Abstract
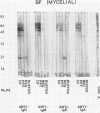
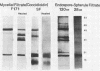
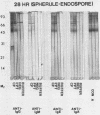
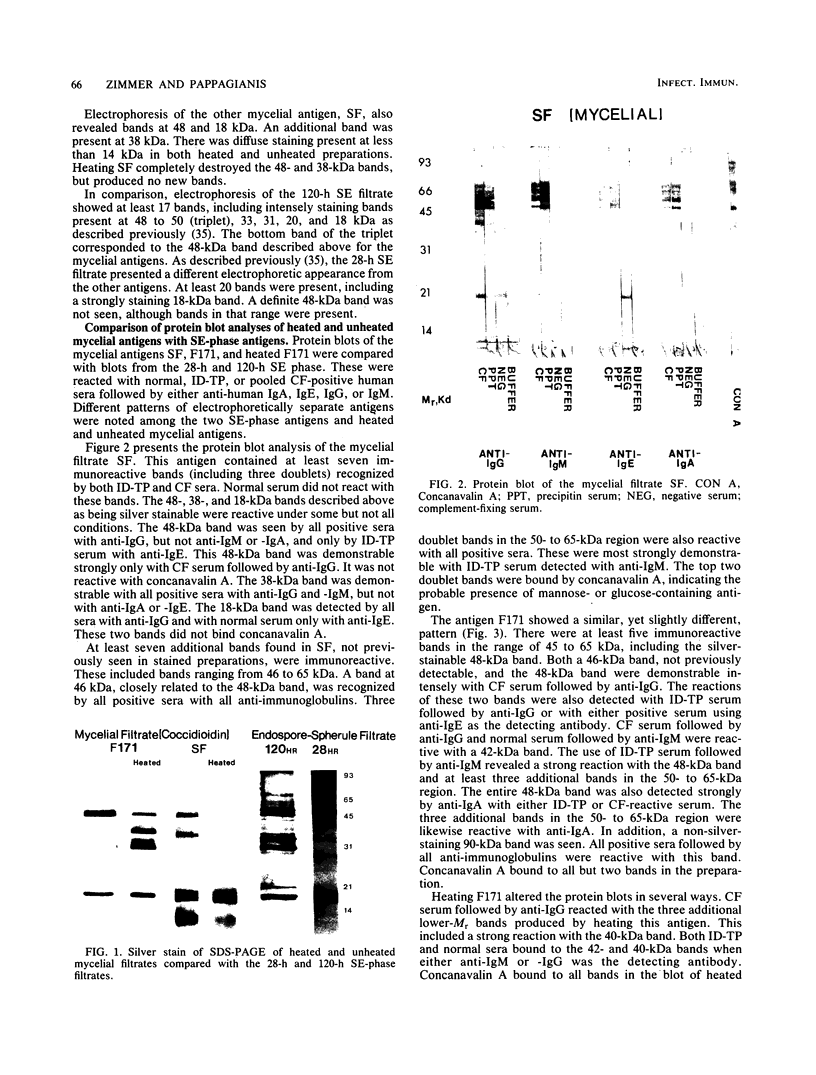
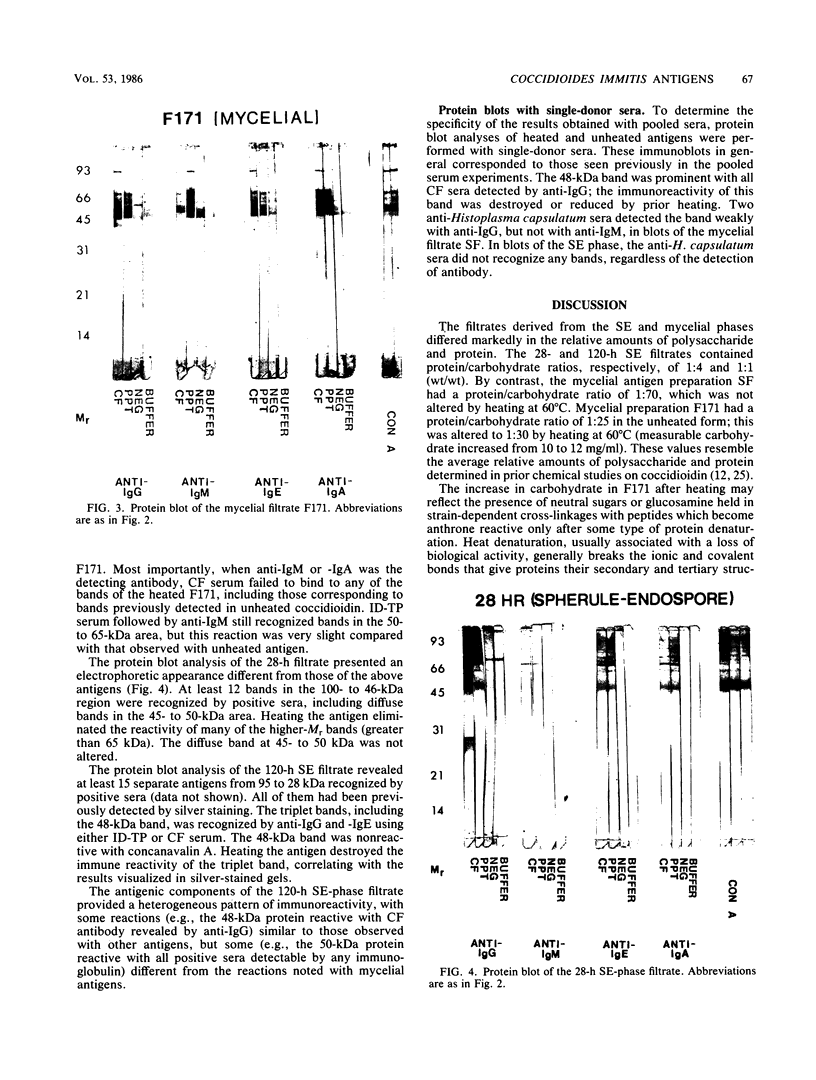
The extracellular proteins produced by Coccidioides immitis during growth of the spherule-endospore-phase and mycelial-phase antigen (coccidioidin) were studied by polyacrylamide gel electrophoresis followed by immunoblot analysis to detect specific serologic function. Filtrates obtained from 28- and 120-h growth of the spherule-endospore phase were compared with each other and with coccidioidin by using negative, immunoglobulin M (IgM) precipitin-positive, or complement fixation-positive pooled and single human sera followed by peroxidase-labeled anti-human IgA, IgE, IgG, or IgM (heavy chain specific) or peroxidase-labeled concanavalin A to detect the reaction. A total of 35 bands was seen in the stained gels. Different patterns were noted among the two spherule-endospore preparations and unheated and heated coccidioidin. At least 15 electrophoretically separate antigens were detected with positive serum ranging in approximate molecular weight (Mr) from 100,000 to 18,000. Most were clustered between 45 and 60 kilodaltons (kDa). Common bands were noted at 48 and 18 kDa. At least one band at 48 kDa was strongly reactive with complement fixation-positive serum demonstrated by reaction with anti-IgG and anti-IgE. In contrast, doublet bands in the 50- to 65-kDa area were highly reactive with IgM precipitin-positive serum detected by anti-IgM. IgM antibodies present in both positive sera reacted with a band at 46 kDa which was not reactive with IgG. Heating the antigens altered the reactivity of many of the antigens, including the 48-kDa band, but not the 46-kDa band.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERMAN R. J., FRIEDMAN L., ROESSLER W. G., SMITH C. E. The virulence and infectivity of twenty-seven strains of Coccidioides immitis. Am J Hyg. 1956 Sep;64(2):198–210. doi: 10.1093/oxfordjournals.aje.a119834. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- CAMPBELL C. C., BINKLEY G. E. Serologic diagnosis with respect to histoplasmosis, coccidioidomycosis, and blastomycosis and the problem of cross reactions. J Lab Clin Med. 1953 Dec;42(6):896–906. [PubMed] [Google Scholar]
- Cox R. A., Arnold D. R. Immunoglobulin E in coccidioidomycosis. J Immunol. 1979 Jul;123(1):194–200. [PubMed] [Google Scholar]
- Cox R. A., Baker B. S., Stevens D. A. Specificity of immunoglobulin E in coccidioidomycosis and correlation with disease involvement. Infect Immun. 1982 Aug;37(2):609–616. doi: 10.1128/iai.37.2.609-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Huppert M., Starr P., Britt L. A. Reactivity of alkali-soluble, water-soluble cell wall antigen of Coccidioides immitis with anti-Coccidioides immunoglobulin M precipitin antibody. Infect Immun. 1984 Feb;43(2):502–507. doi: 10.1128/iai.43.2.502-507.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgenhauer K. Quantitation and specific detection methods after disc electrophoresis of serum proteins. Clin Chim Acta. 1970 Feb;27(2):305–312. doi: 10.1016/0009-8981(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Harrison J. L. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia. 1982 May 22;78(2):107–122. doi: 10.1007/BF00442634. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Pappagianis D., Cobb J. M. Development of vaccines for coccidioidomycosis. Mycopathol Mycol Appl. 1970;41(1):177–185. doi: 10.1007/BF02051493. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., LINDSEY N. J., SMITH C. E., SAITO M. T. ANTIBODIES IN HUMAN COCCIDIOIDOMYCOSIS: IMMUNOELECTROPHORETIC PROPERTIES. Proc Soc Exp Biol Med. 1965 Jan;118:118–122. doi: 10.3181/00379727-118-29773. [DOI] [PubMed] [Google Scholar]
- SALVIN S. B., SMITH R. F. Antigens from the yeast phase of Histoplasma capsulatum. III. Isolation, properties, and activity of a protein-carbohydrate complex. J Infect Dis. 1959 Jul-Aug;105(1):45–53. doi: 10.1093/infdis/105.1.45. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., BEARD R. R., KEPP R. M., CLARK R. W., EDDIE B. U. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Hyg. 1950 Jul;52(1):1–21. doi: 10.1093/oxfordjournals.aje.a119404. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., SIMONS S. A. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc. 1956 Feb 18;160(7):546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- Sawaki Y., Huppert M., Bailey J. W., Yagi Y. Patterns of human antibody reactions in coccidioidomycosis. J Bacteriol. 1966 Jan;91(1):422–427. doi: 10.1128/jb.91.1.422-427.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R. W., Tritschler C., Conant N. F., Lowe E. P. Comparison of Coccidioides immitis arthrospore, mycelium, and spherule cell walls, and influence of growth medium on mycelial cell wall composition. Infect Immun. 1977 Jul;17(1):91–97. doi: 10.1128/iai.17.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]