Abstract
Cell rearrangements require dynamic changes in cell–cell contacts to maintain tissue integrity. We investigated the function of Cdc42 in maintaining adherens junctions (AJs) and apical polarity in the Drosophila melanogaster neuroectodermal epithelium. About one third of cells exit the epithelium through ingression and become neuroblasts. Cdc42-compromised embryos lost AJs in the neuroectoderm during neuroblast ingression. In contrast, when neuroblast formation was suppressed, AJs were maintained despite the loss of Cdc42 function. Loss of Cdc42 function caused an increase in the endocytotic uptake of apical proteins, including apical polarity factors such as Crumbs, which are required for AJ stability. In addition, Cdc42 has a second function in regulating endocytotic trafficking, as it is required for the progression of apical cargo from the early to the late endosome. The Par complex acts as an effector for Cdc42 in controlling the endocytosis of apical proteins. This study reveals functional interactions between apical polarity proteins and endocytosis that are critical for stabilizing dynamic basolateral AJs.
Introduction
Cadherin-based adherens junctions (AJs) are critical elements of intercellular adhesion between epithelial cells. In most epithelia, AJs are organized as an apical belt, the zonula adherens (ZA), which is a component of the apical junctional complex that segregates apical from basolateral membranes in these highly polarized cells. AJs are dynamic structures and, during cell rearrangement, have to rapidly disassemble and reform to maintain ZA continuity and epithelial integrity. Several mechanisms have been proposed to regulate AJ stability, but our understanding of how AJ integrity is maintained during dynamic morphogenetic movements in vivo remains limited (Gumbiner, 2000, 2005; Bryant and Stow 2004; D'Souza-Schorey, 2005; Halbleib and Nelson, 2006).
The Drosophila melanogaster embryo assembles a ZA when the first epithelium forms (Tepass and Hartenstein, 1994; Müller and Wieschaus, 1996). The ZA is maintained in epithelia throughout morphogenesis, during which frequent changes in cell–cell contacts occur. One example is the ventral neuroectoderm, which is an epithelial layer that gives rise to neural and epidermal progenitor cells. About one third of the cells of the neuroectodermal epithelium ingress as individual cells and form neural progenitors (neuroblasts or neural stem cells), whereas the remaining cells retain epithelial character and differentiate into epidermis (Campos-Ortega and Hartenstein, 1997). Zygotic expression of Drosophila epithelial cadherin (DEcad), the major adhesion molecule at the ZA in the Drosophila embryo, is required to maintain AJs in the neuroectoderm, whereas maternal expression of DEcad is sufficient to maintain the ZA in other epithelia that do not undergo cell rearrangements, such as the dorsal ectoderm. Moreover, blocking neuroblast specification and, thus, neuroblast ingression ameliorates the requirement of zygotic DEcad expression to support the integrity of neuroectodermal AJs (Tepass et al., 1996; Uemura et al., 1996). These observations raise the question as to whether specific mechanisms are used to support AJ stability in the neuroectoderm.
Studies in mammalian cell culture have pointed to the GTPases of the Rho family, Rho, Rac, and Cdc42, as one group of AJ regulators (Fukata and Kaibuchi, 2001; Van Aelst and Symons, 2002; Braga and Yap, 2005). Also, the analysis of Rho GTPases in Drosophila suggests that Rho1 is a critical regulator of AJ stability (Bloor and Kiehart, 2002; Fox et al., 2005) and that Cdc42 impacts on AJs through its role as a component of the Par complex that controls epithelial polarity, including ZA formation in early embryos (Hutterer et al., 2004; Macara, 2004). Cdc42 is a regulator of cell polarity in many systems, including yeast, the Caenorhabditis elegans zygote, Drosophila neuroblasts, and in migrating cells (Atwood et al., 2007; Goldstein and Macara, 2007). Cdc42 activates the apical Par complex by binding to Par6. Par6, in turn, recruits atypical PKC (aPKC), which phosphorylates targets such as the polarity proteins Lethal giant larvae or Crumbs (Crb) to promote the formation of the apical membrane and the ZA (Hutterer et al., 2004; Sotillos et al., 2004). Cdc42 may also act through its effector Wiskott-Aldrich syndrome protein (Wasp) to control junction-associated actin (Otani et al., 2006) or may contribute to epithelial organization by regulating vesicle trafficking (for reviews see Cerione, 2004; Ridley 2006).
In this study, we address the function of Cdc42 in promoting the stability of dynamic AJs in the Drosophila neuroectoderm. Our findings suggest that Cdc42 acts through its effector, the Par complex, to modify apical endocytosis at two stages: Cdc42 prevents the endocytotic uptake of apical proteins from the plasma membrane, and it promotes the processing of apical proteins from the early to the late endosomal compartment.
Results
Cdc42 is essential for maintaining AJs in the Drosophila neuroectoderm
To address the function of Rho GTPases in AJ formation and maintenance in vivo, we have expressed dominant-negative (DN) forms of Rho1, Rac1, and Cdc42 in the Drosophila embryo using the Gal4/upstream activation sequence (UAS) system and the ubiquitous driver line da-Gal4. We found that expression of Rho1-DN led to a rather general disruption of AJs, confirming the previous work of others (Bloor and Kiehart, 2002). Rac1-DN showed only minor defects in AJ integrity, which were confined to the vicinity of the ventral midline (unpublished data). Interestingly, expression of Cdc42-DN showed a strong disruption of AJs in the ventral ectoderm. In contrast, AJs in other parts of the ectoderm, the ventral midline, and the dorsal ectoderm remained largely unaffected in these embryos (Fig. 1, A and B). We also expressed constitutively active (CA) forms of all three GTPases in embryos and found that AJs became severely disrupted throughout the ectoderm where they were organized in irregular large clusters (unpublished data). Because of the intriguing differential requirement of Cdc42 in maintaining AJs, we concentrated our further analysis on this GTPase.
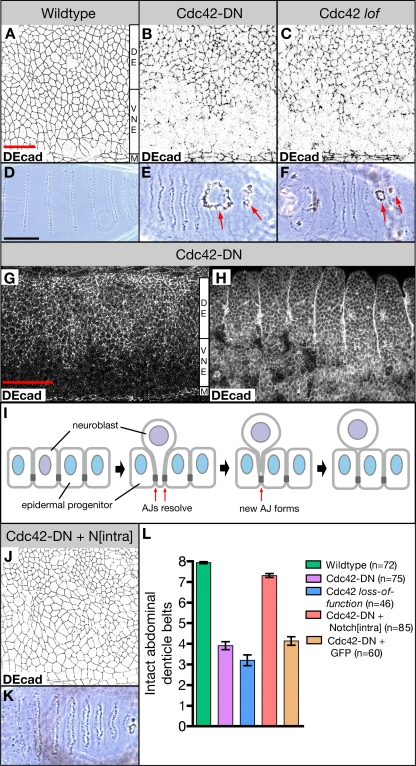
Figure 1.
Ventral ectodermal defects in embryos overexpressing Cdc42-DN and Cdc42 loss-of-function embryos. (A–C) Wild-type embryo (A), embryo overexpressing Cdc42-DN under the control of da-Gal4 (Cdc42-DN; B), and embryo produced by a Cdc423/Cdc426 female (Cdc42 lof; C) labeled for the AJ marker DEcad. (D–F) Ventral cuticle of wild-type embryo (D), Cdc42-DN embryo (E), and Cdc42 loss-of-function embryo (F). Arrows indicate holes on the ventral surface. (G and H) Cdc42-DN embryos labeled for DEcad at stages 11 (G) and 14 (H). (I) Illustration of neuroblast ingression. As a neuroblast (purple) begins to ingress from the ventral neuroectoderm, existing AJs (dark gray) at the neuroblast/epidermal progenitor (blue) boundaries resolve. New AJs form between neighboring epidermal cells. (J and K) DEcad stain (J) and ventral cuticle (K) of embryos expressing both Cdc42-DN and Nintra under the control of da-Gal4. (L) The extent of ventral cuticle defects was quantified by counting the number of intact abdominal denticle belts (mean ± SEM [error bars]). The difference in the number of intact belts is highly significant (P < 0.001) for Cdc42-DN embryos versus wild type, Cdc42 loss-of-function embryos versus wild type, and Cdc42-DN Nintra embryos versus Cdc42-DN embryos. Coexpression of GFP with Cdc42-DN under the control of da-Gal4 did not affect the severity of ventral cuticle defects caused by Cdc42-DN. M, ventral midline; VNE, ventral neuroectoderm; DE, dorsal ectoderm. Bars: (A–C and J) 20 μm; (D–F and K) 100 μm; (G and H) 50 μm.
AJ defects became apparent in da-Gal4 UAS-Cdc42-DN (da>Cdc42-DN) embryos at stage 9 and are most prominent at stage 11 when AJs are compromised in the entire ventral ectoderm, the neuroectoderm, excluding the ventral midline cells (Fig. 1, A, B, and G). About one third of the epithelial cells ingress from the neuroectoderm to become neuroblasts during stages 8–11 (Campos-Ortega and Hartenstein, 1997). At later stages of development of da>Cdc42-DN embryos, AJs are reestablished in large areas of the ventral ectoderm, whereas cells in other ventral regions lose epithelial integrity and degenerate, causing the formation of holes in the ventral epidermis (Fig. 1 H). In contrast, AJs in the dorsal ectoderm and the ventral midline show only minor defects in da>Cdc42-DN embryos. Preparations of the cuticle of da>Cdc42-DN embryos show holes in the ventral cuticle that affect ∼50% of the ventral region (Fig. 1, E and L). To quantify the strength of defects in the ventral epidermis, we counted the number of intact ventral abdominal denticle belts (Fig. 1 L). In contrast to wild-type embryos that display eight intact denticle belts, da>Cdc42-DN embryos have only four intact denticle belts on average. In addition, these embryos display defects in head development and dorsal closure. Also, cdc42 mutant embryos, which have a reduced maternal cdc42 contribution (Genova et al., 2000), display holes in the ventral epidermis and show compromised AJs in the neuroectoderm similar to da>Cdc42-DN embryos (Fig. 1, C, F, and L). The similarity between the defects observed in da>Cdc42-DN embryos and cdc42 mutant embryos suggests that Cdc42-DN specifically disrupts Cdc42 function in this system. As cdc42 mutants still have some maternal cdc42 contribution and the phenotypes of cdc42 mutants and da>Cdc42-DN embryos are similar, it is unlikely that Cdc42-DN expression completely removes Cdc42 function. We were not able to reproduce the observations reported by Hutterer et al. (2004), who showed that expression of Cdc42-DN with strong maternal drivers causes morphological defects before gastrulation or that expression of Cdc42-DN causes the loss of epithelial polarity throughout the embryo. Collectively, our findings suggest a significantly higher requirement for Cdc42 function to stabilize AJs in the neuroectoderm as compared with other regions of the ectoderm.
The coincidence of neuroblast ingression and AJ breakdown in da>Cdc42-DN embryos suggests that the ingression process itself causes the higher Cdc42 requirement. The ingression of epithelial cells requires dynamic AJs to maintain epithelial continuity. A majority of neuroectodermal cells undergo AJ disassembly and reformation as ∼30% of cells of the neuroectodermal epithelium ingress (Fig. 1 I; Campos-Ortega and Hartenstein, 1997). In contrast, cell contacts remain constant in the dorsal ectoderm or the ventral midline cells during neurulation. To test for the possibility that the higher AJ turnover that results from neuroblast ingression requires higher Cdc42 activity, we suppressed neuroblast specification, and thus ingression, through the expression of a CA form of the Notch receptor (Nintra; Lieber et al., 1993; Rebay et al., 1993; Struhl et al., 1993). Notch signaling specifies epidermal progenitor fate and prevents specification of neuroblasts in the Drosophila neuroectoderm (Campos-Ortega, 1993; Artavanis-Tsakonas et al., 1999). Expression of Nintra in da>Cdc42-DN embryos effectively suppressed formation of ventral cuticle holes (Fig. 1, K and L) but not defects in head morphogenesis or dorsal closure (not depicted). Moreover, AJ integrity in the neuroectoderm of stage 11 embryos is dramatically improved in da>Nintra da>Cdc42-DN embryos compared with da>Cdc42-DN animals (Fig. 1 J). To control for the possibility that the introduction of a second UAS construct could suppress the da>Cdc42-DN phenotype, we examined da>GFP da>Cdc42-DN embryos and found that they exhibit the same defects as da>Cdc42-DN embryos (Fig. 1 L). Together, our results suggest that the reformation of AJs that takes place as a result of neuroblast ingression requires high levels of Cdc42 activity.
Cdc42-compromised neuroectodermal cells lose apical membrane–associated proteins
To further characterize the neuroectodermal defects in da>Cdc42-DN embryos, we examined additional molecular makers that highlight AJs and the apical and basolateral membrane of neuroectodermal cells. In addition to DEcad (Fig. 1), we found that the AJ markers Armadillo (Arm; Drosophila β-catenin), Echinoid (Ed), and α-catenin were also lost from the apicolateral membrane, confirming the loss of AJs (Fig. 2, A–D; and not depicted). The apical determinant and transmembrane protein Crb and its binding partner Patj, which are enriched in the marginal zone immediately apical to the AJs, were also depleted from the apical membrane of neuroectodermal cells (Fig. 2, E–G; and not depicted). As with AJ markers, we found that the apical localization of Crb was restored in da>Cdc42-DN embryos that express Nintra (Fig. 2 H).
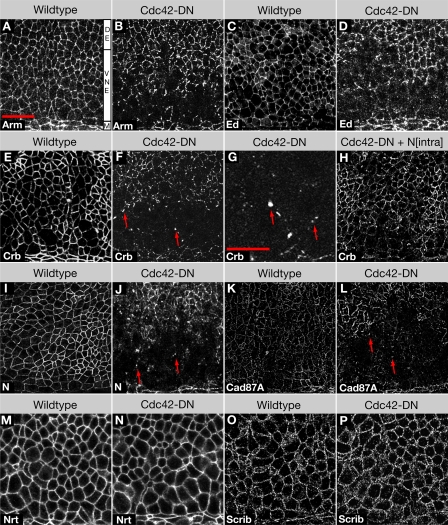
Figure 2.
Embryos expressing Cdc42-DN lose apical and AJ markers from the ventral neuroectoderm. (A and B) Wild-type embryo (A) and Cdc42-DN embryo (B) labeled for the AJ marker Arm. (C and D) Wild-type embryo (C) and Cdc42-DN embryo (D) labeled for the AJ marker Ed. (E–H) Wild-type embryo (E), Cdc42-DN embryos (F and G), and Cdc42-DN Nintra embryo (H) labeled for the apical marker Crb. Arrows indicate cytoplasmic accumulations of Crb. (I and J) Wild-type embryo (I) and Cdc42-DN embryo (J) labeled for the apical marker Notch (N). Arrows indicate cytoplasmic accumulations of Notch. (K and L) Wild-type embryo (K) and Cdc42-DN embryo (L) labeled for the apical marker Cad87A. Arrows indicate cytoplasmic accumulations of Cad87A. (M and N) Wild-type embryo (M) and Cdc42-DN embryo (N) labeled for the basolateral marker Nrt. (O and P) Wild-type embryo (O) and Cdc42-DN embryo (P) labeled for the basolateral marker Scrib. M, ventral midline; VNE, ventral neuroectoderm; DE, dorsal ectoderm. Bars: (A–F and H–P) 20 μm; (G) 10 μm.
To determine whether not only polarity proteins but also other apical proteins are down-regulated, we examined Notch and its ligand Delta, which are also enriched in the marginal zone, and the cadherin Cad87A, which is a marker of the free apical surface (Fung et al., 2008). Again, we found that all three transmembrane proteins were strongly reduced or undetectable in neuroectodermal cells in da>Cdc42-DN embryos (Fig. 2, I–L; and not depicted). Finally, we determined that basolateral proteins, the transmembrane protein Neurotactin (Nrt), and the membrane-associated cytoplasmic adaptor protein Scribble (Scrib) showed a normal distribution in the neuroectoderm of da>Cdc42-DN embryos (Fig. 2, M–P). The general loss of apical and AJ proteins is confined to the neuroectoderm, and only minor defects in the apical localization of these proteins were detected in the ventral midline cells or the dorsal ectoderm. Moreover, apical markers, similar to AJ proteins, showed a normal distribution in ventral epidermal cells that survive to later stages of development. In addition to the loss of apical and AJ proteins from the plasma membrane, we noticed that the apical transmembrane proteins Crb, Notch, Delta, and Cad87A but not the AJ proteins DEcad and Ed accumulated in prominent cytoplasmic puncta in da>Cdc42-DN embryos (Fig. 2 G; see following sections). These puncta were not confined to regions that had lost apical and AJ proteins from their plasma membrane and were seen throughout the ectoderm. Collectively, our findings suggest that reduction of Cdc42 activity causes a loss of apical polarity in the neuroectodermal epithelium as it undergoes dynamic changes in cell–cell contacts.
Cdc42 negatively regulates apical endocytosis
To address the question of the cellular mechanism used by Cdc42 to support apical polarity during Drosophila early neurogenesis, we first examined the distribution of Cdc42. To this end, we generated new Cdc42 antibodies and a transgenic line that expresses GFP-Cdc42 under Gal4/UAS control. Cdc42 antibodies and GFP-Cdc42, as detected with anti-GFP antibodies, showed identical distribution patterns (Fig. 3, A and D–F; and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200807020/DC1), with one exception (see following paragraph). Cdc42 is found in a punctate distribution throughout the cytoplasm of all ectodermal epithelial cells. Cdc42 appears enriched at the plasma membrane, in particular the AJs, where it is also found in small puncta rather than uniformly distributed as suggested by the examination of deconvolved confocal z stacks (see Materials and methods). Thus, Cdc42 colocalized with the apical membrane and the AJs but did not show any obvious apical enrichment.
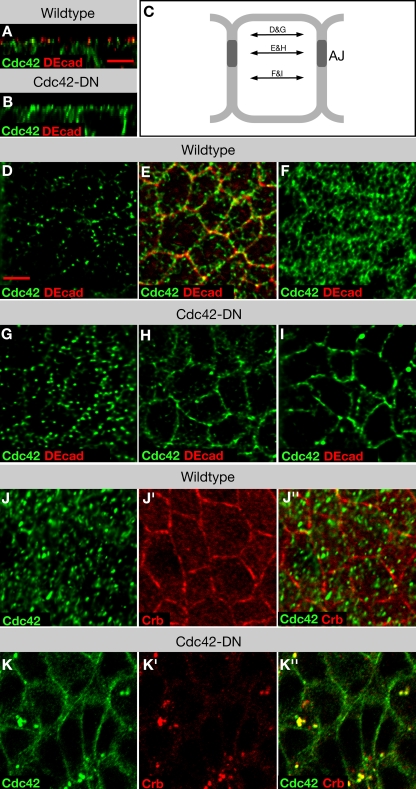
Figure 3.
Localization of Cdc42 in epithelia of wild-type embryos and embryos expressing Cdc42-DN. (A and B) XZ reconstruction of image stacks of neuroectodermal cells of wild-type (A) and Cdc42-DN (B) embryos labeled for DEcad and Cdc42. (C) Schematic of an epithelial cell indicating the focal planes shown in panels D–I. (D–I) Apical membrane (D and G), AJ (E and H), and basolateral membrane (F and I) single-plane views of wild-type (D–F) and Cdc42-DN (G–I) embryos labeled for DEcad and Cdc42. (J and K) Wild-type (J) and Cdc42-DN (K) embryos labeled for Crb and Cdc42. Bars, 5 μm.
We next examined the distribution of Cdc42 in da>Cdc42-DN, da>Cdc42-CA, and da>GFP-Cdc42 embryos using Cdc42 antibodies, thus detecting both the mutant isoforms and endogenous protein. As expected, Cdc42 was overabundant in all three cases. Cdc42-DN–expressing embryos showed mislocalization of Cdc42 at the apical and lateral plasma membrane, an apparent enrichment of apical Cdc42-positive vesicles, and a small number of larger vesicles that could be found anywhere along the apical basal axis (Fig. 3, G–I and K). Embryos expressing Cdc42-CA showed a relatively normal distribution of Cdc42 (Fig. S1), whereas embryos expressing GFP-Cdc42 also displayed normal Cdc42 distribution except for a low frequency of large Cdc42-positive puncta similar to those seen in Cdc42-DN–expressing embryos (Fig. S1). This latter observation raises the possibility that the GFP tag has a slight negative effect on Cdc42 function.
Colocalization experiments revealed that the large Cdc42-positive puncta are the same puncta that accumulate apical membrane proteins in da>Cdc42-DN embryos (Fig. 3, J and K) and are therefore likely vesicular compartments in the biosynthetic or endosomal pathways. To determine whether these vesicles are biosynthetic or endosomal, we exposed live da>Cdc42-DN embryos to FM4-64, a dye that is taken up into cells through endocytosis and labels all endocytotic compartments. FM4-64 colocalized with Crb (Fig. 4, A and B) and other apical proteins to the large cytoplasmic vesicles in da>Cdc42-DN embryos, indicating that these are endocytotic compartments. Quantification of FM4-64 labeling intensity in the neuroectoderm of da>Cdc42-DN embryos compared with wild type indicated that endocytotic uptake of FM4-64 in da>Cdc42-DN embryos is significantly increased (Fig. 4 C). Moreover, although the number of FM4-64–labeled vesicles remained the same in mutant versus wild-type embryos, vesicles in da>Cdc42-DN embryos appeared significantly larger, accounting for the overall increase in labeling intensity. These findings suggest that loss of Cdc42 activity increases endocytosis in neuroectodermal cells.
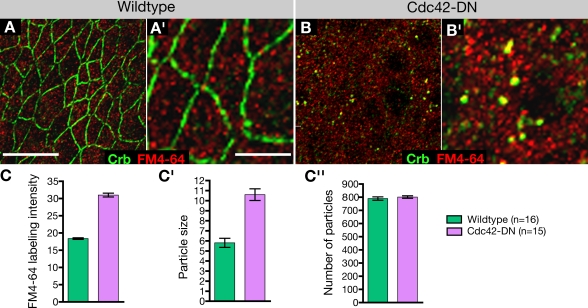
Figure 4.
Internalization of FM4-64 in wild-type embryos and embryos expressing Cdc42-DN. (A and B) Neuroectoderm of wild-type (A and A′) and Cdc42-DN (B and B′) embryos labeled for Crb and the vital dye FM4-64. (C) FM4-64 labeling intensity was measured for individual confocal z stacks comprising ∼16 neuroectodermal cells (mean ± SEM [error bars]). Cdc42-DN embryos showed a substantial increase in FM4-64 uptake, as the observed difference in mean pixel intensity relative to the wild-type control is highly significant (P < 0.001). The differences between Cdc42-DN and wild-type embryos are the result of different particle sizes (P < 0.001; C′), whereas the particle number labeled by FM4-64 remained the same (C′′). Bars: (A and B) 10 μm; (A′ and B′) 5 μm.
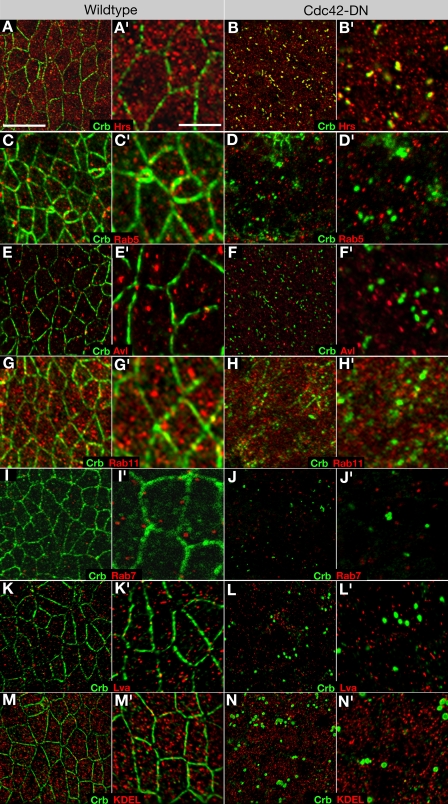
To identify the compartment that accumulates apical proteins in da>Cdc42-DN embryos, we used several markers that highlight different stages in the endocytotic pathway. Double labeling of da>Cdc42-DN embryos with Crb and either Avalanche (Avl), a syntaxin associated with early endosome (Lu and Bilder, 2005), the early endosome marker Rab5 (Wucherpfennig et al., 2003), the recycling endosome marker Rab11 (Dollar et al., 2002; Pelissier et al., 2003), the multivesicular body/late endosome marker Rab7 (Entchev et al., 2000), or Hrs, which marks an early transitory stage between early and late endosomes (Lloyd et al., 2002; Kanwar and Fortini, 2008), showed that only Hrs colocalized with Crb (Fig. 5, A–J) and other apical transmembrane proteins (not depicted). Hrs labels a large number of cytoplasmic puncta. However, colocalization with Crb, Cdc42, and other apical proteins was only seen in approximately one large vesicle per cell in da>Cdc42-DN embryos. These double-labeling experiments were also performed in cdc42 mutant embryos with similar results (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200807020/DC1; and not depicted). Hrs-positive vesicles of similar size were not seen in wild-type control embryos, suggesting that loss of Cdc42 function changes the morphology of endosomes, enlarging them likely by blocking the processing of apical proteins from early to late endosomes. Previous work has found that Cdc42 is associated with the Golgi and implicated Cdc42 in the regulation of exocytosis in some mammalian cells (Kroschewski et al., 1999; Müsch et al., 2001). We did not detect Cdc42 in association with the Golgi, nor did we detect any abnormal accumulation of the seven membrane proteins we examined (Crb, Notch, Delta, Cad87A, DEcad, Ed, and Nrt) in the secretory pathway using markers for the ER (KDEL [Lys-Asp-Glu-Leu]), the Golgi (Lava lamp [Lva]), or the recycling endosome (Rab11), which serves as an exocytotic conduit (Fig. 5, G, H, and K–N; Lock and Stow, 2005; Blankenship et al., 2007). Together, these findings suggest that Cdc42 regulates endocytosis but not exocytosis in Drosophila embryonic epithelia.
Figure 5.
Apical proteins accumulate in an Hrs-positive endocytotic compartment in Cdc42-compromised embryos. (A and B) Wild-type (A and A′) and Cdc42-DN (B and B′) embryos labeled with Crb and the endosome marker Hrs. (C and D) Wild-type (C and C′) and Cdc42-DN (D and D′) embryos labeled for Crb and the early endosome marker Rab5. (E and F) Wild-type (E and E′) and Cdc42-DN (F and F′) embryos labeled for Crb and the early endosome marker Avl. (G and H) Wild-type (G and G′) and Cdc42-DN (H and H′) embryos labeled for Crb and the recycling endosome marker Rab11. (I and J) Embryos expressing the late endosome marker UAS-YFP-Rab7 under the control of da-Gal4 (I and I′) and embryos expressing both UAS-Cdc42-DN and UAS-YFP-Rab7 under the control of da-Gal4 (J and J′) labeled for Crb and GFP. (K and L) Wild-type (K and K′) and Cdc42-DN (L and L′) embryos labeled for Crb and the Golgi marker Lva. (M and N) Wild-type (M and M′) and Cdc42-DN (N and N′) embryos labeled for Crb and the ER marker KDEL. Bars: (A–N) 10 μm; (A′–N′) 5 μm.
Cdc42-compromised embryos displayed abnormally large endosomes containing apical cargo in both the neuroectoderm (0.95 per cell; n = 42), which had lost apical polarity, and in the dorsal ectoderm at a similar frequency (0.92 per cell; n = 56). GFP-Cdc42–expressing embryos, in comparison, contained 0.4 enlarged Cdc42-, Crb-, and Hrs-positive compartments per cell (Fig. S1). Moreover, similar, abnormally large endosomes were seen at other stages of development such as cellularization, when no defects in epithelial polarity were apparent. We conclude that loss of Cdc42 function leads to the accumulation of apical transmembrane proteins in an abnormally enlarged endosomal compartment. Thus, Cdc42 function is required not only for preventing catastrophic loss of apical proteins in the neuroectoderm but also for the processing of apical proteins through the endocytotic pathway in all epithelia we examined.
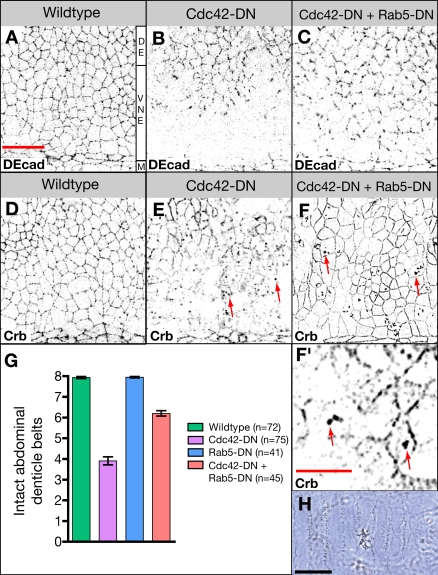
To further support the hypothesis that Cdc42 activity negatively regulates apical endocytosis, we asked whether a reduction in endocytosis can ameliorate the phenotype of da>Cdc42-DN embryos and reconstitute apical protein localization in neuroectodermal cells. We coexpressed Rab5-DN with Cdc42-DN. Rab5 is a Rab GTPase critical for early steps in endocytosis, mediating the fusion of endocytotic vesicles with the early endosome. da>Rab5-DN embryos are lethal and displayed a defective cuticle, but no ventral holes were observed (Fig. 6 G). Neuroectodermal cells of da>Cdc42-DN da>Rab5-DN embryos showed a substantial improvement of normal localization of apical proteins, including Crb and DEcad (Fig. 6, A–F). Corresponding to the normalization of apical polarity during neurogenesis, the terminal phenotype of da>Cdc42-DN is significantly ameliorated by coexpression of Rab5-DN (Fig. 6, G and H). Furthermore, we note that expression of Rab5-DN does not prevent the formation of abnormally enlarged endosomes that accumulate apical proteins in da>Cdc42-DN embryos (Fig. 6 F). This, together with the observation that enlarged apical endosomes are seen in tissues that have not lost apical polarity, again suggests that Cdc42 acts independently in two steps of the apical endocytotic pathway: as a negative regulator of apical endocytosis and as a positive regulator of early to late endosomal processing.
Figure 6.
Inhibition of apical endocytosis rescues defects in Cdc42-compromised embryos. (A–C) Wild-type embryo (A), Cdc42-DN embryo (B), and embryo expressing both Cdc42-DN and Rab5-DN (C) labeled for DEcad. (D–F) Wild-type (D), Cdc42-DN (E), and Cdc42-DN, Rab5-DN (F and F′) embryos labeled for Crb. Arrows point to enlarged endosomes. (G) The extent of ventral cuticle defects was quantified by counting the number of intact abdominal denticle belts (mean ± SEM [error bars]). For Cdc42-DN Rab5-DN embryos, the difference in the mean number of intact belts relative to Cdc42-DN embryos is statistically significant (P < 0.001). (H) Ventral cuticle of Cdc42-DN, Rab5-DN embryo. M, ventral midline; VNE, ventral neuroectoderm; DE, dorsal ectoderm. Bars: (A–F) 20 μm; (F′) 10 μm; (H) 100 μm.
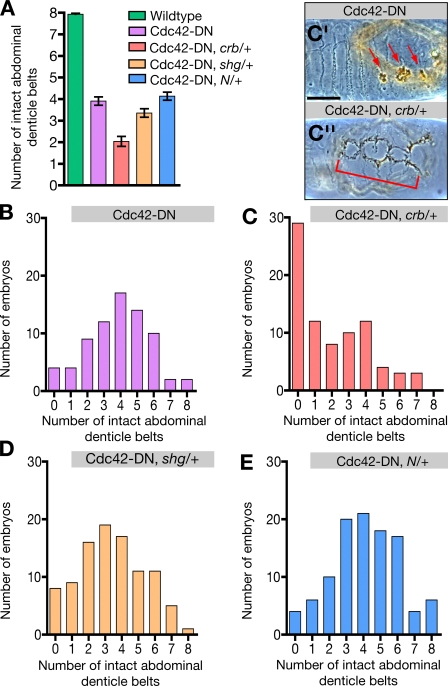
Mammalian Rab5 has been associated with both apical and basolateral endocytosis in epithelial cells (Bucci et al., 1994). In Drosophila epithelial cells, however, Rab5 appears to have an essential function only in apical endocytosis, as Rab5-null epithelial cells accumulate apical markers such as Crb and Notch at the plasma membrane but not basolateral markers such as DEcad (Lu and Bilder, 2005). This would imply that the endocytosis of apical proteins, which is counteracted by Rab5-DN, is the primary effect of the loss of Cdc42 function and that the failure to maintain AJs is secondary to the loss of apical polarity regulators such as Crb (Fig. 2, F and G) or the Par proteins (see following section), which are known to control AJ assembly and stability (Tepass et al., 2001; Knust and Bossinger, 2002). To further test this model, we studied functional interactions between Cdc42 and Crb, DEcad, or Notch by reducing the gene copy of crb, shotgun (shg; the gene encoding DEcad), and Notch from two to one in a da>Cdc42-DN background. The interactions were evaluated and quantified by examining the degree to which the ventral cuticle was disrupted (Fig. 7). Removal of a single copy of crb, shg, or Notch in a wild-type background does not cause lethality or defects in the embryonic epidermis. We did not find interactions with Notch (Fig. 7, A and E), which is consistent with the fact that Cdc42-compromised embryos did not show a Notch-like phenotype (Genova et al., 2000). In contrast, loss of one copy of shg in da>Cdc42-DN embryos led to a significant enhancement of the da>Cdc42-DN phenotype, and the loss of a single copy of crb caused a dramatic enhancement of the da>Cdc42-DN phenotype (Fig. 7, A–D). Fig. 7 A compares the mean phenotypic strength of different genotypes. This representation underestimates the actual strength of the phenotypic enhancement, as only an estimated 50% of embryos evaluated for each genotype are double mutants, whereas the other 50% are da>Cdc42-DN embryos. Therefore, we also present our data by listing the number of embryos in each phenotypic class (Fig. 7, B–E; 0 = no ventral epidermis; 8 = normal ventral epidermis). The majority of da>Cdc42-DN, crb/+ embryos apparently lack abdominal denticle bands and in fact lack most of their ventral epidermis (Fig. 7 C). The strong interaction between Cdc42 and crb compared with the weak interaction between Cdc42 and shg is consistent with the model that the loss of Crb from the membrane is the key event in response to Cdc42 down-regulation that compromises the ability of the neuroectoderm to maintain AJs. Collectively, our findings suggest that Cdc42 negatively regulates the frequency of apical endocytosis to promote apical polarity and, as a consequence, the stability of AJs during cell rearrangement.
Figure 7.
crb and shg enhance the Cdc42-DN phenotype. Quantification of ventral cuticle defects in wild-type; Cdc42-DN; Cdc42-DN, crb/+; Cdc42-DN, shg/+; and Cdc42-DN, Notch/+ embryos. Embryo collections of double-mutant genotypes contained a predicted 50% of double mutants and 50% of Cdc42-DN embryos. (A) The number of intact abdominal denticle belts is presented as mean ± SEM (error bars). For Cdc42-DN, crb/+ and Cdc42-DN, shg/+ embryos, the difference in the mean number of intact belts relative to Cdc42-DN embryos is statistically significant (P < 0.001). For Cdc42-DN, Notch/+ embryos, there is no significant difference relative to Cdc42-DN embryos. (B) Distribution of ventral cuticle phenotypes in a population of Cdc42-DN embryos. (C) Distribution of ventral cuticle phenotypes in a population in which 50% of embryos are predicted to be Cdc42-DN and 50% are predicted to be Cdc42-DN, crb11a22/+. (C′) Ventral cuticle of a Cdc42-DN embryo. Arrows indicate holes in the ventral surface. (C′′) Ventral cuticle of an embryo from a Cdc42-DN, crb/+ population with severely enhanced ventral defects (indicated by bracket). (D) Distribution of ventral cuticle phenotypes in a population in which 50% of embryos are predicted to be Cdc42-DN and 50% are predicted to be Cdc42-DN, shgR69/+. (E) Distribution of ventral cuticle phenotypes in a population in which 50% of embryos are predicted to be Cdc42-DN and 50% are predicted to be Cdc42-DN, N55e11/+. Bar, 100 μm.
The Par complex acts downstream of Cdc42 in regulating the apical endocytotic pathway
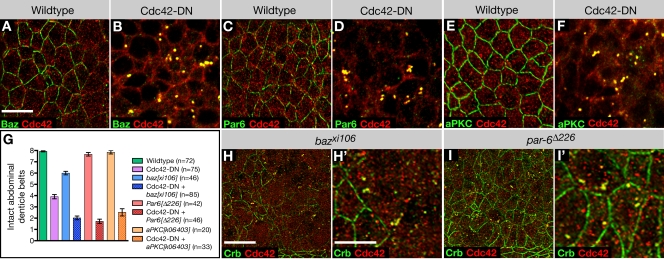
Cdc42 can contribute to endocytosis through regulation of its downstream effector Wasp and actin polymerization (for reviews see Cerione, 2004; Ridley 2006). Also, a second Cdc42 effector, the Par complex, was recently implicated in the regulation of endocytosis in C. elegans and human HeLa cells (Balklava et al., 2007). This study suggested that Cdc42 and the Par proteins Par3, Par6, and aPKC are required to promote endocytosis. However, the function of Cdc42 and Par proteins in endocytosis in epithelial cells was not investigated. To address the question whether Drosophila Par proteins cooperate with Cdc42 in the regulation of endocytosis in neuroectodermal cells, we first examined the distribution of Bazooka (Baz; Drosophila Par3), Par6, and aPKC in da>Cdc42-DN embryos. We found that all three Par proteins are strongly depleted from the apical membrane of neuroectodermal cells and colocalized with Cdc42 at enlarged apical endosomes similar to apical transmembrane proteins such as Crb (Fig. 8, A–F). Normal apical localization but abnormal accumulation of Par proteins at apical endosomes was also observed in other ectodermal epithelial cells that displayed normal apical polarity in da>Cdc42-DN embryos. The loss of both the Crb and Par complexes, which can act redundantly in promoting epithelial polarity (Tanentzapf and Tepass, 2002), from the apical membrane explains the inability of neuroectodermal cells to maintain their AJs.
Figure 8.
Cdc42 interacts with the Par complex to regulate apical endocytosis. (A and B) Wild-type (A) and Cdc42-DN (B) embryos labeled for Baz and Cdc42. (C and D) Wild-type (C) and Cdc42-DN (D) embryos labeled for Par6 and Cdc42. (E and F) Wild-type (E) and Cdc42-DN (F) embryos labeled for aPKC and Cdc42. (G) Cdc42-DN ventral defects are enhanced by the loss of zygotic aPKC, baz, or par6. The extent of ventral cuticle defects was quantified by counting the number of intact abdominal denticle belts (mean ± SEM [error bars]). For all double-mutant combinations, the difference in the mean number of intact belts relative to Cdc42-DN embryos is statistically significant (P < 0.001). (H and I) baz mutant embryos (H and H′) and par6 mutant embryos (I and I′) labeled for Crb and Cdc42. Bars: (A–F, H′, and I′) 5 μm; (H and I) 10 μm.
We next studied embryos in which da>Cdc42-DN is combined with baz, Par6, or aPKC mutations. Mutations in all three Par genes strongly enhance the ventral cuticle defects in da>DN-Cdc42 embryos, suggesting that Cdc42 and Par proteins cooperate in supporting apical polarity of neuroectodermal cells (Fig. 8 G). baz, Par6, and aPKC have strong maternal contributions of expression, and loss of maternal and zygotic expression for all three genes leads to strong epithelial defects at early stages of development, preventing a meaningful analysis of neurulation-stage embryos (Müller and Wieschaus, 1996; Wodarz et al., 2000; Petronczki and Knoblich, 2001; Rolls et al., 2003). Zygotic mutants for aPKC are not embryonic lethal and die at larval stages. par6 zygotic mutants are embryonic lethal but do not show defects in the ventral cuticle or loss of apical polarity in neuroectodermal cells. However, these embryos display enlarged endosomes that accumulate apical cargo (Fig. 8 I). Finally, baz zygotic mutants show prominent cuticle defects, including ventral holes similar to da>Cdc42-DN embryos. Also, large portions of the neuroectoderm of baz mutants show loss of apical polarity, and an accumulation of apical proteins in enlarged endosomes is seen in all ectodermal cells similar to da>Cdc42-DN embryos (Fig. 8 H). Together, these data suggest that the Par complex cooperates with Cdc42 in regulation of the apical endocytotic pathway. The fact that apical polarity seems intact but early to late endosomal processing is compromised in zygotic par6 mutant embryos provides further evidence that apical endocytosis and the processing of apical cargo through the endocytotic pathway may require distinct regulatory inputs from the Cdc42–Par complex and that the latter is more sensitive to the reduction in Cdc42–Par complex function.
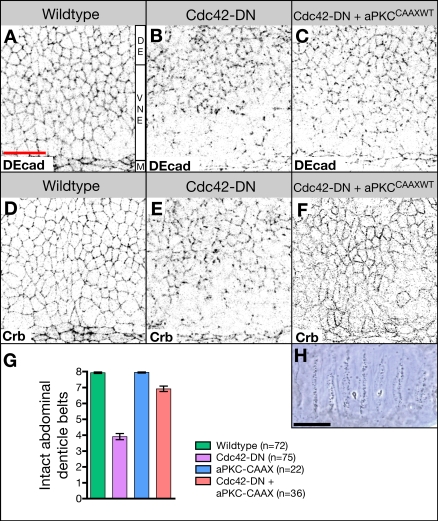
To test whether the Par complex acts downstream and, thus, most likely as an effector complex of Cdc42 in the regulation of endocytosis, we coexpressed a CA form of aPKC (aPKCCAAXWT; Sotillos et al., 2004) and Cdc42-DN. Expression of aPKCCAAXWT strongly suppresses the phenotype of da>Cdc42-DN embryos; localization of apical proteins, including Crb and DEcad (Fig. 9, A–F), is partially restored, and ventral cuticle defects are ameliorated (Fig. 9, G and H). Moreover, the formation of enlarged endosomes containing an abnormal accumulation of apical cargo is completely abolished (Fig. 9 F and not depicted). We conclude that aPKC acts downstream of Cdc42 in the regulation of apical endocytosis.
Figure 9.
Activated aPKC rescues defects in Cdc42-DN embryos. (A–C) Wild-type embryo (A), Cdc42-DN embryo (B), and embryo expressing both Cdc42-DN and aPKCCAAXWT under the control of da-Gal4 (C) labeled with DEcad. (D–F) Wild-type (D), Cdc42-DN (E), and Cdc42-DN, aPKCCAAXWT (F) embryos labeled for Crb. (G) The extent of ventral cuticle defects was quantified by counting the number of intact abdominal denticle belts (mean ± SEM [error bars]). For Cdc42-DN, aPKCCAAXWT embryos, the difference in the mean number of intact belts relative to Cdc42-DN embryos is statistically significant (P < 0.001). (H) Ventral cuticle of da-Gal4, Cdc42-DN, aPKCCAAXWT embryo. M, ventral midline; VNE, ventral neuroectoderm; DE, dorsal ectoderm. Bars: (A–G) 20 μm; (H) 100 μm.
Discussion
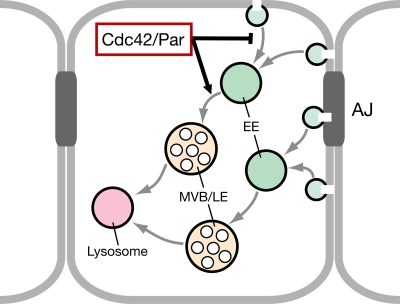
Our findings support a model (Fig. 10) posing that Cdc42 regulates two distinct steps of endocytosis in Drosophila epithelial cells. Cdc42 function slows the removal of apical proteins from the plasma membrane by decreasing endocytotic uptake, and it accelerates the processing of apical cargo from the early to the late endosome. Previous work has suggested that Cdc42 might promote endocytosis through its effector Wasp and the regulation of actin polymerization (for review see Ridley, 2006). However, our observations that (a) embryos with compromised Par complex function show similar endocytotic defects as embryos with reduced Cdc42 function and that (b) endocytotic defects in embryos with compromised Cdc42 function can be rescued by the expression of an active form of aPKC indicate that the Par complex acts as an effector of Cdc42 to regulate endocytosis in the Drosophila ectodermal epithelium. The targets of aPKC in the endocytotic pathway are unknown at present, and we cannot rule out that they include actin regulators.
Figure 10.
Model of the Cdc42–Par complex function in the regulation of the apical endocytotic pathway. Our data suggest that Cdc42 and the Par complex cooperate to regulate two distinct steps in the endocytosis of apical membrane components. The Cdc42–Par complex inhibits endocytosis from the plasma membrane and also promotes progression from the early endosome (EE) to the multivesicular body/late endosome (MVB/LE). Apical and basolateral (including AJ) proteins follow distinct endocytotic routes.
We propose that aPKC has at least two distinct phosphorylation targets that regulate the apical endocytotic pathway. One of the two targets of aPKC is likely to control the uptake of apical membrane proteins or their effective recycling back to the apical membrane. Apical proteins did not accumulate in the Rab11-positive recycling endosome in Cdc42-compromised embryos, suggesting that the Cdc42–Par complex could regulate entry into the recycling pathway but not the progression through this pathway back to the apical membrane. The suppression of Cdc42-DN–induced loss of apical proteins by the coexpression of Rab5-DN is consistent with both an endocytotic or recycling defect given that this experiment does not allow us to determine whether the Cdc42–Par complex acts upstream or downstream of Rab5. However, the simplest explanation for the substantial increase in uptake of the endocytotic tracer FM4-64 in Cdc42-compromised neuroectodermal cells would be an increase in the frequency of endocytosis. A second aPKC target is likely associated with the Hrs-positive endosome, which marks the transition from early endosome to multivesicular body formation. Attractive candidates would be the ESCRT (endosomal sorting complex required for transport) complexes that regulate multivesicular body formation. Defects in the function of these complexes can also lead to abnormally enlarged endosomal compartments (Hurley, 2008). It is remarkable that loss of Cdc42 and Par complex function causes the appearance of a single (on average), enlarged endosomal compartment per cell containing apical cargo.
Recently, the first evidence was published for the involvement of the Par complex in the regulation of endocytosis. Balklava et al. (2007) showed that the Par complex, similar to Cdc42, is required for effective endocytosis in C. elegans oocytes and coelomocytes as well as human HeLa cells. This study identifies Cdc42 and Par proteins as positive regulators of endocytotic uptake of clathrin-dependent cargo or the recycling of clathrin-independent cargo. Cdc42 and Par proteins might act to stabilize apical proteins by promoting recycling. However, we did not detect colocalization of Cdc42 or apical proteins in either wild-type or da>Cdc42-DN embryos with the Rab11-positive apical recycling endosome, suggesting that the Cdc42–Par complex regulates surface uptake directly. This is also consistent with the reconstitution of apical proteins at the plasma membrane in da>Cdc42-DN da>Rab5-DN embryos. In addition to previous work, we show that the expression of an active form of aPKC suppressed the defects seen in da>Cdc42-DN embryos, suggesting that the Par complex acts downstream and, thus, likely as an effector complex of Cdc42 in endocytosis similar to other aspects of cell polarization.
Cdc42 has been implicated as either a positive or negative regulator in several aspects of vesicle trafficking in both the biosynthetic or endocytotic/recycling pathways (Kroschewski et al., 1999; Garrett et al., 2000; Cohen et al., 2001; Müsch et al., 2001; Wu et al., 2003; Schmidt et al., 2006; Wells et al., 2006; for review see Cerione, 2004), and Cdc42 can contribute to clathrin- and dynamin-dependent and -independent endocytotic pathways (Sabharanjak et al., 2002; Balklava et al., 2007; Mayor and Pagano, 2007). This diversity of Cdc42 requirements in vesicle trafficking suggests that its function is highly cell type and/or context dependent. Our work further extends the notion that Cdc42 has context-dependent functions, as we demonstrate that the highly dynamic AJs of the Drosophila neuroectoderm are susceptible to reduced Cdc42 activity, whereas AJs in morphogenetically “silent” regions of epithelia do not critically depend on normal levels of Cdc42 function. The rescue of neuroectodermal AJs in Cdc42-compromised embryos upon blocking neuroblast specification and ingression suggests that the essential role of Cdc42 in stabilizing AJs is not a general property of the neuroectoderm but a direct consequence of the breakdown and reformation of AJs upon neuroblast ingression. It remains to be seen whether Cdc42 becomes activated in response to cell rearrangement in the neuroectoderm or whether GTP-Cdc42 levels are uniform throughout the ectoderm, and an essential requirement for active Cdc42 only develops in response to cell rearrangement.
Cdc42 was reported to localize to the Golgi complex in mammalian cells, which is consistent with its function in Golgi to ER and Golgi to basolateral surface vesicle trafficking (for reviews see Cerione, 2004; Ridley, 2006). We did not find colocalization of Cdc42 as detected by Cdc42 antibodies or GFP-Cdc42 with Golgi markers or markers that label other components of the biosynthetic pathway in Drosophila embryonic epithelial cells. Moreover, Cdc42-compromised embryos did not accumulate apical or basolateral membrane proteins in the biosynthetic pathway, which is in contrast, for example, to embryos that lack exocyst function (Blankenship et al., 2007). Thus, we did not find any evidence for a function of Cdc42 in secretion in the Drosophila ectoderm. However, a minor defect in exocytosis such as a change in the kinetics of vesicle release from the Golgi (Müsch et al., 2001) may have been below our detection threshold. Alternatively, a role of Cdc42 in exocytosis may have not been detected because we did not study embryos that completely lacked Cdc42 function.
Cdc42 localizes to a population of small puncta throughout the cytoplasm and plasma membrane–associated puncta that are enriched at the level of AJs. Expression of Cdc42-DN causes a relocalization of Cdc42; a more uniform labeling is observed along the lateral membrane, AJs, and marginal zone. In the cytoplasm, Cdc42 remains distributed in small puncta, which, however, now appear enriched in the apical cytoplasm, and Cdc42 is seen associated with an abnormally enlarged Hrs-positive (but Rab11, Rab5, Avl, and Rab7 negative) endosomal compartment. The N17 DN mutations of Rho and Ras GTPases are believed to have a higher affinity for guanine nucleotide exchange factors (GEFs) than the normal protein but remain GDP bound and thus inactive, suggesting that DN proteins such as Cdc42-DN act by binding and sequestering GEFs (Feig, 1999). A GEF that activates Cdc42 at the apical membrane or along the apical endocytotic pathway remains to be identified.
Several findings are consistent with the model that endocytotic regulation of apical proteins rather than endocytosis of AJ proteins is the immediate result of the Cdc42–Par complex function: (a) the fact that reduced Rab5 function rescues the loss of apical polarity observed in da>Cdc42-DN embryos, although Rab5 is essential for endocytosis of apical proteins but not AJ proteins in Drosophila epithelial cells (Lu and Bilder, 2005); (b) the tight association of Cdc42 effectors Par6 and aPKC with the apical membrane (Wodarz et al., 2000; Petronczki and Knoblich, 2001); and (c) the strong genetic interaction between Cdc42-DN and crb compared with the weak interactions between Cdc42-DN and shg. Thus, the loss of AJs in the neuroectoderm is presumably a secondary consequence of the loss of apical polarity proteins in Cdc42-compromised embryos. This model is also consistent with the observation that apical (e.g., Crb) and basolateral (e.g., DEcad or Ed) membrane protein follow different endocytotic routes. Despite the loss of DEcad and Ed from the membrane, these proteins are not detected in the abnormally enlarged Hrs-positive endosomes that accumulate apical proteins. Apical/tight junction proteins and basolateral/AJ proteins also take distinct endocytotic routes in mammalian cells (Macara and Spang, 2006). Independent regulatory mechanisms for apical and basolateral endocytosis may make important contributions to morphogenesis. For example, neuroblast ingression, a typical epithelial mesenchymal transition, requires the coordinated loss of apical polarity. This process may be initiated by a deactivation of Cdc42, which precipitates the rapid endocytosis of the apical membrane, including apical polarity proteins and a subsequent loss of AJs, most likely also through endocytosis.
A gradual loss of epithelial AJs is also observed in the ventricular zone of the mouse neuroepithelium mutant for Cdc42 (Cappello et al., 2006). This remarkable similarity to the Cdc42-compromised Drosophila embryonic neuroectoderm suggests that during mammalian neurogenesis, the movement of self-renewing progenitor cells from the ventricular zone epithelium to the subventricular zone also involves dynamic changes in AJs. The stability of these AJs and apical polarity likely depends on the Cdc42–Par complex function and, as a consequence, the maintenance of cells within the ventricular zone (Cappello et al., 2006). It will be interesting to determine whether the Cdc42–Par complex–dependent regulation of endocytosis contributes to the maintenance of the mammalian neuroepithelium.
Materials and methods
Drosophila genetics
The following stocks were used in this study: da-Gal4 (Wodarz et al., 1995), UAS-Cdc42.N17, UAS-Cdc42.V12, UAS-Rac1.N17, UAS-Rac1.V12 (Luo et al., 1994), UAS-Rho1.N19 (Strutt et al., 1997), UAS-Rho1.V14 (Lee et al., 2000), Cdc423, Cdc426 (gift from R. Fehon, University of Chicago, Chicago, IL; Genova et al., 2000), UAS-Nintra (Lieber et al., 1993), UAS-mCD8-GFP, UAS-Rab5.S43N (gift from M. Gonzalez-Gaitan, University of Geneva, Geneva, Switzerland; Entchev et al., 2000), UAS-aPKCCAAXWT (gift from C. Doe, University of Oregon, Eugene, OR; Sotillos et al., 2004), UAS-YFP-Rab7 (Zhang et al., 2007), bazxi106 (Müller and Wieschaus, 1996), par-6Δ226 (Petronczki and Knoblich, 2001), aPKCk06403 (Wodarz et al., 2000; Rolls et al., 2003), crb11a22 (Tepass et al., 1990), N55e11 (Lindsley and Zimm, 1992), and shgR69 (Godt and Tepass, 1998). The following stable stocks were generated: UAS-Cdc42.N17; UAS-Nintra, UAS-Cdc42.N17; UAS-mCD8-GFP, UAS-Cdc42.N17 UAS-Rab5.S43N, UAS-Cdc42.N17 UAS-aPKCCAAXWT, bazxi106/FM7 Kr-GFP; UAS-Cdc42.N17, par6Δ226/FM7 Kr-GFP; UAS-Cdc42.N17, aPKCk06403 UAS-Cdc42.N17/CyO Kr-GFP and aPKCk06403/Cyo Kr-GFP; and da-Gal4. Overexpression of UAS constructs was accomplished by crossing to da-Gal4 and allowing embryos to develop at 29°C until the desired stage. Cdc42 loss-of-function embryos were generated by first crossing Cdc423 females with Cdc426 males to generate heteroallelic (Cdc423/Cdc426) females that provide a low maternal contribution of Cdc42 as previously described (Genova et al., 2000).
Antibody production
GST-Cdc42 (gift from A. Wilde, University of Toronto, Toronto, Canada) was expressed in bacteria, purified by standard methods, and injected into guinea pigs. Serum GP21c was used for immunocytochemistry at a dilution of 1:500.
Production of GFP-Cdc42
Cdc42 was subcloned into pENTR-TOPO (Invitrogen) using the following primers: forward (5′-CACCATGCAAACCATCA-3′) and reverse (5′-TAAGAATTTGCACTTCCTTTTCT-3′). Cdc42 was then transferred using Gateway Technology (Invitrogen) into pPGW-attB, an expression vector that adds a GFP tag to the N terminus. pPGW-attB was produced by adding an attB site (gift from M. Calos, Stanford University School of Medicine, Stanford, CA; Groth et al., 2004) into the NsiI site of pPGW (Drosophila Gateway Vector Collection; http://www.ciwemb.edu/labs/murphy/Gateway%20vectors.html). Transgenic animals were produced by Genetic Services, Inc. using flies carrying attP2 (Groth et al., 2004).
Immunocytochemistry
Drosophila embryos were fixed as previously described (Tepass et al., 1990) except for Arm stainings, for which embryos were heat fixed (Tepass, 1996), and Cdc42 stainings, for which the fixation solution contained 6% formaldehyde in PEMS buffer (0.1 M Pipes, 2 μM MgSO4, and 50 μM EGTA). The primary antibodies used were anti-Cdc42 (GP21c), 1:50 rat anti-DEcad, 1:100 mouse anti-Arm, 1:20 mouse anti-Nrt, 1:1,000 mouse anti-Notch F461.3B, 1:100 rat anti–α-catenin, 1:100 mouse anti-Delta (Developmental Studies Hybridoma Bank), 1:100 rat anti-Crb (Pellikka et al., 2002), 1:1,000 rat anti-Ed (gift from L. Nilson, McGill University, Montreal, Quebec, Canada; Laplante and Nilson, 2006), 1:1,000 guinea pig anti-Cad87A (gift from D. Godt, University of Toronto, Toronto, Ontario, Canada; Fung et al., 2008), 1:500 guinea pig anti-Scrib (gift from D. Bilder, University of California, Berkeley, Berkeley, CA), 1:200 guinea pig anti-Hrs (gift from H. Bellen, Baylor College of Medicine, Houston, TX; Lloyd et al., 2002), 1:500 chicken anti-Avl (gift from D. Bilder; Lu and Bilder, 2005), 1:1,000 rabbit anti-Rab11 (gift from D.F. Ready, Purdue University, West Lafayette, IN; Satoh et al., 2005), 1:150 rabbit anti-aPKC C-20 (Santa Cruz Biotechnology, Inc.), 1:1,000 rabbit anti-Baz, 1:500 guinea pig anti-Par6 (gift from A. Wodarz, University of Göttingen, Göttingen, Germany), 1:500 rabbit anti-PatJ (Tanentzapf et al., 2000), 1:50 rabbit anti-Rab5 (gift from M. Gonzalez-Gaitan; Wucherpfennig et al., 2003), rabbit anti-Lva (gift from W. Sullivan, University of California, Santa Cruz, Santa Cruz, CA; Sisson et al., 2000), 1:200 mouse anti-KDEL(10C4) (Abcam), and 1:500 anti-GFP–Alexa Fluor 488 (Invitrogen). Secondary antibodies were conjugated to Alexa Fluor 488 (Invitrogen), Cy3, or Cy5 (Jackson ImmunoResearch Laboratories). Fixed embryos were mounted in VECTASHIELD (Vector Laboratories).
FM4-64 labeling of Drosophila embryos
Embryos were gradually chilled to 10°C on apple agar and dechorionated in ice-cold 50% bleach for 4 min. Embryos were washed with ice-cold water and incubated in 5 μg/ml FM4-64–FX (Invitrogen) in 0.9% NaCl over a heptane phase for 10 min at 4°C. Embryos were then fixed in 6% paraformaldehyde in PEMS buffer over a heptane phase for 10 min at 4°C and devitillinized by hand. A shortened immunohistochemical protocol was followed to avoid excessive washing of the FM4-64 dye. Fixed embryos were given three washes of 10 min each in PBT (1.3 M NaCl, 0.07 M Na2HPO4, 0.03 M NaH2PO4, and 0.3% Triton X-100), a 30-min incubation in PBT plus 2% normal goat serum and 2% BSA (PBTB), and 1 h in primary antibody. Embryos were then given three washes of 10 min each in PBT, 30 min of incubation in PBTB, and 1 h in secondary antibody. Embryos were then given three washes of 10 min each in PBT and were mounted in VECTASHIELD.
Image acquisition, processing, and statistical analysis
Fixed embryos were imaged with a confocal microscope (LSM 510; Carl Zeiss, Inc.) with Plan Apochromat 63× NA 1.4 and Plan Neofluar 40× NA 1.3 objectives at room temperature using LSM 510 software (Carl Zeiss, Inc.). Confocal z stacks were deconvolved with Volocity software (PerkinElmer). All images are deconvolved confocal stacks unless otherwise indicated. Fluorescence intensity measurements were taken using ImageJ software (National Institutes of Health) from deconvolved confocal stacks. Measurements of the size and number of particles were taken using the analyze particles function in ImageJ on deconvolved confocal stacks that were first processed by conversion to 8-bit grayscale and automatic thresholding. Mean fluorescence intensity, mean particle size, and number of particles were measured for each of n confocal stacks (each generated from a different embryo and pooled from at least four independent experiments), and the data are represented as the mean ± SEM. For ventral cuticle defects, intact denticle belts were counted per embryo for n embryos, and the data are represented as mean ± SEM. For the counting of enlarged endosomes, Cdc42- and Crb-positive compartments were counted per cell for n epithelial cells (from deconvolved confocal stacks generated from at least five different embryos and pooled from at least two independent experiments), and the data are represented as mean ± SEM. Statistical significance was determined using Student's t test.
Online supplemental material
Fig. S1 shows the distribution of Cdc42-CA and GFP-Cdc42 in epithelial cells. Fig. S2 shows that apical polarity markers are lost from the plasma membrane of the ventral neuroectoderm and accumulate in endosomal compartments in Cdc42 loss-of-function embryos. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200807020/DC1.
Supplementary Material
Acknowledgments
We are grateful to H. Bellen, D. Bilder, M. Calos, C. Doe, R. Fehon, D. Godt, M. Gonzalez-Gaitan, L. Nilson, D.F. Ready, W. Sullivan, A. Wilde, A. Wodarz, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for reagents. We thank T. Harris for assistance with deconvolution microscopy.
This work was supported by a grant from the National Cancer Institute of Canada to U. Tepass.
Abbreviations used in this paper: AJ, adherens junction; aPKC, atypical PKC; Arm, Armadillo; Avl, Avalanche; Baz, Bazooka; CA, constitutively active; Crb, Crumbs; DEcad, Drosophila epithelial cadherin; DN, dominant negative; Ed, Echinoid; GEF, guanine nucleotide exchange factor; Lva, Lava lamp; Nrt, Neurotactin; Scrib, Scribble; shg, shotgun; UAS, upstream activation sequence; Wasp, Wiskott-Aldrich syndrome protein; ZA, zonula adherens.
References
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. [DOI] [PubMed] [Google Scholar]
- Atwood, S.X., C. Chabu, R.R. Penkert, C.Q. Doe, and K.E. Prehoda. 2007. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J. Cell Sci. 120:3200–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balklava, Z., S. Pant, H. Fares, and B.D. Grant. 2007. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 9:1066–1073. [DOI] [PubMed] [Google Scholar]
- Blankenship, J.T., M.T. Fuller, and J.A. Zallen. 2007. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120:3099–3110. [DOI] [PubMed] [Google Scholar]
- Bloor, J.W., and D.P. Kiehart. 2002. Drosophila RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis. Development. 129:3173–3183. [DOI] [PubMed] [Google Scholar]
- Braga, V.M., and A.S. Yap. 2005. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr. Opin. Cell Biol. 17:466–474. [DOI] [PubMed] [Google Scholar]
- Bryant, D.M., and J.L. Stow. 2004. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14:427–434. [DOI] [PubMed] [Google Scholar]
- Bucci, C., A. Wandinger-Ness, A. Lütcke, M. Chiariello, C.B. Bruni, and M. Zerial. 1994. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc. Natl. Acad. Sci. USA. 91:5061–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega, J.A. 1993. Mechanisms of early neurogenesis in Drosophila melanogaster. J. Neurobiol. 24:1305–1327. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J.A., and V. Hartenstein. 1997. The Embryonic Development of Drosophila melanogaster. Springer, Berlin. 405 pp.
- Cappello, S., A. Attardo, X. Wu, T. Iwasato, S. Itohara, M. Wilsch-Bräuninger, H.M. Eilken, M.A. Rieger, T.T. Schroeder, W.B. Huttner, et al. 2006. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 9:1099–1107. [DOI] [PubMed] [Google Scholar]
- Cerione, R.A. 2004. Cdc42: new roads to travel. Trends Cell Biol. 14:127–132. [DOI] [PubMed] [Google Scholar]
- Cohen, D., A. Müsch, and E. Rodriguez-Boulan. 2001. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2:556–564. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C. 2005. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 15:19–26. [DOI] [PubMed] [Google Scholar]
- Dollar, G., E. Struckhoff, J. Michaud, and R.S. Cohen. 2002. Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation. Development. 129:517–526. [DOI] [PubMed] [Google Scholar]
- Entchev, E.V., A. Schwabedissen, and M. González-Gaitán. 2000. Gradient formation of the TGF-beta homolog Dpp. Cell. 103:981–991. [DOI] [PubMed] [Google Scholar]
- Feig, L.A. 1999. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1:E25–E27. [DOI] [PubMed] [Google Scholar]
- Fox, D.T., C.C. Homem, S.H. Myster, F. Wang, E.E. Bain, and M. Peifer. 2005. Rho1 regulates Drosophila adherens junctions independently of p120ctn. Development. 132:4819–4831. [DOI] [PubMed] [Google Scholar]
- Fukata, M., and K. Kaibuchi. 2001. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2:887–897. [DOI] [PubMed] [Google Scholar]
- Fung, S., F. Wang, M. Chase, D. Godt, and V. Hartenstein. 2008. Expression profile of the cadherin family in the developing Drosophila brain. J. Comp. Neurol. 506:469–488. [DOI] [PubMed] [Google Scholar]
- Garrett, W.S., L.M. Chen, R. Kroschewski, M. Ebersold, S. Turley, S. Trombetta, J.E. Galán, and I. Mellman. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 102:325–334. [DOI] [PubMed] [Google Scholar]
- Genova, J.L., S. Jong, J.T. Camp, and R.G. Fehon. 2000. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev. Biol. 221:181–194. [DOI] [PubMed] [Google Scholar]
- Godt, D., and U. Tepass. 1998. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 395:387–391. [DOI] [PubMed] [Google Scholar]
- Goldstein, B., and I.G. Macara. 2007. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 13:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth, A.C., M. Fish, R. Nusse, and M.P. Calos. 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 166:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2000. Regulation of cadherin adhesive activity. J. Cell Biol. 148:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6:622–634. [DOI] [PubMed] [Google Scholar]
- Halbleib, J.M., and W.J. Nelson. 2006. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20:3199–3214. [DOI] [PubMed] [Google Scholar]
- Hurley, J.H. 2008. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 20:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer, A., J. Betschinger, M. Petronczki, and J.A. Knoblich. 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell. 6:845–854. [DOI] [PubMed] [Google Scholar]
- Kanwar, R., and M.E. Fortini. 2008. The big brain aquaporin is required for endosome maturation and notch receptor trafficking. Cell. 133:852–863. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Knust, E., and O. Bossinger. 2002. Composition and formation of intercellular junctions in epithelial cells. Science. 298:1955–1959. [DOI] [PubMed] [Google Scholar]
- Kroschewski, R., A. Hall, and I. Mellman. 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1:8–13. [DOI] [PubMed] [Google Scholar]
- Laplante, C., and L.A. Nilson. 2006. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development. 133:3255–3264. [DOI] [PubMed] [Google Scholar]
- Lee, T., C. Winter, S.S. Marticke, A. Lee, and L. Luo. 2000. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 25:307–316. [DOI] [PubMed] [Google Scholar]
- Lieber, T., S. Kidd, E. Alcamo, V. Corbin, and M.W. Young. 1993. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 7:1949–1965. [DOI] [PubMed] [Google Scholar]
- Lindsley, D.L., and G.G. Zimm. 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego. 1133 pp.
- Lloyd, T.E., R. Atkinson, M.N. Wu, Y. Zhou, G. Pennetta, and H.J. Bellen. 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signalling in Drosophila. Cell. 108:261–269. [DOI] [PubMed] [Google Scholar]
- Lock, J.G., and J.L. Stow. 2005. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 16:1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H., and D. Bilder. 2005. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7:1232–1239. [DOI] [PubMed] [Google Scholar]
- Luo, L., Y.J. Liao, L.Y. Jan, and Y.N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: DRac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787–1802. [DOI] [PubMed] [Google Scholar]
- Macara, I.G. 2004. Par proteins: partners in polarization. Curr. Biol. 14:R160–R162. [PubMed] [Google Scholar]
- Macara, I.G., and A. Spang. 2006. Closing the GAP between polarity and vesicle transport. Cell. 125:419–421. [DOI] [PubMed] [Google Scholar]
- Mayor, S., and R.E. Pagano. 2007. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, H.A., and E. Wieschaus. 1996. Armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch, A., D. Cohen, G. Kreitzer, and E. Rodriguez-Boulan. 2001. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 20:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani, T., T. Ichii, S. Aono, and M. Takeichi. 2006. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 175:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier, A., J.P. Chauvin, and T. Lecuit. 2003. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr. Biol. 13:1848–1857. [DOI] [PubMed] [Google Scholar]
- Pellikka, M., G. Tanentzapf, M. Pinto, C. Smith, C.J. McGlade, D.F. Ready, and U. Tepass. 2002. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 416:143–149. [DOI] [PubMed] [Google Scholar]
- Petronczki, M., and J.A. Knoblich. 2001. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 3:43–49. [DOI] [PubMed] [Google Scholar]
- Rebay, I., R.G. Fehon, and S. Artavanis-Tsakonas. 1993. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 74:319–329. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. 2006. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16:522–529. [DOI] [PubMed] [Google Scholar]
- Rolls, M.M., R. Albertson, H.P. Shih, C.Y. Lee, and C.Q. Doe. 2003. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 162:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak, S., P. Sharma, R.G. Parton, and S. Mayor. 2002. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell. 2:411–423. [DOI] [PubMed] [Google Scholar]
- Satoh, A.K., J.E. O'Tousa, K. Ozaki, and D.F. Ready. 2005. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 132:1487–1497. [DOI] [PubMed] [Google Scholar]
- Schmidt, M.H., K. Husnjak, I. Szymkiewicz, K. Haglund, and I. Dikic. 2006. Cbl escapes Cdc42-mediated inhibition by downregulation of the adaptor molecule betaPix. Oncogene. 25:3071–3078. [DOI] [PubMed] [Google Scholar]
- Sisson, J.C., C. Field, R. Ventura, A. Royou, and W. Sullivan. 2000. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J. Cell Biol. 151:905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos, S., M.T. Díaz-Meco, E. Caminero, J. Moscat, and S. Campuzano. 2004. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl, G., K. Fitzgerald, and I. Greenwald. 1993. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 74:331–345. [DOI] [PubMed] [Google Scholar]
- Strutt, D.I., U. Weber, and M. Mlodzik. 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 387:292–295. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., and U. Tepass. 2002. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5:46–52. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., C. Smith, J. McGlade, and U. Tepass. 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass, U. 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177:217–225. [DOI] [PubMed] [Google Scholar]
- Tepass, U., and V. Hartenstein. 1994. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161:563–596. [DOI] [PubMed] [Google Scholar]
- Tepass, U., C. Theres, and E. Knust. 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799. [DOI] [PubMed] [Google Scholar]
- Tepass, U., E. Gruszynski-DeFeo, T.A. Haag, L. Omatyar, T. Török, and V. Hartenstein. 1996. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 10:672–685. [DOI] [PubMed] [Google Scholar]
- Tepass, U., G. Tanentzapf, R. Ward, and R. Fehon. 2001. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35:747–784. [DOI] [PubMed] [Google Scholar]
- Uemura, T., H. Oda, R. Kraut, S. Hayashi, Y. Kotaoka, and M. Takeichi. 1996. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10:659–671. [DOI] [PubMed] [Google Scholar]
- Van Aelst, L., and M. Symons. 2002. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 16:1032–1054. [DOI] [PubMed] [Google Scholar]
- Wells, C.D., J.P. Fawcett, A. Traweger, Y. Yamanaka, M. Goudreault, K. Elder, S. Kulkarni, G. Gish, C. Virag, C. Lim, et al. 2006. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 125:535–548. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., U. Hinz, M. Engelbert, and E. Knust. 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 82:67–76. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., A. Ramrath, A. Grimm, and E. Knust. 2000. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 150:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W.J., S. Tu, and R.A. Cerione. 2003. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell. 114:715–725. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig, T., M. Wilsch-Bräuninger, and M. González-Gaitán. 2003. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161:609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., K.L. Schulze, P.R. Hiesinger, K. Suyama, S. Wang, M. Fish, M. Acar, R.A. Hoskins, H.J. Bellen, and M.P. Scott. 2007. Thirty-one flavors of Drosophila rab proteins. Genetics. 176:1307–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.