Abstract
Neurons have high densities of voltage-gated Na+ channels that are restricted to axon initial segments and nodes of Ranvier, where they are responsible for initiating and propagating action potentials. New findings (Bréchet, A., M.-P. Fache, A. Brachet, G. Ferracci, A. Baude, M. Irondelle, S. Pereira, C. Leterrier, and B. Dargent. 2008. J. Cell Biol. 183:1101–1114) reveal that phosphorylation of several key serine residues by the protein kinase CK2 regulates Na+ channel interactions with ankyrin G. The presence of CK2 at the axon initial segment and nodes of Ranvier provides a mechanism to regulate the specific accumulation and retention of Na+ channels within these important domains.
Neurons process and encode information through synaptic activity that results in the generation and propagation of action potentials (APs). Voltage-gated Na+ (Nav) and K+ (Kv) channels, highly enriched at the axon initial segment (AIS) and nodes of Ranvier (Fig. 1 A), are central to this activity. Electrophysiological measurements suggest that AIS Nav channel density is ∼50 times that of proximal dendrites (Kole et al., 2008), and in myelinated axons, nodal Nav channel densities are at least 25 times those of the internode (Shrager, 1987). How do neurons restrict Nav channels to the AIS and nodes? Experiments indicate that the cytoskeletal scaffold ankyrin G (ankG) is responsible for clustering Nav and Kv channels. For example, loss of ankG by RNA interference or in mutant mice blocks AIS and nodal clustering of ion channels (Zhou et al., 1998; Dzhashiashvili et al., 2007; Hedstrom et al., 2007), and efforts to identify AIS localization determinants in Nav channels revealed a highly conserved ankG-binding AIS-targeting motif in the II-III linker domain (Garrido et al., 2003; Lemaillet et al., 2003). Intriguingly, KCNQ2/3 Kv channels, also enriched at the AIS, appear to have independently evolved an AIS-targeting motif nearly identical in sequence to that found in Nav channels (Pan et al., 2006).
Figure 1.
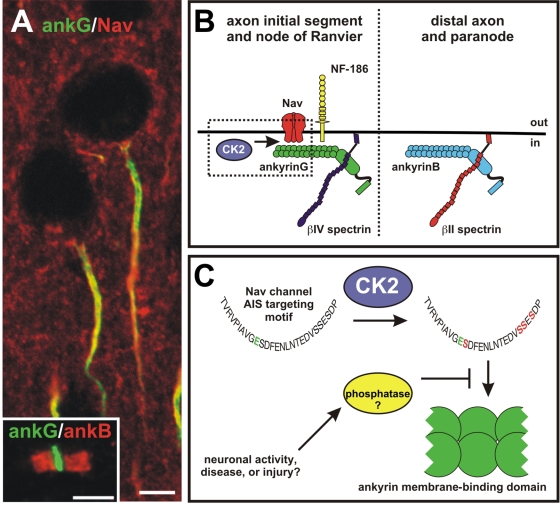
Spatially restricted CK2 activity increases the affinity of Nav channels for ankG. (A) AISs have high densities of Nav channels (red) and ankG (green). (A, inset) Nodes of Ranvier have complementary distributions of ankyrins, with ankB at paranodes (red) and ankG at nodes (green). Bars, 5 μm. (B) CK2 is restricted to the AIS and nodes of Ranvier, where it promotes the interaction between Nav channels and ankG. AnkG also interacts with neurofascin-186 (NF-186) in a phosphorylation-dependent manner, and is linked to the cytoskeleton through βIV spectrin. In contrast, distal axons and paranodes contain ankB that is linked to the cytoskeleton through βII spectrin. CK2 is not present in these regions, resulting in a much lower affinity between Nav channels and ankB. (C) CK2 phosphorylates four serine residues in the AIS-targeting motif. This increases the affinity of the AIS-targeting motif for the ankyrin MBD by 1,000-fold. It is possible that the density of channels in the axon and at the AIS could be dynamically regulated through increasing or decreasing levels of CK2, or through the activity of yet-to-be identified phosphatases.
Besides ankG, axons also have high levels of ankyrin B (ankB). These similar cytoskeletal scaffolds occupy complementary regions of the axon (Fig. 1 B), such that in myelinated axons, ankB is restricted to paranodes, whereas ankG is only found at nodes (Fig. 1 A, inset; Ogawa et al., 2006); in hippocampal neurons, ankG is found only at the AIS, whereas ankB is found throughout the distal axon (Boiko et al., 2007). Because both ankB and ankG share a highly conserved membrane-binding domain (MBD) that interacts with the AIS-targeting motif, it has been difficult to understand how Nav channels are restricted only to regions with ankG. In the current issue, Bréchet et al. (see p. 1101) help to resolve this conundrum by demonstrating that spatially regulated phosphorylation of several key serine residues in the AIS-targeting motif strongly enhances its affinity for the MBD.
Phosphorylation (and dephosphorylation) of AIS and nodal proteins has been shown previously to play important roles in regulating AIS assembly. For example, phosphorylated p65/RelA and IκBα, an inhibitor of the nuclear factor κB (NFκB) transcription factor, are enriched at the AIS and nodes of Ranvier (Schultz et al., 2006), and inhibition of IκBα phosphorylation disrupts AIS formation (Sanchez-Ponce et al., 2008). In contrast, rather than promote binding, phosphorylation of a tyrosine residue conserved in L1 family cell adhesion molecules enriched at the AIS (e.g., neurofascin-186 and NrCAM) blocks their interaction with ankyrins (Garver et al., 1997). It may be that phosphorylation is a common mechanism for regulating interactions among AIS proteins.
To investigate this possibility and to further define the mechanism of Nav channel clustering and interaction with ankG, Bréchet et al. (2008) performed a structure function analysis of the AIS-targeting motif using a Kv2.1-Nav1.2 chimera that included only the AIS-targeting motif of Nav1.2. They found that by combining a glutamate mutation (Nav1.2 E1111A) with mutation of single or multiple serines in the AIS-targeting motif (Nav1.2 S1112A, S1123-24A, or S11126A; Fig. 1 C), AIS recruitment of the mutant chimera was completely abolished. Subsequent sequence analysis suggested that these serines were potential phosphorylation sites for the protein kinase CK2, and in vitro phosphorylation assays confirmed this prediction. Using surface plasmon resonance, they then showed that CK2 modulated the association of the AIS-targeting motif with the ankyrin MBD. In fact, CK2-mediated phosphorylation increased the affinity of the AIS-targeting motif for the ankyrin MBD ∼1,000-fold! The relevance of the phosphorylation for Nav channel clustering was further demonstrated by showing that the Kv2.1-Nav1.2 chimera could compete for endogenous Nav channels at the AIS, but phosphorylation-deficient chimeras could not.
Protein kinase CK2's role in regulating Nav channel clustering was further confirmed by demonstrating that CK2 is highly enriched at the AIS of hippocampal neurons both in vitro and in vivo, and at central nervous system and peripheral nervous system nodes, where it colocalizes with Nav channels and ankG. Thus, CK2 is spatially restricted to sites where it can promote the binding of Nav channels to ankG. Inhibition of CK2 in cultured hippocampal neurons caused a decrease in the density of Nav channels at the AIS. Surprisingly, CK2 inhibition also caused a reduction in the amount of ankG at the AIS, which suggests either that CK2 directly regulates ankG stability (possibly through βIV spectrin) or that Nav channels can also influence the stability of ankG. This latter possibility is consistent with the observation that silencing of Nav channel expression in motor neurons by RNA interference reduces AIS ankG clustering (Xu and Shrager, 2005).
It is tempting to speculate that the enrichment of Nav channels only in ankG-rich regions might be further ensured by an ankB-interacting phosphatase. This would provide an additional layer of regulation, ensuring that Nav channels are spatially restricted and only interact with ankG. Because Nav channels can also bind to ankB-MBD, inappropriate phosphorylation of the serines within the AIS-targeting motif could result in ectopic clustering of channels along the axon, as well as hyperexcitability. Alternatively, failure to appropriately phosphorylate channels (or increased activity of phosphatases) could lead to a neuron in a hypoexcitable state and conduction block. It will be interesting to determine if diseases or injuries that lead to altered cellular excitability might not result from inappropriate Nav channel phosphorylation or dephosphorylation (Fig. 1 C). Although ankG and ankB are very similar in their MBDs, ankB contains a unique insertion that facilitates intramolecular binding between ankB's N-terminal membrane-binding and C-terminal domains. Rather than dephosphorylation of the Nav channel, ankG specificity may be a consequence of ankB's MBD being occupied by its own C-terminal domain (Abdi et al., 2006).
Although the results of Bréchet et al. (2008) provide important insights into the mechanisms of AIS assembly, as any good paper should, they also raise additional questions. For example, how is CK2 restricted to the AIS? Does its localization depend on ankG? Because KCNQ2/3 Kv channels share a common AIS-targeting motif, does CK2 also regulate the localization of these Kv channels? Further, it is also important to note that mutation of the serines alone (whether single or multiple) did not affect the localization of Kv2.1-Nav1.2 chimeras unless these mutations were combined with the glutamate (Nav1.2 E1111A) mutation. The significance of E111A remains obscure but suggests that phosphorylation is not the only mechanism regulating Nav channel clustering. It is also intriguing to note that additional levels of subcellular specialization exist among Nav channels at the AIS. For example, although ankG extends throughout the AIS in retinal ganglion cells, Nav1.1 occupies a microdomain in the proximal AIS (close to the cell body), whereas Nav1.6 channels occupy the complementary distal AIS (Van Wart et al., 2007). The reasons for this additional degree of specialization are unknown, and it will be interesting to determine if phosphorylation contributes to formation of these microdomains.
Finally, perhaps the most intriguing implication of this work is the potential for dynamic regulation of Nav channel density. Because action potential initiation depends on the density of Nav (and Kv) channels, modulation of AIS channel numbers by regulating CK2 activity or through competing phosphatases (Fig. 1 C) could be an important way to dynamically regulate the biophysical properties of the spike-generating machinery. If true, besides synaptic plasticity, AIS plasticity may be another way in which neurons can strengthen or modify neural circuits.
References
- Abdi, K.M., P.J. Mohler, J.Q. Davis, and V. Bennett. 2006. Isoform specificity of ankyrinB: a site in the divergent C-terminal domain is required for intramolecular association. J. Biol. Chem. 281:5741–5749. [DOI] [PubMed] [Google Scholar]
- Boiko, T., M. Vakulenko, H. Ewers, C.C. Yap, C. Norden, and B. Winckler. 2007. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J. Neurosci. 27:590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet, A., M.-P. Fache, A. Brachet, G. Ferracci, A. Baude, M. Irondelle, S. Pereira, C. Leterrier, and B. Dargent. 2008. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interaction with ankyrin G. J. Cell Biol. 183:1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhashiashvili, Y., Y. Zhang, J. Galinska, I. Lam, M. Grumet, and J.L. Salzer. 2007. Nodes of Ranvier and axon initial segments are ankyrin G–dependent domains that assemble by distinct mechanisms. J. Cell Biol. 177:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido, J.J., P. Giraud, E. Carlier, F. Fernandes, A. Moussif, M.P. Fache, D. Debanne, and B. Dargent. 2003. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 300:2091–2094. [DOI] [PubMed] [Google Scholar]
- Garver, T.D., Q. Ren, S. Tuvia, and V. Bennett. 1997. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J. Cell Biol. 137:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom, K.L., X. Xu, Y. Ogawa, R. Frischknecht, C.I. Seidenbecher, P. Shrager, and M.N. Rasband. 2007. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 178:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole, M.H., S.U. Ilschner, B.M. Kampa, S.R. Williams, P.C. Ruben, and G.J. Stuart. 2008. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 11:178–186. [DOI] [PubMed] [Google Scholar]
- Lemaillet, G., B. Walker, and S. Lambert. 2003. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 278:27333–27339. [DOI] [PubMed] [Google Scholar]
- Ogawa, Y., D.P. Schafer, I. Horresh, V. Bar, K. Hales, Y. Yang, K. Susuki, E. Peles, M.C. Stankewich, and M.N. Rasband. 2006. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J. Neurosci. 26:5230–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z., T. Kao, Z. Horvath, J. Lemos, J.-Y. Sul, S.D. Cranstoun, M.V. Bennett, S.S. Scherer, and E.C. Cooper. 2006. A common ankyrin-G-based mechanism retains KCNQ and Nav channels at electrically active domains of the axon. J. Neurosci. 26:2599–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ponce, D., M. Tapia, A. Munoz, and J.J. Garrido. 2008. New role of IKK alpha/beta phosphorylated IkappaB alpha in axon outgrowth and axon initial segment development. Mol. Cell. Neurosci. 37:832–844. [DOI] [PubMed] [Google Scholar]
- Schultz, C., H.G. Konig, D. Del Turco, C. Politi, G.P. Eckert, E. Ghebremedhin, J.H. Prehn, D. Kogel, and T. Deller. 2006. Coincident enrichment of phosphorylated IkappaBalpha, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol. Cell. Neurosci. 33:68–80. [DOI] [PubMed] [Google Scholar]
- Shrager, P. 1987. The distribution of sodium and potassium channels in single demyelinated axons of the frog. J. Physiol. 392:587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart, A., J.S. Trimmer, and G. Matthews. 2007. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 500:339–352. [DOI] [PubMed] [Google Scholar]
- Xu, X., and P. Shrager. 2005. Dependence of axon initial segment formation on Na+ channel expression. J. Neurosci. Res. 79:428–441. [DOI] [PubMed] [Google Scholar]
- Zhou, D., S. Lambert, P.L. Malen, S. Carpenter, L.M. Boland, and V. Bennett. 1998. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]