduced in comparison to that of WT/GFP→WT mice. This alludes to an essential role for β3 integrin signaling in the recruitment of CD45+ cells from BM into sites of angiogenesis.
Figure 5.
Identification of tumor infiltrating BMDCs. (A) Immunofluorescent detection of GFP (a and d) and CD45 (Ab and Ae) along with the merged image (Ac and Af). Sections are of B16F10 origin in WT mice after BMT with WT/GFP (a–c) or DiYF/GFP (d–f) donor marrow. (B) Immunofluorescent detection of GFP (green) and F4/80 (red) in B16F10 tumor sections from WT mice after BMT with WT/GFP (a) or DiYF/GFP (c) donor marrow. Blood vessels marked as V. (b and d) Higher magnifications of enclosed boxes in a and c, respectively. (C) Immunofluorescent detection of CD45+F4/80+Gr-1+CD3 (a and e) and GFP (b and f) along with merged images of B16F10 (c and g) and RM1 (d and h) tumor sections from WT mice after BMT with WT/GFP (a–c) or DiYF/GFP (e–h) donor marrow. Arrows indicate GFP-positive cells that are negative for CD45+F4/80+Gr-1+CD3. Bars, 100 μm.
The majority of GFP+ cells were located at the periphery of tumors and immunofluorescent visualization of the macrophage marker F4/80 indicated a good portion of these cells to be positive (33.6% in WT mice and 45.0% in DiYF mice; Fig. S3 A); however, the extent of perivascularly colocalized F4/80+ and GFP+ cells was low (6.1% in WT mice and 17.0% in DiYF mice; Fig. 5 B). Macrophage depletion with clodronate further reduced tumor size in DiYF mice (Fig. S3 E). Limited numbers of other hematopoietic cell types, such as granulocytes and monocytes (both are Gr-1 positive) and T-lymphocytes (CD3 positive) were located near the periphery of tumors but no obvious differences between DiYF/GFP→WT and WT/GFP→WT mice were found. By using a combination of anti-CD45, -F4/80, -CD3, and -Gr-1 antibodies for immunofluorescence analysis, along with visualization of GFP, we found that >95% of GFP+ cells in B16F10 tumors and >97% in RM1 tumors were positive for at least one of these markers (Fig. 5 C). Hence, the majority of BMD tumor-infiltrating cells were of hematopoietic origin.
Reduced SDF-1 concentration in tumor tissues of DiYF mice leads to impaired recruitment of CXCR4+ BMDCs
A key factor for BMDC retention in angiogenic tissues is SDF-1, a chemokine produced by, but not limited to, mural cells such as perivascular fibroblasts and smooth muscle cells (Kucia et al., 2005; Orimo et al., 2005; Grunewald et al., 2006). DiYF angiogenic defects are accompanied by reduced numbers of SMA-positive cells, which may result in decreased levels of local and systemic SDF-1. Therefore, the SDF-1 content in tumors and plasma from WT and DiYF mice were determined. Although SDF-1 plasma levels in tumor-bearing WT and DiYF mice were closely matched (Fig. S4 A, available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1), tumor concentrations of SDF-1 in DiYF mice were ∼50% less than that of WT mice (Fig. 6 A). SDF-1 levels in leukocytes, BM cells, endothelial cell culture medium, and smooth muscle cell culture medium were similar for WT and DiYF mice (Fig. S4, B and C). Transplantation of WT marrow into DiYF mice normalized the levels of SDF-1 in tumor tissues (Fig. 6 B).
Figure 6.
The SDF-1/CXCR4 axis regulates angiogenesis in DiYF mice. (A) Levels of SDF-1 in WT and DiYF B16F10 tumors. Data represent mean ± SEM (duplicate measurements of n = 10 [WT] and n = 8 [DiYF] tumors each group). *, P < 0.05. (B) Levels of SDF-1 in WT and DiYF B16F10 tumors after BMT with WT donor marrow. Data represent mean ± SEM (duplicate measurements of n = 6 per group). (C) Immunofluorescent detection of GFP (a, d, and g) and CXCR4 (b, e, and h) along with the merged image (c, f, and i). Sections are of RM1 origin in WT (a–c and g–i) and DiYF (d–f) mice after BMT with WT/GFP (a–f) or DiYF/GFP (g–i) donor marrow. (D) Peripheral blood analysis of β3+ and CXCR4+ cells in nontumor- and B16F10 tumor-bearing WT and DiYF mice. (E) Percentage of circulating CXCR4+ cells in nontumor- and B16F10 tumor-bearing WT and DiYF mice. Data represent mean ± SEM (n = 5 per group). (F) Percentage of circulating CXCR4+β3+ cells in B16F10 tumor-bearing WT and DiYF mice. Data represent mean ± SEM (n = 5 per group). **, P < 0.01.
SDF-1 has been shown to mediate the recruitment and retention of CXCR4+ BMDCs to angiogenic blood vessels (Jin et al., 2006). Immunohistochemical staining of tumor tissues for CXCR4 showed that the majority of GFP+ cells in tumor sections were also CXCR4 positive (Fig. 6 C). As noted previously in Fig. 5, there was an apparent defect in recruitment of CXCR4+ cells to tumor tissues of animals with DiYF but not WT BM regardless of the host tissue genotype (Fig. 6 C). To ensure that the discrepancy between apparent quantities of tumor resident GFP+CXCR4+ cells was not caused by variations in systemic availability of transplant groups, FACS analysis including differential blood cell counts was used to determine the levels of circulating CXCR4+ cells as well as cell populations in the blood of WT and DiYF mice or mice with implanted tumors. Our data showed no significant differences in circulating cell populations (Fig. S4, D and E) except for those expressing CXCR4. Approximately 10% of circulating leukocytes were CXCR4+. Of these, ∼30% expressed β3 integrin (Fig. 6 D). The amount of circulating CXCR4+ cells in DiYF blood was marginally higher than in WT blood (Fig. 6 E). The presence of tumors almost doubled the amounts of CXCR4+ cells in the circulation of both WT and DiYF mice. Although the percentages of CXCR4+ cells in DiYF and WT blood were slightly different in nontumor-bearing mice, the percentage of β3-positive CXCR4+ cells in DiYF mice was significantly higher in DiYF tumor-bearing mice compared with WT tumor-bearing mice (Fig. 6 F). Analysis of cells flushed from bones of WT and DiYF mice indicated no differences in CD34+, CXCR4+, or Sca1+ cell quantities in nontumor- and tumor-bearing mice (Fig. S4 F). These data indicate that the release of BMDCs into the circulation is normal or above normal in DiYF mice and, therefore, does not account for the observed defects in BMDC recruitment to sites of neovascularization and alludes to potential retention deficiencies of recruited DiYF BMDCs.
Other parameters such as MMP-2 and MMP-9 as well as osteoclast activity are known to influence BMDC mobilization in vivo (Heissig et al., 2002; Kollet et al., 2006; Cheng et al., 2007). We found elevated levels of total and active MMP-2 and MMP-9 in BM aspirates from WT and DiYF mice bearing tumors compared with nontumor-bearing counterparts; however, there were no differences between WT and DiYF mice not bearing or bearing tumors (Fig. S5, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1). Moreover, osteoclast activity as determined by circulating levels of bone-specific tartrate-resistant acid phosphatase (TRAcP) 5b were found to be similar between WT and DiYF animals in tumor-bearing mice (Fig. S5 C). As such, the impaired accumulation of BMDCs at angiogenic sites in DiYF mice is not a consequence of defects in their release from BM.
Collectively, these data emphasize the importance of the SDF-1/CXCR4 axis in BMDC recruitment and the importance of BMDC β3 integrin function in this process. As mobilization of BMDCs is similar in WT and DiYF mice, it is easy to speculate that β3 integrin on BM cells is crucial to recruitment processes after mobilization such as transendothelial migration and retention of recruited cells.
β3 function is crucial for adhesion and endothelial transmigration of CXCR4+ cells
Many integrins are known to control cellular adhesion and transmigration through endothelium, which, in turn, controls lymphocyte homing and subsequent immune responses (Mackay, 1995). It is possible that the DiYF mutation of β3 integrins could affect adhesion of circulating BM-derived CXCR4+ cells to the endothelial lining as well as their transmigration. To investigate this possibility, CXCR4+ cells were isolated from peripheral blood of WT and DiYF mice and their ability to adhere and transmigrate through an endothelial monolayer was assessed. The presence of tumors promoted a threefold increase in the adhesion of WT CXCR4+ cells to endothelium but had no effect on the adherence of DiYF CXCR4+ cells (Fig. 7 A). Likewise, DiYF CXCR4+ cells had an impaired ability to transmigrate through the endothelial monolayer compared with WT CXCR4+ cells (Fig. 7 B). However, WT CXCR4+ cell migration was twofold that of DiYF cells without tumor and increased in the presence of tumor, whereas the presence of tumor had no notable effect on DiYF CXCR4+ cell migration. These data illustrate the importance of fully functional β3 integrin signaling in processes associated with recruitment into and retention of recruited BM-derived CXCR4+ cells at angiogenic sites.
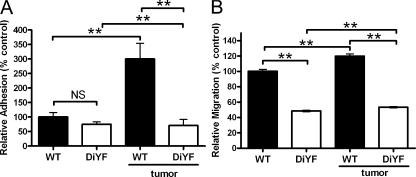
Figure 7.
Circulating CXCR4+ cells from DiYF mice exhibit impaired adhesion to and migration through endothelium. (A) Relative number of adherent CXCR4+ cells from the peripheral blood of nontumor- and B16F10 tumor-bearing WT and DiYF after plating on an endothelial monolayer. Data represent mean ± SEM and are representative of nine independent experiments. (B) Relative number of endothelial monolayer transmigrating CXCR4+ cells from the peripheral blood of nontumor- and B16F10 tumor-bearing WT and DiYF after plating on an endothelial monolayer. Data represent mean ± SEM and are representative of five independent experiments. **, P < 0.01.
Discussion
Our previous studies showed that defective β3 integrin signaling in DiYF mice resulted in inhibition of tumor growth and angiogenesis in vivo and impaired endothelial cell responses in vitro (Mahabeleshwar et al., 2006). The present study was initiated as a result of a preliminary finding wherein transplantation of WT BM completely rescued defective angiogenesis in DiYF mice. The findings presented in this paper are depicted in Fig. 8 with the key points summarized as follows. The course of tumor- and wound-associated angiogenic responses are dependent on BM cell β3 integrin function and not resident host tissue. Defects in platelet function stemming from the DiYF mutation are not causative of the angiogenic defects displayed by DiYF mice. The recruitment of DiYF BMDCs to sites of neovascularization is compromised compared with that of WT BMDCs. The majority of recruited BMDCs are negative for endothelial or smooth muscle cell markers, but appear to express CD45, Gr1, CD3, and CXCR4, indicating the hematopoietic origin of these cells. SDF-1 levels are reduced in tumors grown in DiYF mice compared with WT, a condition corrected by BMT. Impaired β3 integrin activity has little effect on the process of BMDC release into the circulation; however, DiYF CXCR4+ BMDCs exhibit defective adhesion and transmigration through endothelial monolayers compared with WT counterparts. These data indicate that β3 integrin activity on circulating CXCR4+ BMDCs, but not β3 integrin on resident endothelial cells or platelets, plays a governing role in angiogenesis through regulating BMDC recruitment to angiogenic sites and adherence to and transmigration through endothelium.
Figure 8.
Model depicting steps in the angiogenic process affected by defective β3 integrin as exemplified by the DiYF mouse. Although impaired β3 function in the DiYF mouse does not affect the mobilization of BMDCs from the BM niche, it weakens adhesion and decreases migration functions, which, in turn, reduce the overall recruitment and retention of BMDCs, resulting in elevated levels of circulating CXCR4+ BMDCs. This leads to dampened vascular sprouting and an inhibition of angiogenesis. Defective endothelial cell function in DiYF mice appears to play a recessive role in this process. Low levels of SDF-1, a result of this angiogenic defect, further potentiate the reduced recruitment and retention of CXCR4+ cells.
A definitive role for β3 integrin in the regulation of angiogenesis remains elusive. Conflicting data regarding β3 integrin's role in the development of neovasculature have been reported. Although αvβ3 inhibitors define a proangiogenic function (Brooks et al., 1994a,b), studies using β3 knockout mice suggest negative regulatory angiogenic properties (Reynolds et al., 2002). Interestingly, increased tumor vascularization and tumor growth in β3-null mice could be reversed by marrow repopulation with WT donor marrow (Taverna et al., 2004), thus implicating BM as a key factor in the neovascularization processes. Although the impaired angiogenesis exhibited by DiYF knockin mice alludes to a proangiogenic role for β3 integrin in angiogenic responses similar to that described using inhibitors of αvβ3, this impairment can be rescued through BMT with WT marrow, a response mirrored in β3 knockout mice.
Thus, results of both β3 knockin and knockout models suggest pathological angiogenesis is dependent on the function of this integrin on BM cells rather than residential endothelium. Because both knockout and knockin of β3 integrin are known to affect the function of platelet αIIbβ3, an obvious explanation for the results of BMT studies would be the regulatory role of platelet αIIbβ3 in angiogenesis. Indeed, platelets were recently shown to regulate pathological vascularization in certain models (Brill et al., 2004; Italiano et al., 2007). In addition, thrombocytopenia, as well as the inhibition of platelet aggregation by a highly specific αIIbβ3 integrin antagonist, resulted in a reduction of retinal neovascularization in a hypoxia-induced mouse model of angiogenesis (Rhee et al., 2004). Considering these data, impaired platelet functions of DiYF mice should elicit angiogenic defects and stunted tumor growth, both of which should be capable of being abrogated or reduced through the depletion of circulating platelets. Platelet depletion reduced the angiogenic response in both WT and DiYF mice; however, the absence of circulating platelets failed to normalize the angiogenic and tumor growth differences between WT and DiYF mice. Thus, it appears that platelets are not solely responsible for the effects of BMT. This finding is in agreement with prior work suggesting that αIIbβ3 inhibitors fail to modify blood vessel development or platelet–tumor interactions in vitro (Brill et al., 2004; Kisucka et al., 2006). Therefore, in DiYF mice there is a functional defect in other types of BMDCs that substantially contribute to the angiogenic phenotype.
Our results using BMT of GFP-expressing WT and DiYF mice revealed recruitment deficiencies of DiYF BMDCs in comparison to WT BMDCs. Several recent studies have demonstrated stimulatory roles for BMDCs in angiogenic processes (De Palma et al., 2005; Orimo et al., 2005; Grunewald et al., 2006), potentially explaining the overall reduced angiogenic response observed in DiYF mice. These reports define distinctly different roles of BMDCs in angiogenesis with some purporting direct incorporation of BMDCs into developing vasculature (Lyden et al., 2001; Peters et al., 2005), whereas others illustrate a supporting, paracrine role of these BMDCs (De Palma et al., 2005; Grunewald et al., 2006). In our model, BMDCs were found to be adjacent to endothelial cells or to SMA-positive pericytes and outside of the laminin basement membrane, similar to the reports of others (Ziegelhoeffer et al., 2004). With little to no recruited BMDCs displaying endothelial or pericyte characteristics, results from our model suggest a more supporting, paracrine-like function of BMDCs in angiogenesis. At the same time, we observed that the majority of recruited BMDCs appear to be of hematopoietic origin as they express CD45, F4/80, CD3, and Gr1. Similar papers by others emphasize the role of hematopoietic cells in angiogenic in vivo models (Ziegelhoeffer et al., 2004; Grunewald et al., 2006; Jin et al., 2006). In addition, we, along with others (Grunewald et al., 2006), have found that CXCR4 appears to be a marker expressed on the vast majority of recruited BMDCs. Therefore, in our model, BMDCs have a prominent stimulatory role in neovascularization without direct incorporation into blood vessels. Moreover, the functional activity of β3 integrin on BMDCs is crucial for their recruitment.
Because BMDC recruitment to neovascularizing sites is a multistep process (Lindbom and Werr, 2002; Grunewald et al., 2006; Lamagna and Bergers, 2006; Vestweber, 2007), we assessed whether impaired activity of β3 integrin influences the process of BMDC release from the BM niche into the circulation. The latter process is known to be triggered by angiogenic factors produced by injured tissues and tumors (Hattori et al., 2002; Lindbom and Werr, 2002; Grunewald et al., 2006; Kopp et al., 2006). β3 integrin is crucial for osteoclast-mediated bone resorption (Zhao et al., 2005), which, in turn, contributes to the release of cells from the BM niche into blood (Kollet et al., 2006). However, similar to the experiments by Zhao et al. (2005), we observed no major differences in bone resorptive function of tumor-bearing WT and DiYF mice, indicating that the DiYF mutation does not impair β3 integrin activity on osteoclasts. Thus, even though DiYF mice exhibited higher numbers of circulating CXCR4+ BMDCs compared with WT, it appears that β3 integrin activity affects not the release of CXCR4+ cells from BM, but rather their recruitment from the circulation into tissues. Vascular walls present a barrier to circulating cells for relocating from the blood stream to sites of angiogenesis. The recruitment of BMDCs into surrounding tissues involves sequential interactions with endothelium and extravascular tissue components (Lindbom and Werr, 2002; Schenkel et al., 2004; Millan et al., 2006). First, transmigrating BMDCs need to establish transient and dynamic adhesive contacts with endothelium. Previous studies have shown that αvβ3 integrin serves as a heterotypic ligand for CD31 (Buckley et al., 1996), a molecular marker for endothelial cells, and controls the arrest and extravasation of hematopoietic cells (Lindbom and Werr, 2002). Moreover, αvβ3 integrin on monocytes, neutrophils, and other leukocyte subsets plays a key role in the transition between tight adhesion to the VE and subsequent diapedesis (Weerasinghe et al., 1998; Lindbom and Werr, 2002). Our previous study demonstrated that the DiYF mutation impairs adhesion and migration of endothelial cells (Mahabeleshwar et al., 2006). In this study, we found that this mutation affected the ability of BM-derived CXCR4+ cells to adhere to and transmigrate through an endothelial monolayer. Therefore, functional activity of β3 integrin is crucial for the adhesion and transmigration of BMDCs at sites of neovascularization, which, in turn, is crucial for the angiogenesis-dependent responses of tumor growth and wound healing.
Angiogenic factors released by peripheral tissues promote mobilization of BMDCs, especially CXCR4+ cells from the BM niche (Hattori et al., 2002; Hiratsuka et al., 2002; Grunewald et al., 2006; Kopp et al., 2006). Indeed, our data showed higher CXCR4+ cell numbers in the circulating blood of both tumor-bearing WT and DiYF mice compared with nontumor-bearing mice. SDF-1, along with its receptor CXCR4, is a key chemoattractant for hematopoietic progenitor/stem cell mobilization, trafficking, recruitment, and retention (Petit et al., 2007). As perivascular cells, these recruited CXCR4+ hematopoietic cells promote and stabilize neovessels by releasing angiogenic factors and providing physical support (Petit et al., 2007). In fact, defective vascularization was noted in SDF-1 or CXCR4 knockout embryos (Ara et al., 2005). In our model, all of the vasculature-associated BMDCs were CXCR4 positive, indicating that these cells were recruited by its ligand, SDF-1. Elevated plasma SDF-1 levels stimulates CXCR4+ BMDC mobilization (Hattori et al., 2001; Heissig et al., 2002). We did not find any notable differences in plasma SDF-1 levels in WT or DiYF mice. Other factors associated with BMDC mobilization and osteoclast function, such as BM resident MMP-2 and MMP-9 or serum TRAcP levels, were also found to be similar in WT and DiYF mice. However, increased blood CXCR4+ or CXCR4+β3+ cell numbers were observed in DiYF or tumor-bearing DiYF mice compared with WT or tumor-bearing WT mice. Therefore, decreased recruitment of BMDCs into tissues and defective adhesive and migratory capacities and hence retention of DiYF CXCR4+ BMDCs were causal in the increased levels of circulating CXCR4+ or CXCR4+β3+ cells in β3 integrin–defective DiYF or tumor-bearing DiYF mice. Interestingly, we found that the levels of SDF-1 in tumors from DiYF mice were lower than tumor-bearing WT mice. The major source of SDF-1 in peripheral tissues are VE cells, epithelial cells, stromal fibroblasts, and SMA-positive myofibroblasts (Fedyk et al., 2001; Orimo et al., 2005; Ruiz et al., 2006). Thus, in our model, defective recruitment of BMDCs and impaired angiogenesis were accompanied by reduced SDF-1 expression, which, in turn, further augmented the angiogenic defect in DiYF mice. Transplantation of WT BM restored these defects, including SDF-1 levels, in DiYF mice.
In sum, this study establishes a new role for β3 integrin in the regulation of angiogenesis, one that may influence not only the interpretations of numerous preclinical studies using αvβ3 inhibitors but also the development of new anti- as well as proangiogenic strategies.
Materials and methods
Mice
8–12-wk-old WT or DiYF mice were on a C57BL/6 background as described previously (Law et al., 1999). WT/GFP mice were purchased from The Jackson Laboratory. DiYF/GFP mice were bred from DiYF and GFP heterozygote mice. All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Subcutaneous tumor and wound healing models
Mice were injected subcutaneously with 106 B16F10 melanoma or RM1 prostate tumor cells (Chen et al., 2005). Tumors were harvested 9 (RM1 in WT and DiYF mice), 11 (B16F10 and RM1 in BMT WT and DiYF mice), or 13 d (B16F10 in BMT WT and DiYF mice) after implantation, weighed, and processed for histological and immunohistochemical staining.
A back punch wound healing model was used as described elsewhere (Hoffman et al., 2006). Digital photographs were taken at the time of injury and on days 3, 7, 10, and 13 thereafter. Pictures were taken using a microscope (SMZ 1000; Nikon) equipped with a digital camera (DXM1200F; Nikon). The wound area was analyzed by tracing the wound margin from images and calculating pixel area with ImagePro Plus software (v5.1; Media Cybernetics). Wound healing area was calculated as a percentage of area of the original wound.
For BMT studies, mice (recipients) were lethally irradiated (9 Gy) followed by BM reconstitution by tail vein injection with 107 BM cells isolated from the donor femurs. 8 wk after BMT, mice were used for tumor or wound healing experiments.
Platelet depletion and tumor implantation
Mice were injected subcutaneously with 106 B16F10 tumor cells and then randomly grouped into two groups. 30 min after tumor implantation, the experimental group received an intravenous infusion of rat anti-mouse GPIbα (Emfret Analytics), whereas the control group received rat IgG (both at 2 μg/g of body weight) in 200 μl of sterile PBS. Injections of antibodies were repeated on days 3 and 6 to maintain low platelet counts. Platelet numbers in circulating blood were monitored using tail vein blood. Tumors were removed and analyzed on day 9.
Murine SDF-1 immunoassay
SDF-1α content in mouse plasma or tumor lysate was determined by an SDF-1 ELISA kit (R&D Systems). ELISA was performed using ELISA Kinetic microplate reader (MDS Analytical Technologies). Total protein content was quantified by Bradford assay to equalize protein loading.
Flow cytometry
Erythrocytes were removed from blood samples using a mouse erythrocyte lysing kit (R&D Systems). The remaining cell population (106) was stained with phycoerythrin-conjugated rat anti-mouse CXCR4 antibody (R&D Systems) to determine CXCR4+ cell quantities. Alternatively, double staining was performed with phycoerythrin-conjugated anti-mouse CXCR4 and rabbit anti-human β3 integrin as primary antibodies (Santa Cruz Biotechnology, Inc.) and Alexa Fluor 488–conjugated goat anti–rabbit IgG as the secondary antibody. Cells were permeabilized with 0.2% Triton X-100 for 5 min before β3 integrin staining. FACS analyses were performed using FACSCalibur and data were analyzed using CellQuest Software (version 3.8; BD).
Adhesion and transmigration of BMDCs
Peripheral blood cells were isolated and stained with a phycoerythrin-conjugated anti-mouse CXCR4 antibody and sorted with a FACSVantage SE station (BD). CXCR4+ cells were collected and further stained with FAST DiI (Invitrogen). Endothelial cells were isolated from WT mouse lung microvasculature as described previously (Mahabeleshwar et al., 2006) and cultured as monolayers on fibronectin-coated plates or in the upper chamber of a Boyden-type migration chamber. DiI-stained CXCR4+ BMDCs (104) were added on top of endothelial monolayers and incubated for 1 (adhesion) or 18 h (transmigration) at 37°C in humidified 5% CO2 air. Dishes of adherent cells were fixed with 4% paraformaldehyde, washed, and photographed with an inverted fluorescence microscope (DMIRB; Leica). Numbers of DiI-positive attached and spread cells on the endothelial cell monolayer were counted. Alternatively, transmigrated cells in the lower chamber of the migration chamber were collected and counted with an inverted fluorescent microscope equipped with a camera (Retiga EXi; QImaging).
Histology and immunohistochemistry
Tissues were fixed with 4% paraformaldehyde and embedded in paraffin or in OCT freezing medium. Histological and immunohistochemical staining was performed using standard techniques as described previously (Chen et al., 2005). The following primary antibodies were used: rat anti-mouse CD31 (Research Diagnostics Inc.), rat anti-mouse F4/80 (AbD Serotec), rat anti-mouse Gr-1 (BD), Cy3-α–SMA (Sigma-Aldrich), rat anti-mouse CXCR4 (R&D Systems), rabbit anti-NG2 (Millipore), rabbit anti-GFP (AnaSpec, Inc.), rabbit anti-laminin (Sigma-Aldrich), rat anti-mouse CD45 (BD), rabbit anti-goat VE-Cadherin (Santa Cruz Biotechnology, Inc.), and rat anti-mouse CD3 (eBioscience). All antibodies were used at a 1:100 dilution except for rabbit anti-GFP (1:500) and rabbit anti-laminin (1:1,000). Chromogenic visualization followed the Vectastain ABC kit protocol (Vector Laboratories). Secondary antibodies fluorescently labeled with Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) were used for immunofluorescence at a 1:200 dilution. All the slides were mounted with Vectashield mounting medium with DAPI (Vector Laboratories). Specimens were photographed using a research microscope system (DMR; Leica) or a laser-scanning confocal microscope (DMRXE TCS SP2; Leica), equipped with a camera (Retiga EXi). Quantitative analysis of the positively stained area or density was performed using ImagePro Plus software as described previously (Chen et al., 2005).
Western blot analysis
BM cells were isolated by flushing the femurs and tibiae of WT and DiYF mice with ice-cold PBS. Cells were collected by centrifugation at 2,000 rpm for 5 min and lysed in buffer composed of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM iodoacetamide, 2 mM phenylmethylsulfonyl fluoride, 2 mM EDTA, 10 mM NaF, 10 mM Na2P2O7, 10 μg/ml leupeptin, 4 μg/ml pepstatin, and 0.1 U/ml aprotinin. Cell lysates were cleared and supernatants were assayed for protein concentration using the Bradford protein assay method (Bio-Rad Laboratories). Cell lysates were denatured using Laemmli sample buffer, and proteins were separated by SDS-PAGE and then probed with the indicated antibody. Where appropriate, nitrocellulose membranes were stripped and blotted with anti-actin antibody as a loading control. Antibodies used were anti–MMP-2 and –MMP-9 (Santa Cruz Biotechnology, Inc.) and anti–β-actin (Sigma-Aldrich).
Measurement of MMP-2, MMP-9, and osteoclast activity
BM resident MMP-2 and MMP-9 levels were assessed using BM aspirate lysates from nontumor- and tumor-bearing WT and DiYF mice. Protein content and assay volumes were equalized before using the measurement kit (R&D Systems). Osteoclast activity was measured by quantification of TRAcP 5b in the serum of tumor-bearing WT and DiYF mice using a commercially available ELISA kit (Immunodiagnostic Systems, Inc).
Statistical analysis
The statistical difference between groups was determined using a two-tailed Student's t test or analysis of variance. P-values of 0.05 or less were regarded as statistically significant.
Online supplemental material
Fig. S1 shows that decreased angiogenesis in DiYF mice is caused by impaired integrin β3 activation in BMDCs. Fig. S2 shows that infiltration of BMDCs is integrin β3 dependent. Fig. S3 shows the localization of F4/80-positive macrophages in a B16F10 tumor implanted in WT and DiYF mice transplanted with GFP-BM. Fig. S4 shows the SDF-1 levels in the plasma from WT and DiYF mice. Fig. S4 also shows the number of different types of circulating cells in blood in WT and DiYF mice. Fig. S5 shows MMP-2 and MMP-9 contents in BM in WT and DiYF mice with or without B16F10 tumors. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200802179/DC1.
Supplementary Material
Acknowledgments
We acknowledge financial support from the National Institutes of Health (grants HL071625, CA126847, and HL073311).
The authors have no conflicting financial interests.
W. Feng's present address is The First Affiliated Hospital, School of Medicine, Xi'an Jiaotong University, Xi'an, Shaanxi 710061, China.
Abbreviations used in this paper: BM, bone marrow; BMDC, BM-derived cell; BMT, BM transplantation; NG2, neuro/glial cell 2 chondroitin proteoglycan; SDF-1, stromal derived factor-1; SMA, smooth muscle actin; TRAcP, tartrate-resistant acid phosphatase; VE, vascular endothelium; WT, wild type.
References
- Ara, T., K. Tokoyoda, R. Okamoto, P.A. Koni, and T. Nagasawa. 2005. The role of CXCL12 in the organ-specific process of artery formation. Blood. 105:3155–3161. [DOI] [PubMed] [Google Scholar]
- Blystone, S.D., M.P. Williams, S.E. Slater, and E.J. Brown. 1997. Requirement of integrin beta3 tyrosine 747 for beta3 tyrosine phosphorylation and regulation of alphavbeta3 avidity. J. Biol. Chem. 272:28757–28761. [DOI] [PubMed] [Google Scholar]
- Brill, A., H. Elinav, and D. Varon. 2004. Differential role of platelet granular mediators in angiogenesis. Cardiovasc. Res. 63:226–235. [DOI] [PubMed] [Google Scholar]
- Brooks, P.C., R.A. Clark, and D.A. Cheresh. 1994. a. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 264:569–571. [DOI] [PubMed] [Google Scholar]
- Brooks, P.C., A.M. Montgomery, M. Rosenfeld, R.A. Reisfeld, T. Hu, G. Klier, and D.A. Cheresh. 1994. b. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 79:1157–1164. [DOI] [PubMed] [Google Scholar]
- Buckley, C.D., R. Doyonnas, J.P. Newton, S.D. Blystone, E.J. Brown, S.M. Watt, and D.L. Simmons. 1996. Identification of alpha v beta 3 as a heterotypic ligand for CD31/PECAM-1. J. Cell Sci. 109:437–445. [DOI] [PubMed] [Google Scholar]
- Burger, J.A., and T.J. Kipps. 2006. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 107:1761–1767. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P. 2002. Integrin indecision. Nat. Med. 8:14–16. [DOI] [PubMed] [Google Scholar]
- Chandhoke, S.K., M. Williams, E. Schaefer, L. Zorn, and S.D. Blystone. 2004. Beta 3 integrin phosphorylation is essential for Arp3 organization into leukocyte alpha V beta 3-vitronectin adhesion contacts. J. Cell Sci. 117:1431–1441. [DOI] [PubMed] [Google Scholar]
- Chen, J., P.R. Somanath, O. Razorenova, W.S. Chen, N. Hay, P. Bornstein, and T.V. Byzova. 2005. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 11:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X.W., M. Kuzuya, K. Nakamura, K. Maeda, M. Tsuzuki, W. Kim, T. Sasaki, Z. Liu, N. Inoue, T. Kondo, et al. 2007. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ. Res. 100:904–913. [DOI] [PubMed] [Google Scholar]
- De Nichilo, M.O., and G.F. Burns. 1993. Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate alpha v integrin expression on cultured human macrophages. Proc. Natl. Acad. Sci. USA. 90:2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma, M., M.A. Venneri, R. Galli, S.L. Sergi, L.S. Politi, M. Sampaolesi, and L. Naldini. 2005. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 8:211–226. [DOI] [PubMed] [Google Scholar]
- Eliceiri, B.P., and D.A. Cheresh. 1999. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J. Clin. Invest. 103:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri, B.P., and D.A. Cheresh. 2001. Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 13:563–568. [DOI] [PubMed] [Google Scholar]
- Epstein, R.J. 2004. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat. Rev. Cancer. 4:901–909. [DOI] [PubMed] [Google Scholar]
- Fedyk, E.R., D. Jones, H.O. Critchley, R.P. Phipps, T.M. Blieden, and T.A. Springer. 2001. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J. Immunol. 166:5749–5754. [DOI] [PubMed] [Google Scholar]
- Grunewald, M., I. Avraham, Y. Dor, E. Bachar-Lustig, A. Itin, S. Jung, S. Chimenti, L. Landsman, R. Abramovitch, and E. Keshet. 2006. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 124:175–189. [DOI] [PubMed] [Google Scholar]
- Hattori, K., B. Heissig, K. Tashiro, T. Honjo, M. Tateno, J.H. Shieh, N.R. Hackett, M.S. Quitoriano, R.G. Crystal, S. Rafii, and M.A. Moore. 2001. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 97:3354–3360. [DOI] [PubMed] [Google Scholar]
- Hattori, K., B. Heissig, Y. Wu, S. Dias, R. Tejada, B. Ferris, D.J. Hicklin, Z. Zhu, P. Bohlen, L. Witte, et al. 2002. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat. Med. 8:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig, B., K. Hattori, S. Dias, M. Friedrich, B. Ferris, N.R. Hackett, R.G. Crystal, P. Besmer, D. Lyden, M.A. Moore, et al. 2002. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 109:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka, S., K. Nakamura, S. Iwai, M. Murakami, T. Itoh, H. Kijima, J.M. Shipley, R.M. Senior, and M. Shibuya. 2002. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2:289–300. [DOI] [PubMed] [Google Scholar]
- Hoffman, M., A. Harger, A. Lenkowski, U. Hedner, H.R. Roberts, and D.M. Monroe. 2006. Cutaneous wound healing is impaired in hemophilia B. Blood. 108:3053–3060. [DOI] [PubMed] [Google Scholar]
- Italiano, J.E., Jr., J.L. Richardson, S. Patel-Hett, E. Battinelli, A. Zaslavsky, S. Short, S. Ryeom, J. Folkman, and G.L. Klement. 2007. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentialy released. Blood. 111:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, D.K., K. Shido, H.G. Kopp, I. Petit, S.V. Shmelkov, L.M. Young, A.T. Hooper, H. Amano, S.T. Avecilla, B. Heissig, et al. 2006. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat. Med. 12:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori, M., T. Kawaguchi, M.S. Berger, and R.O. Pieper. 2006. Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growth and angiogenesis. J. Biol. Chem. 281:37256–37264. [DOI] [PubMed] [Google Scholar]
- Khakoo, A.Y., and T. Finkel. 2005. Endothelial progenitor cells. Annu. Rev. Med. 56:79–101. [DOI] [PubMed] [Google Scholar]
- Kisucka, J., C.E. Butterfield, D.G. Duda, S.C. Eichenberger, S. Saffaripour, J. Ware, Z.M. Ruggeri, R.K. Jain, J. Folkman, and D.D. Wagner. 2006. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. USA. 103:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet, O., A. Dar, S. Shivtiel, A. Kalinkovich, K. Lapid, Y. Sztainberg, M. Tesio, R.M. Samstein, P. Goichberg, A. Spiegel, et al. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12:657–664. [DOI] [PubMed] [Google Scholar]
- Kopp, H.G., C.A. Ramos, and S. Rafii. 2006. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr. Opin. Hematol. 13:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia, M., R. Reca, K. Miekus, J. Wanzeck, W. Wojakowski, A. Janowska-Wieczorek, J. Ratajczak, and M.Z. Ratajczak. 2005. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 23:879–894. [DOI] [PubMed] [Google Scholar]
- Lamagna, C., and G. Bergers. 2006. The bone marrow constitutes a reservoir of pericyte progenitors. J. Leukoc. Biol. 80:677–681. [DOI] [PubMed] [Google Scholar]
- Law, D.A., F.R. DeGuzman, P. Heiser, K. Ministri-Madrid, N. Killeen, and D.R. Phillips. 1999. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 401:808–811. [DOI] [PubMed] [Google Scholar]
- Lindbom, L., and J. Werr. 2002. Integrin-dependent neutrophil migration in extravascular tissue. Semin. Immunol. 14:115–121. [DOI] [PubMed] [Google Scholar]
- Lyden, D., K. Hattori, S. Dias, C. Costa, P. Blaikie, L. Butros, A. Chadburn, B. Heissig, W. Marks, L. Witte, et al. 2001. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7:1194–1201. [DOI] [PubMed] [Google Scholar]
- Mackay, C. 1995. Lymphocyte migration. A new spin on lymphocyte homing. Curr. Biol. 5:733–736. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar, G.H., W. Feng, D.R. Phillips, and T.V. Byzova. 2006. Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 203:2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar, G.H., W. Feng, K. Reddy, E.F. Plow, and T.V. Byzova. 2007. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 101:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan, J., L. Hewlett, M. Glyn, D. Toomre, P. Clark, and A.J. Ridley. 2006. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8:113–123. [DOI] [PubMed] [Google Scholar]
- Orimo, A., P.B. Gupta, D.C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V.J. Carey, A.L. Richardson, and R.A. Weinberg. 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. [DOI] [PubMed] [Google Scholar]
- Peters, B.A., L.A. Diaz, K. Polyak, L. Meszler, K. Romans, E.C. Guinan, J.H. Antin, D. Myerson, S.R. Hamilton, B. Vogelstein, et al. 2005. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 11:261–262. [DOI] [PubMed] [Google Scholar]
- Petit, I., D. Jin, and S. Rafii. 2007. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 28:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen, S., J. Palm, D. Rossi, N. Kaskenpaa, I. Rajantie, S. Yla-Herttuala, K. Alitalo, I.L. Weissman, and P. Salven. 2008. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl. Acad. Sci. USA. 105:6620–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, L.E., L. Wyder, J.C. Lively, D. Taverna, S.D. Robinson, X. Huang, D. Sheppard, R.O. Hynes, and K.M. Hodivala-Dilke. 2002. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8:27–34. [DOI] [PubMed] [Google Scholar]
- Reynolds, A.R., L.E. Reynolds, T.E. Nagel, J.C. Lively, S.D. Robinson, D.J. Hicklin, S.C. Bodary, and K.M. Hodivala-Dilke. 2004. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 64:8643–8650. [DOI] [PubMed] [Google Scholar]
- Rhee, J.S., M. Black, U. Schubert, S. Fischer, E. Morgenstern, H.P. Hammes, and K.T. Preissner. 2004. The functional role of blood platelet components in angiogenesis. Thromb. Haemost. 92:394–402. [DOI] [PubMed] [Google Scholar]
- Ruiz, d.A.C., A. Luttun, and P. Carmeliet. 2006. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 124:18–21. [DOI] [PubMed] [Google Scholar]
- Schenkel, A.R., Z. Mamdouh, and W.A. Muller. 2004. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5:393–400. [DOI] [PubMed] [Google Scholar]
- Shojaei, F., X. Wu, A.K. Malik, C. Zhong, M.E. Baldwin, S. Schanz, G. Fuh, H.P. Gerber, and N. Ferrara. 2007. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25:911–920. [DOI] [PubMed] [Google Scholar]
- Shojaei, F., C. Zhong, X. Wu, L. Yu, and N. Ferrara. 2008. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 18:372–378. [DOI] [PubMed] [Google Scholar]
- Taverna, D., H. Moher, D. Crowley, L. Borsig, A. Varki, and R.O. Hynes. 2004. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc. Natl. Acad. Sci. USA. 101:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto, T., M. Yamato, Y. Shiratsuchi, M. Terasawa, J. Yang, K. Nishida, Y. Kobayashi, and T. Okano. 2006. Expression of Integrin beta3 is correlated to the properties of quiescent hemopoietic stem cells possessing the side population phenotype. J. Immunol. 177:7733–7739. [DOI] [PubMed] [Google Scholar]
- Vestweber, D. 2007. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 218:178–196. [DOI] [PubMed] [Google Scholar]
- Weerasinghe, D., K.P. McHugh, F.P. Ross, E.J. Brown, R.H. Gisler, and B.A. Imhof. 1998. A role for the αvβ3 integrin in the transmigration of monocytes. J. Cell Biol. 142:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis, S.M., J.N. Lindquist, L.A. Barnes, K.M. Lutu-Fuga, J. Cui, M.R. Wood, and D.A. Cheresh. 2007. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 109:1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L., L.M. DeBusk, K. Fukuda, B. Fingleton, B. Green-Jarvis, Y. Shyr, L.M. Matrisian, D.P. Carbone, and P.C. Lin. 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 6:409–421. [DOI] [PubMed] [Google Scholar]
- Zhao, H., H. Kitaura, M.S. Sands, F.P. Ross, S.L. Teitelbaum, and D.V. Novack. 2005. Critical role of beta3 integrin in experimental postmenopausal osteoporosis. J. Bone Miner. Res. 20:2116–2123. [DOI] [PubMed] [Google Scholar]
- Ziegelhoeffer, T., B. Fernandez, S. Kostin, M. Heil, R. Voswinckel, A. Helisch, and W. Schaper. 2004. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 94:230–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.