Abstract
Overexpression of ATP-binding cassette (ABC) drug transporters that actively efflux a variety of amphipathic compounds can cause multidrug resistance (MDR) in cancer cells, which is a major obstacle in the success of cancer chemotherapy. The development of synthetic small molecule compounds or the identification of natural products that block ABC transporter-mediated efflux has been the conventional approach used to combat MDR. The strategy of using chemosensitizers, however, has not been successful in clinical cancer chemotherapy. Therefore, alternative approaches to identify or to synthesize compounds that can induce selective toxicity in cancer cells overexpressing one or more ABC transporters have been undertaken. This review summarizes the recent advances in identifying strategies to restore sensitivity to chemotherapeutics in multidrug resistant cancer cells.
Keywords: ATP-binding cassette transporters, Multidrug resistance, Chemosensitizers, Modulators, Collateral sensitivity
THE FUNCTION AND SIGNIFICANCE OF ATP-BINDING CASSETTE TRANSPORTERS IN THE DEVELOPMENT OF MULTIDRUG RESISTANCE IN CANCER CELLS
Multidrug resistance (MDR) in cancer is a phenomenon that occurs when cancer cells become simultaneously resistant to structurally unrelated chemotherapeutic agents. MDR in cancer patients will eventually lead to the failure of cancer treatment. Several cellular mechanisms can be responsible for MDR, such as reduced apoptosis, advanced DNA damage repair mechanisms or altered drug metabolism. However, the most common mechanism of resistance is the active efflux of drugs by ATP-binding cassette (ABC) transporters. These transporters have important physiological roles in mammalian cells, which have been extensively reviewed [1–4].
ABC drug transporters
ABC transporters are membrane proteins consisting of both transmembrane domains (TMDs) and distinctive nucleotide-binding domains (NBDs), which generate energy from ATP hydrolysis to actively transport a variety of compounds across the membrane [4]. These transporters belong to the ABC protein superfamily which is subdivided into seven distinct subfamilies (ABCA-ABCG) based on sequence homology and domain organization. Among others, three members of the ABC transporter family, ABCB1 (P-glycoprotein or Pgp), ABCC1 (MRP1) and ABCG2 (MXR, BCRP), appear to play an important role in the development of MDR in cancer cells. Several members of this family are capable of actively transporting a wide range of substrates including ions, sugars, amino acids, lipids, toxins and anticancer drugs. Essentially, when these ABC drug transporters are overexpressed in cancer cells, they can confer cross-resistance to multiple drugs of differing chemical classes by actively effluxing cytotoxic drugs, thus reducing the accumulated amount of drug below the effective chemotherapeutic level and resulting in MDR [5–7]. In addition, at least 15 genetic disease conditions are associated with defects in 20 members of the ABC superfamily, such as Cystic fibrosis (ABCC7), Tangier Disease (ABCA1), Dubin-Johnson syndrome (ABCC2) and Pseudoxanthoma elasticum (ABCC6) [1].
P-glycoprotein (ABCB1)
ABCB1 was the first human ABC drug transporter identified and has been studied extensively [8]. It transports a broad variety of compounds, including some of the most popular anticancer drugs such as taxanes, anthracyclines and Vinca alkaloids [8]. Since all attempts to obtain crystals of human ABCB1 suitable for X-ray crystallography have failed thus far, the structure of ABCB1 (and other human ABC drug transporters) is predicted based on biochemical studies, mutational analysis and structural information from bacterial homologs such as Sav1866 [9]. Although a low resolution structure based on electron microscopy has been described [10, 11], the predicted structure of human ABCB1 is believed to consist of 2 halves, each with one transmembrane domain (TMD) containing six transmembrane helices and 1 NBD, with helices 4, 5 and 6 in the N-terminal half and helices 10, 11 and 12 in the C-terminal half to form the transport substrate site(s) [12–14].
ABCC1 and ABCG2
ABCC1 was the first member of the MRP family that was found to be linked with MDR by Cole et al. [15]. Structurally, ABCC1 is predicted to have three TMDs containing17 transmembrane helices. Unlike ABCB1, it has one additional TMD with five transmembrane helices within its N-terminal region [16, 17]. In addition to transporting its physiological substrate such as oxidized glutathione (GSSG) [18] or LTC4 [19, 20], ABCC1 is capable of transporting anticancer drugs such as anthracyclines and mitoxantrone, as well as drugs conjugated to glutathione- (GSH), sulfate- or glucuronate [21]. A more recently identified drug transporter is ABCG2, also known as ABCP [22], breast cancer resistance protein [23] or mitoxantrone resistance protein [24], which is a half-transporter with one NBD and one TMD consisting of 6 transmembrane helices. Unlike ABCB1 and ABCC1, ABCG2 must dimerize to function, and it is believed to function as a homodimer or oligomer. [25]. Similar to ABCB1 and ABCC1, ABCG2 transports a variety of drugs, including anthracyclines, mitoxantrone, topotecan, etoposide, prazosin and flavopiridol [26–30] as well as other compounds such as riboflavin [31] and sterols [32, 33]. ABCG2 is expressed on stem cells of both normal and cancer lineages [34].
Clinical significance
Cancer treatment often involves the use of chemotherapeutic agents; yet these drugs are not always effective. This loss of efficacy is predominantly correlated with the overexpression of ABC drug transporters [5], which was first described in the early 1970’s [35]. Various tumors such as renal cell, adrenocortical, colon and hepatocellular cancers express ABCB1 and are principally chemoresistant [36]. In contrast, exposure to chemotherapeutics causes an upregulation of ABC transporters on relapse of disease in cancers with low baseline expression of these transporters. Expression of ABC transporters is well documented in patients with leukemia; in acute myelocytic leukemia (AML) 30% of patients express ABCB1 while over 50% express ABCB1 at relapse. Plasschaert and colleagues showed that ABCG2 has higher expression and is functionally more active in acute lymphoblastic leukemia (ALL) B-lineage than in T-lineage samples [37]. In their report, the wild type ABCG2 gene was found in the samples which transported Rhodamine 123. Although the expression of ABCB1 plays an important role in MDR for leukemia, there are discrepancies in studies performed to evaluate the importance of ABCG2 in this cancer (reviewed in [7]). Expression of ABCG2 has been reported in a variety of other cancers and is most prominent in colon, stomach and esophageal cancer [38].
In addition to causing multidrug resistance, ABC transporters have a great impact on the pharmacokinetics of chemotherapeutic agents. Both ABCB1 and ABCG2 are expressed at the blood-brain barrier and in the gastrointestinal tract. Expression of ABC transporters in these biological barriers limits the absorption of various compounds in these tissues. The oral bioavailability of a number of anticancer agents is altered by ABC transporter expression. [39–42]. ABCG2 has also been identified in the apical membrane of alveolar epithelial cells in lactating mammary glands of mice, cows and humans [43] and there serves to transfer drugs and xenotoxins into breast milk. Although ABCG2 plays a protective role in the mammary glands, paradoxically, carcinogens and toxins are concentrated and passed on to the infant by ABCG2. In contrast, ABCG2 localized to the placenta effluxes compounds away from the fetus and plays a role in the maternal-fetal barrier [44]. Investigators have reported a variety of compounds such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and riboflavin which have been secreted by ABCG2 into breast milk [31, 43, 45, 46]. There have been many recent reviews which focus on the clinical significance of ABC transporters in the pharmacokinetics of drugs [6, 47, 48]. In the following sections, we summarize the strategies employed by researchers to combat MDR in cancer.
RE-SENSITIZING MDR CANCER CELLS TO ANTICANCER DRUGS BY DEVELOPING INHIBITORS TO ABC DRUG TRANSPORTERS
Ideally, the most direct and easiest way to restore drug sensitivity in MDR cancer cells caused by ABC drug transporters is to block transporter-mediated drug efflux. Since 1980, researchers have been searching for both broad-spectrum and specific modulators that can reverse MDR in cancer cells. Tremendous efforts have been made to discover and synthesize such inhibitors/modulators. Several examples of ABC drug transporter inhibitors which have been discovered or synthesized are listed in Table 1. In addition, alternative strategies such as regulating expression of drug transporters or using drugs that specifically target drug transporters are also discussed later.
Table 1.
Inhibitors of major ABC drug transporters, ABCB1 (Pgp), ABCC1 (MRP1) and ABCG2
| Modulator | ABC drug transporter(s) affected | Reference |
|---|---|---|
| Verapamil | ABCB1
ABCC1 |
[51]
[145] |
| Cyclosporine A | ABCB1
ABCC1 ABCG2 |
[55, 56]
[145] [60, 61] |
| SDZ PSC 833 | ABCB1 | [63, 64] |
| S9788 | ABCB1 | [62] |
| MS-209 | ABCB1
ABCC1 |
[146]
[145, 147] |
| Biricodar/VX-710 | ABCB1
ABCC1 ABCG2 |
[148, 149]
[145] [149] |
| GF120918/Elacridar | ABCB1
ABCG2 |
[68]
[76] |
| OC-144-093/ONT-093 | ABCB1 | [69] |
| Tariquidar/XR 9576 | ABCB1 | [70, 73] |
| LY 335979/Zosuquidar | ABCB1 | [150] |
| Laniquidar/R101933 | ABCB1 | [151] |
| Mitotane | ABCB1 | [152] |
| WK-X-34 | ABCB1
ABCG2 |
[153]
[154] |
| Annamycin | ABCB1
ABCC1 |
[155]
[156] |
| MK-571 | ABCC1 | [85] |
| Fumitremorgin C | ABCG2 | [86] |
| Ko143 | ABCG2 | [87] |
| PK11195 | ABCB1
ABCC1 ABCG2 |
[157]
[157] [157] |
| Disulfiram | ABCB1
ABCC1 |
[158]
[158] |
| Antimalarials: (Quinine, quinidine, mefloquine, chloroquine, primaquine, pyronaridine) | ABCB1
ABCC1 |
[159–161]
[162, 163] |
| Polyphenols: (Quercetin, silymarin, hesperetin, daidzein; resveratrol, naringenin, genistein) | ABCB1
ABCC1 ABCG2 |
[90]
[89, 164] [88, 165] |
| Curcumin | ABCB1
ABCC1 ABCG2 |
[91]
[93] [92] |
| Ginsenoside | ABCB1
ABCG2 |
[166]
[167] |
Designing or finding potent chemosensitizers that are selective, low in intrinsic toxicity and highly effective has been more difficult than expected. However, how to tackle drug resistance using available pharmacological and structural information to select or design new inhibitors (reviewed in [3, 49, 50]) is now much clearer. It is agreed in principle that an inhibitor/chemosensitizer must be able to increase intracellular anticancer drug levels, restore drug sensitivity and/or interfere with photoaffinity labeling of a particular drug transporter [49]. The first ABCB1 chemosensitizer was identified in 1981 by Tsuruo et al. when the calcium channel blocker verapamil was found to re-sensitize vincristine-resistant P388 leukemia cells to vincristine and vinblastine [51]. A subsequent study presented direct evidence that verapamil restored Vinca-alkaloid toxicity by increasing its accumulation in resistant cells [52]. However, verapamil at its highest tolerable concentration failed to enhance vinblastine efficacy in a phase I clinical trial carried out in 1985 [53]. Several years later, the immunosuppressant cyclosporine A (CsA) was shown to completely re-sensitize a resistant variant of human T-cell acute lymphatic leukemia cell line to vincristine and daunorubicin. Furthermore, CsA was also effective against doxorubicin resistance in solid tumors [54]. It was thereafter used as a benchmark for ABCB1 inhibitors for in vitro studies due to its high potency and low intrinsic toxicity [55, 56]. Unfortunately, similarly to verapamil in clinical trials, CsA failed to achieve clinical inhibition of ABCB1 at the concentrations tested [57–59]. More recently, CsA was also shown to block ABCG2-mediated efflux and restore drug sensitivity in ABCG2 overexpressing cells [60, 61]. It is important to note that both verapamil and CsA are transported by ABCB1 and thus, they modulate the efflux function by competing for the substrate binding site(s).
After the failure of these 1st generation ABCB1 inhibitors, the quantitative structural activity relationship approach was used to generate the 2nd generation of ABCB1 inhibitors such as SDZ PSC833 (Valspodar) and S9788. SDZ PSC833 is a non-immunosuppressive CsA derivative developed in 1991, and S9788 is a triazine that was designed based on the chemical structure of verapamil [62, 63]. Disappointingly, despite being much more potent than CsA in in vitro testing [64], serious complications arose in clinical trials when SDZ PSC833 was used in combination with anticancer drugs [65, 66]. It emerged that SDZ PSC833 partially impairs drug metabolism and elimination, significantly reduces the systemic clearance of anticancer drugs and consequently elevates toxicity [65, 66]. More recently, SDZ PSC833 was tested on patients with recurring or refractory multiple myeloma, but again failed to improve the treatment [67].
GF120918 (Elacridar), OC144-093 (Ontogen), XR9576 (Tariquidar) and LY335979 (Zosuquidar) are 3rd generation ABCB1 inhibitors (see Table 1). They were synthesized in an attempt to improve on the 2nd generation inhibitors [68–71] and are reported to be more selective and work in the nanomolar concentration range [72–74]. LY335979 very potently and specifically inhibit ABCB1 function [75]. It was able to reduce tumor mass and prolong survival in mice engrafted with drug resistant human tumors [75]. On the other hand, GF120918 [68, 76] and the anthranilamide derivative XR9576 [72, 73, 77] inhibit not only ABCB1, but also ABCG2-mediated transport. GF120918 sensitized human MDR sarcoma MES-Dx5 cells and improved topotecan bioavailability in mice [28, 39]. Phase I and II clinical trials have been and are being performed on some of these 3rd generation inhibitors [78–81], and results are very promising [82–84].
ABCC1 [15] and ABCG2 [23] are more recently identified ABC drug transporters. Therefore, data on them are not as extensive as that for ABCB1. In 1995, a leukotriene LTD4 receptor antagonist MK-571 was discovered by Gekeler et al. to inhibit ABCC1-mediated transport without any effects on ABCB1 [85]. Being low in intrinsic toxicity, relatively potent and specific, MK-571 is thus still the benchmark inhibitor to block ABCC1-mediated drug transport. Soon after the discovery of ABCG2, a fungal toxin Fumitremorgin C (FTC), was shown to inhibit ABCG2-mediated transport [86]. FTC is both highly potent and specific, but with undesirable neurotoxic effects in vivo. Subsequent studies resulted in the development of Ko143, a new tetracyclic analog of FTC that is even more potent and specific yet non-toxic, which is ideal for future clinical studies [87].
Furthermore, many natural products have shown promising chemosensitizing effects on ABC drug transporters. Compounds such as curcumin, ginsenoside, some polyphenols and anti-malarials are derived from natural sources and show low intrinsic toxicity. Many of them demonstrate broad-spectrum modulatory effects on more than one ABC drug transporter (Table 1). For example, plant polyphenols [88–90] and curcumin [91–93] have been reported to modulate all three major ABC drug transporters: ABCB1, ABCC1 and ABCG2. However, regardless of the source of the inhibitors, unpredictable pharmacokinetic drug interactions, simultaneous involvement of several drug transporters in tumor tissues, as well as the variability in drug transporter expression levels among individuals, remain major obstacles to using modulators to restore drug sensitivity in the clinic.
ALTERNATIVE APPROACHES TO REVERSING MDR
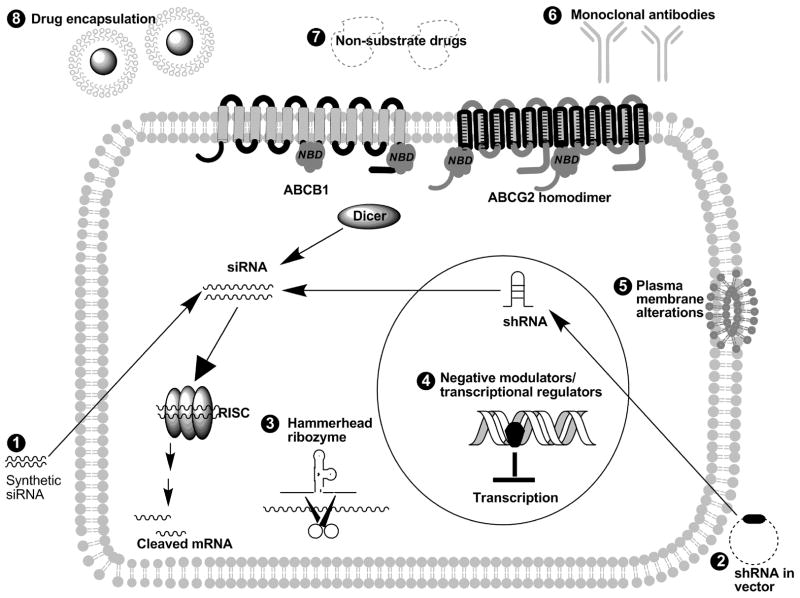
With the lack of success in inhibiting multidrug resistance using traditional drug inhibitors, investigators have designed novel compounds to circumvent ABC transporters by a variety of mechanisms. One popular method is to target mRNA. This can be accomplished by antisense oligonucleotides, hammerhead ribozymes, and siRNA. In addition, investigators have developed transcriptional regulators, agents to alter the plasma membrane (see Fig. (1)) as well as compounds that selectively target MDR cancer cells (Table 2).
Fig. 1. Schematic of the various methods used to circumvent MDR mediated by ABC transporters.
Gene silencing of ABC transporters can be performed using synthetic siRNA(1), shRNA in a vector (2), and hammerhead ribozyme (3). Numerous negative modulators/transcriptional regulators (4) can inhibit transcription of these transporters in the nucleus. MDR can also be modulated by plasma membrane alterations (5). In addition, monoclonal antibodies (6), non-substrate drugs (7), and drug encapsulation (8) have also been used to evade MDR in cancer cells.
Table 2.
Compounds that selectively target cells overexpressing ABC drug transporters
| Compound (Reference) | Class of compound | Cell lines | Transporter overexpressed |
|---|---|---|---|
| Verapamil/nifedipine/calmodulin inhibitor Trifluoperazine [134–136] | Calcium channel blockers | CHO MDR cells | ABCB1 |
| Verapamil (S-isomer) [168, 169] | Calcium channel blocker | BHK-21 | ABCC1 |
| Bisdioxopiperazine dexrazoxane/ICRF-187 [170] | Topoisomerase II inhibitor | K/VP.5 | ABCB1 |
| Tunicamycin [171] | N-linked glycosylation inhibitor | KB-C-1 | ABCB1 |
| Gemcitabine [172] | Deoxycytidine analog | H69/DAU; NYH/VM | ABCB1 |
| Gemcitabine [141] | Deoxycytidine analog | 2R120; 2R160; SW1573/S1; SW1573/S1; GLC4/ADR; 2780AD; KB-8-5; BROmdr | ABCB1; ABCC1 |
| Cytosine arabinoside [172] | Deoxycytidine analog | H69/DAU; NYH/VM | ABCB1 |
| 5-fluorouracil [142] | Thymidylate synthase inhibitor | KB-C-1; KB-V-1; KB-A-1 | ABCB1 |
| LY294002 [140] | PI3-kinase inhibitor | KB-V-1 | ABCB1 |
| NSC73306 [137] | Thiosemicarbazone | KB-V-1; KB-8-5; KB-8-5-11; NCI/ADR-RES | ABCB1 |
| KP772/FFC24 [139] | Lanthanum compound | GLC4/ADR; HL60/ADR; KB-C-1; MCF-7/bcrp | ABCB1; ABCC1; ABCG2 |
| Celecoxib [143] | COX-2 inhibitor | HT-29-dx; PN1A-NIH3T3 | ABCB1 |
| NBDHEX [173] | Glutathione-S-transferase inhibitor | CEM-VBL10; CEM-VBL100; U-2 OS/DX | ABCB1 |
Antisense oligonucleotides
Antisense oligonucleotides are an alternative method to inhibit the expression of ABC transporters. The mechanisms by which these oligonucleotides function are complex and have not been fully elucidated [94]. Phosphorothioate oligonucleotides are the first-generation of anti-sense molecules. They are more resistant to nucleases, but may produce pharmacological effects unrelated to the anti-sense effects. One major concern regarding these molecules is cellular uptake, and investigators have demonstrated that administration with Lipofectin is necessary to obtain partial gene silencing of ABCB1 [95]. In addition, uptake enhancing modifications such as 5′ cholesterol-conjugation produced improved silencing without the need for cationic lipids. Concentrations in the low micromolar range are necessary to reduce protein levels by half [96]. Kang et al. report that chimeric hexitol nucleic acid gapmer oligonucleotides are effective at inhibiting ABCB1 gene expression at nanomolar concentrations [97].
Hammerhead ribozymes
Ribozymes are catalytic RNAs that have intrinsic endoribonucleolytic cleavage activity which can be used to target a specific mRNA at a specific position containing a NUX motif, where N is any nucleotide and X is A, C or U. Investigators have designed ribozymes to target ABCB1[98], ABCG2 [99], and ABCC2 [100]. These three ribozymes were then combined in a multitarget multiribozyme (MTMR) containing three trans-acting hammerhead ribozymes directed against ABCB1, ABCG2 and ABCC2, three cis-acting ABCB1 ribozymes, and three ABCB1 homologous spacer sequences [101]. This self-contained MTMR undergoes autocatalytic self-cleavage by the cis-acting ribozymes to free the trans-acting ribozymes to act on the targeted transcripts. This novel approach was able to cleave the ABC transporter-specific transcripts in drug-resistant cancer cell lines.
RNA interference (siRNA)
Small interfering RNAs (siRNAs) are used to target ABC transporters at the mRNA level. This double-stranded RNA, normally between 19–21 nucleotides in length, is designed to enhance the degradation of the single-stranded RNA sequence of the desired gene. Dicer, the RNAse III enzyme, processes the double-stranded RNA into siRNA that incorporates into the multiprotein RNA-induced silencing complex (RISC) which cleaves the targeted mRNA [102]. siRNA possesses several advantages over antisense oligonucleotides, which include ease of delivery, lower concentrations needed for gene silencing, and the ability to silence genes at any stage in development. The transient silencing effects of siRNA are however, one drawback of this technology. Investigators have shown that both synthetic and vector-based expression of siRNA can specifically decrease expression of ABCB1 and ABCB4 in paclitaxel-resistant ovarian cancer cells [103]. siRNA has also been utilized to modulate expression of ABCC2 and ABCC3 in primary hepatocytes [104], ABCG2 in BeWo cells [105], and ABCB1 using a combination of siRNAs [106]. The half-life of the ABCB1 mRNA (4 hours), and protein (16 hours) [107], allows for an increase in transcript and protein level to original levels 7 days after siRNA administration. To extend the silencing effects of siRNA, others have engineered an H1-RNA gene promoter-driven expression vector encoding anti-ABCB1 [108], anti-ABCC2 [109] and anti-ABCG2 [110] short hairpin RNA (shRNA), which shows the highest efficacy to date in gene-silencing. The efficiency of gene knock-down depends on the delivery into the cell, and studies report that an adenoviral-based delivery of shRNA is superior to adenoviral delivery of ribozymes [111]. Others studies show that attenuated Salmonella typhimurium has potential as an in vivo delivery vector for ABCB1 siRNA in a human tongue squamous cell cancer mouse model [112]. In addition, a transposon-based Sleeping Beauty (SB)-based RNAi system produces stable and durable silencing of ABCB1 [113]. This non-viral siRNA transposon-based SB vector was utilized to show that silencing of ABCB1 causes increases in imatinib intracellular levels in chronic myeloid leukemia cells [114] and that two proteasome inhibitors used to treat relapsed or refractory multiple myeloma are substrates for ABCB1 [115]. Investigators have used a retroviral-mediated shRNAi for ABCB1 in vivo and provided documentation of the effect in the intact animal using bioluminescence [116]. Stein et al. have recently reported a complete reversal of the MDR phenotype in vivo using an intratumoral jet-injection of anti-ABCB1 short hairpin RNA-encoding plasmid DNA [117].
Transcriptional regulation
Investigators have also identified a number of transcriptional regulators of ABC transporters. For instance, transcriptional decoys have been employed to decrease drug resistance. In one such study, investigators used phosphorothioate-modified antisense oligonucleotides targeted at the transcriptional MED-1(Multiple start site Element Downstream) element of the human ABCB1 gene promoter to modulate multidrug resistance [118]. The human MED-1 cis-element is unique because it has an inverted sequence compared to the consensus MED-1 sequence in other TATA-less promoters which allows for targeted silencing of the ABCB1 MED-1. Investigators have also utilized LANCL2, a gene overexpressed in 20% of all glioblastomas, which transcriptionally suppresses ABCB1 due to decreased ABCB1 promoter activity [119]. Others have constructed the K2-5F repressor consisting of five Zif DNA-binding domains directed against the ABCB1 promoter (SP1/EGR1/WT1) and two KRAB-A domains [120]; ponasterone induction of K2-5F caused reduction in cell surface expression, in total protein and in mRNA of ABCB1 [121]. Scala et. al. showed that 8-CL-c-AMP, a type I cAMP-dependent protein kinase (PKA) inhibitor, downregulated ABCB1 expression in a dose dependent manner, indicating a role for PKA in ABCB1 promoter activity [122]. Inhibition of Protein Kinase C (PKC) can also prevent ABCB1 upregulation by extracellular stimuli [123]. A natural marine product, Et743, is an inhibitor of ABCB1 transcription due to blockade of promoter activation [124]. Lastly, NF-κB has also been implicated in ABCB1 regulation [125]. Some researchers believe that ABCC1 is down regulated by p53, possibly by reducing Sp1 binding [126] while others have reported that the transcription factor complex AP-1 regulates ABCC1 [127].
Plasma membrane alterations
There are also agents that modify the plasma membrane and thus prevent ABCB1 overexpression. Fatty acid-polyethylene glycol fatty acid diesters have been used to block multidrug resistance in Ara C-treated cells [128]. These diesters work on the cell surface and were able to prevent both short-term ABCB1 expression and the multidrug resistance phenotype in cells that survived the initial treatment with chemotherapeutics.
Encapsulation of drugs
Enhancements in drug delivery systems have also been used to circumvent ABC transporters. One such advance is the use of encapsulation to deliver drugs in a tumor site-directed manner. For instance, investigators have developed poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles to co-administer paclitaxel and ceramide, an apoptosis modulator [129]. Nanoparticles with paclitaxel and ceramide were able to sensitize multidrug resistant cells to the same concentrations of paclitaxel to which the drug-sensitive cells were susceptible. Liposomal formulations of doxorubicin have also been successfully developed to bypass ABC transporters [130].
Antibodies
Antibodies have been used to combat multidrug resistance. Palmitoylated synthetic peptides of the extracellular loops of ABCB1 were reconstituted in liposomes with or without Lipid A, then resuspended in alum [131]. Interestingly, the mice did not show auto-immune symptoms; nevertheless, immunization increased survival half-time by 77% and efficacy of chemotherapy against P388 R cells. In vitro studies of such cells administered the sera of immunized mice showed similar cytotoxic results.
Drugs that are not substrates for the major ABC drug transporters
Presently, the pharmaceutical industry and the Food and Drug Administration (FDA) are aware of the alterations that ABC transporters can cause in the pharmacokinetics as well as in the efficacy of drugs. Thus, potential drug compounds are commonly tested to determine if they are substrates for ABC transporters. Consequently, medicinal chemists have been synthesizing analogues of drugs to circumvent multidrug resistance mediated by ABC transporters. For instance, camptothecin analogs were designed and tested for ABCG2 substrate specificity [132]. Compounds with low polarity were not found to be substrates for either ABCG2 or ABCB1. In addition, drugs designed to be substrates for solute carriers, a superfamily of membrane transport proteins (SLCs) recently shown to be important in the pharmacokinetics of drugs [133], may facilitate the uptake of chemotherapeutics and avoid MDR. The role of solute carriers in cancer chemotherapy has recently been reviewed elsewhere [133]. However, there are no conclusive experimental data available yet on overexpression of solute carriers in drug resistant tumor tissue.
COMPOUNDS SPECIFICALLY TARGETING MDR CANCER CELLS
Despite tremendous efforts to develop inhibitors (both specific and broad-spectrum) and to discover natural products modulators of ABC drug transporters, there are still no inhibitors currently used in clinical treatment. Verapamil, one of the first ABCB1 modulators found to effectively reverse MDR mediated by ABCB1, was also one of the first compounds to show high collateral toxicity towards ABCB1-overexpressing Chinese hamster ovary cells. This “hypersensitivity” or “collateral sensitivity” provides researchers with an alternative approach that can be used to combat MDR caused by the overexpression of ABC drug transporters. Numerous research groups are searching for compounds with substantial collateral toxicity towards MDR cell lines which can induce apoptosis more specifically in MDR cell lines. These compounds show potential for use in the clinic, either alone or in combination with already existing cancer chemotherapeutics. Examples of compounds exhibiting collateral sensitivity are listed in Table 2.
In 1987, Cano-Gauci and Riordan discovered that ABCB1-overexpressing Chinese hamster ovary (CHO) cells were hypersensitive to calcium channel blockers such as verapamil, nifedipine and the calmodulin inhibitor trifluoperazine [134]. This hypersensitivity is both calcium-independent and independent of intracellular verapamil accumulation [134]. Subsequent studies by Warr et al. in 1988 demonstrated that in addition to calcium channel blockers, vincristine-resistant CHO cells are also hypersensitive to the membrane active agent quinidine sulfate, suggesting that calcium channels are not the primary target of this hypersensitivity or collateral toxicity [135]. More recently, Karwatsky et al. focused on the mechanistic aspect of the observed hypersensitivity of MDR CHO cells to verapamil [136]. It was concluded that apoptosis was caused by elevating the production of reactive oxygen species (ROS) in MDR cells. Moreover, this collateral toxicity was correlated with high ATPase activity, independent of p53 activity, and could be inhibited by the overexpression of Bcl-2 [136].
Recently, Ludwig et al. identified a small molecule thiosemicarbazone, NSC73306, which can induce cytotoxicity specifically in cancer cells overexpressing functional ABCB1 [137]. The hypersensitivity is correlated directly with both the increased ABCB1 function and MDR. Moreover, culturing MDR cells in the presence of NSC73306 resulted in the loss of ABCB1 expression and consequent loss of the MDR phenotype. Despite the correlation of NSC73306 cytotoxicity with the expression and function of ABCB1, there is no evidence to show direct interaction of NSC73306 with ABCB1. Considering that NSC73306 also possesses divalent metal chelation properties and that this effect is not limited to Pgp-expressing CHO cells, it is likely to have a distinct mechanism of collateral sensitivity unlike that of verapamil, even though the final phenomenon is somewhat similar. Similarly, Heffeter et. al. discovered a new lanthanum compound, KP772, with anticancer properties in both in vitro and in vivo assays [138], which also has preferential cytotoxicity towards cancer cells overexpressing either ABCB1 (Pgp), ABCC1 (MRP1) or ABCG2 [139]. It was suggested that KP772 induces significantly more apoptosis and cell cycle arrest in cells overexpressing these ABC transporters, explaining hypersensitivity in MDR cancer cells. These observations are consistent with those of Nicholson et al., who reported that a phosphatidylinositol-3-kinase inhibitor (LY294002) induced apoptosis preferentially (6-fold higher) in ABCB1-overexpressing KB-V1 cells [140] compared to drug-sensitive cells. Interestingly, comparably to NSC73306, long term exposure to KP772 resulted in the loss of ABCB1 protein expression in ABCB1-overexpressing cells, and no evidence of direct interaction of KP772 with these ABC transporters was established. Similarly, Bergman et al. observed increases in sensitivity to gemcitabine by both ABCB1- and ABCC1-overexpressing cells, which could be abolished completely by verapamil [141]. It was suggested that this hypersensitivity is due to enhanced cellular stress caused by the overexpression of ABCB1 or ABCC1, resulting in increased gemcitabine metabolism and sensitivity in these MDR cells.
In addition to the above-mentioned compounds, several compounds similar to 5-fluorouracil induce significantly greater apoptosis in MDR carcinoma cells than in drug-sensitive cells after 48 hours of exposure [142]. More recently, celecoxib, a specific inhibitor of COX-2 activity, was also shown to have the same property. Fantappie et al. demonstrated that in MDR cells celecoxib reduces expression of ABCB1, Bcl-xL, and Bcl-2. Celecoxib also induces the translocation of Bax from the cytosol to the mitochondria and cytochrome c into the cytosol, which in turn activates caspase-3-dependent apoptosis [143].
By using a developed method that correlates the activity of candidate anticancer drugs with gene expression of ABCB1 in 60 diverse cancer cell lines used by the National Cancer Institute (NCI-60) [144], it is now possible to identify compounds that are selectively cytotoxic to ABCB1-overexpressing cancer cells. Though the exact mechanism(s) of these compounds is still unclear, several very different, but plausible mechanisms have been proposed. These agents may interfere with the PI3-kinase/PKB pathway, in turn affecting downstream intracellular signaling pathways and inducing apoptosis in MDR cells [140], or they may induce apoptosis by enhanced production of ROS in MDR cells [136]. It is feasible that different compounds have different mechanisms to achieve the same result.
CONCLUSIONS
ABC transporters provide the body vital protection from foreign compounds; however, their overexpression in cancer cells poses a major obstacle to cancer therapy. This is the main cause of treatment failure in cancer patients. Due to the clinical impact of MDR, investigators continue to search for a safe yet effective inhibitor of these transporters. Although some success has been seen in vitro, until now, these results have not been translated to the clinic. A new wave of compounds that have potent collateral toxicity towards MDR cells may serve as the long sought treatment of MDR in cancer chemotherapy. By advances in understanding the molecular pharmacology of these compounds, especially their effects on signaling pathways, successful in vivo inhibition of MDR may soon be realized.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute, NIH, Center for Cancer Research. AMC was supported by the NIGMS Pharmacology Research Associate (PRAT) Program. We thank Dr. Zuben E. Sauna for his helpful suggestions and Mr. George Leiman for his editorial assistance.
Abbreviations
- ABC
ATP-binding cassette
- CsA
cyclosporine A
- FTC
Fumitremorgin C
- MDR
multidrug resistance
- MRP
multidrug-resistance protein
- Pgp
P-glycoprotein
- TMD
transmembrane domain
References
- 1.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 2.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 6.Hardwick LJ, Velamakanni S, van Veen HW. The emerging pharmacotherapeutic significance of the breast cancer resistance protein (ABCG2) Br J Pharmacol. 2007;151:163–174. doi: 10.1038/sj.bjp.0707218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 9.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg MF, Callaghan R, Ford RC, Higgins CF. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg MF, Callaghan R, Modok S, Higgins CF, Ford RC. Three-dimensional structure of P-glycoprotein: the transmembrane regions adopt an asymmetric configuration in the nucleotide-bound state. J Biol Chem. 2005;280:2857–2862. doi: 10.1074/jbc.M410296200. [DOI] [PubMed] [Google Scholar]
- 12.Loo TW, Clarke DM. Identification of residues in the drug-binding domain of human P-glycoprotein. Analysis of transmembrane segment 11 by cysteine-scanning mutagenesis and inhibition by dibromobimane. J Biol Chem. 1999;274:35388–35392. doi: 10.1074/jbc.274.50.35388. [DOI] [PubMed] [Google Scholar]
- 13.Loo TW, Clarke DM. Determining the structure and mechanism of the human multidrug resistance P-glycoprotein using cysteine-scanning mutagenesis and thiol-modification techniques. Biochim Biophys Acta. 1999;1461:315–325. doi: 10.1016/s0005-2736(99)00165-0. [DOI] [PubMed] [Google Scholar]
- 14.Loo TW, Clarke DM. The transmembrane domains of the human multidrug resistance P-glycoprotein are sufficient to mediate drug binding and trafficking to the cell surface. J Biol Chem. 1999;274:24759–24765. doi: 10.1074/jbc.274.35.24759. [DOI] [PubMed] [Google Scholar]
- 15.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 16.Bakos E, Evers R, Szakacs G, Tusnady GE, Welker E, Szabo K, de Haas M, van Deemter L, Borst P, Varadi A, Sarkadi B. Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem. 1998;273:32167–32175. doi: 10.1074/jbc.273.48.32167. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg MF, Mao Q, Holzenburg A, Ford RC, Deeley RG, Cole SP. The structure of the multidrug resistance protein 1 (MRP1/ABCC1). Crystallization and single-particle analysis. J Biol Chem. 2001;276:16076–16082. doi: 10.1074/jbc.M100176200. [DOI] [PubMed] [Google Scholar]
- 18.Hirrlinger J, Konig J, Keppler D, Lindenau J, Schulz JB, Dringen R. The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem. 2001;76:627–636. doi: 10.1046/j.1471-4159.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 19.Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–5242. [PubMed] [Google Scholar]
- 20.Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 21.Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580:1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 23.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 25.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 26.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59:4237–4241. [PubMed] [Google Scholar]
- 27.Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999;59:5938–5946. [PubMed] [Google Scholar]
- 28.Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, Pluim D, Beijnen JH, Schellens JH. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–941. [PubMed] [Google Scholar]
- 29.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 30.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding Cassette Half-Transporter, ABCG2 (MXR/BCRP/ABCP1), in Flavopiridol-resistant Human Breast Cancer Cells. Clin Cancer Res. 2001;7:145–152. [PubMed] [Google Scholar]
- 31.van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol. 2007;27:1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albrecht C, Elliott JI, Sardini A, Litman T, Stieger B, Meier PJ, Higgins CF. Functional analysis of candidate ABC transporter proteins for sitosterol transport. Biochim Biophys Acta. 2002;1567:133–142. doi: 10.1016/s0005-2736(02)00608-9. [DOI] [PubMed] [Google Scholar]
- 33.Janvilisri T, Venter H, Shahi S, Reuter G, Balakrishnan L, van Veen HW. Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003;278:20645–20651. doi: 10.1074/jbc.M301358200. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 35.Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973;323:466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- 36.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plasschaert SL, van der Kolk DM, de Bont ES, Kamps WA, Morisaki K, Bates SE, Scheffer GL, Scheper RJ, Vellenga E, de Vries EG. The role of breast cancer resistance protein in acute lymphoblastic leukemia. Clin Cancer Res. 2003;9:5171–5177. [PubMed] [Google Scholar]
- 38.Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, Germa-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 39.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 40.Seamon JA, Rugg CA, Emanuel S, Calcagno AM, Ambudkar SV, Middleton SA, Butler J, Borowski V, Greenberger LM. Role of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitor. Mol Cancer Ther. 2006;5:2459–2467. doi: 10.1158/1535-7163.MCT-06-0339. [DOI] [PubMed] [Google Scholar]
- 41.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, Daw N, Jenkins JJ, 3rd, Gilbertson R, Germain GS, Harwood FC, Houghton PJ. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 43.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 44.Young AM, Allen CE, Audus KL. Efflux transporters of the human placenta. Adv Drug Del Rev. 2003;55:125–132. doi: 10.1016/s0169-409x(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 45.van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci. 2006;27:10–16. doi: 10.1016/j.tips.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 46.van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis. 2006;27:123–130. doi: 10.1093/carcin/bgi176. [DOI] [PubMed] [Google Scholar]
- 47.Chan LM, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci. 2004;21:25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Merino V, Jimenez-Torres NV, Merino-Sanjuan M. Relevance of multidrug resistance proteins on the clinical efficacy of cancer therapy. Curr Drug Deliv. 2004;1:203–212. doi: 10.2174/1567201043334650. [DOI] [PubMed] [Google Scholar]
- 49.McDevitt CA, Callaghan R. How can we best use structural information on P-glycoprotein to design inhibitors? Pharmacol Ther. 2007;113:429–441. doi: 10.1016/j.pharmthera.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273–286. doi: 10.2174/138161206775201965. [DOI] [PubMed] [Google Scholar]
- 51.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–1972. [PubMed] [Google Scholar]
- 52.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982;42:4730–4733. [PubMed] [Google Scholar]
- 53.Benson AB, 3rd, Trump DL, Koeller JM, Egorin MI, Olman EA, Witte RS, Davis TE, Tormey DC. Phase I study of vinblastine and verapamil given by concurrent iv infusion. Cancer Treat Rep. 1985;69:795–799. [PubMed] [Google Scholar]
- 54.Twentyman PR, Fox NE, White DJ. Cyclosporine A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987;56:55–57. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldberg H, Ling V, Wong PY, Skorecki K. Reduced cyclosporine accumulation in multidrug-resistant cells. Biochem Biophys Res Commun. 1988;152:552–558. doi: 10.1016/s0006-291x(88)80073-1. [DOI] [PubMed] [Google Scholar]
- 56.Slater L, Sweet P, Wetzel M, Stupecky M, Osann K. Comparison of cyclosporine A, verapamil, PSC-833 and cremophor EL as enhancing agents of VP-16 in murine lymphoid leukemias. Leuk Res. 1995;19:543–548. doi: 10.1016/0145-2126(95)00029-n. [DOI] [PubMed] [Google Scholar]
- 57.Bartlett NL, Lum BL, Fisher GA, Brophy NA, Ehsan MN, Halsey J, Sikic BI. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J Clin Oncol. 1994;12:835–842. doi: 10.1200/JCO.1994.12.4.835. [DOI] [PubMed] [Google Scholar]
- 58.Berman E, McBride M, Lin S, Menedez-Botet C, Tong W. Phase I trial of high-dose tamoxifen as a modulator of drug resistance in combination with daunorubicin in patients with relapsed or refractory acute leukemia. Leukemia. 1995;9:1631–1637. [PubMed] [Google Scholar]
- 59.Verweij J, Herweijer H, Oosterom R, van der Burg ME, Planting AS, Seynaeve C, Stoter G, Nooter K. A phase II study of epidoxorubicin in colorectal cancer and the use of cyclosporine-A in an attempt to reverse multidrug resistance. Br J Cancer. 1991;64:361–364. doi: 10.1038/bjc.1991.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta A, Dai Y, Vethanayagam RR, Hebert MF, Thummel KE, Unadkat JD, Ross DD, Mao Q. Cyclosporine A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006;58:374–383. doi: 10.1007/s00280-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 61.Pawarode A, Shukla S, Minderman H, Fricke SM, Pinder EM, O’Loughlin KL, Ambudkar SV, Baer MR. Differential effects of the immunosuppressive agents cyclosporine A, tacrolimus and sirolimus on drug transport by multidrug resistance proteins. Cancer Chemother Pharmacol. 2007;60:179–188. doi: 10.1007/s00280-006-0357-8. [DOI] [PubMed] [Google Scholar]
- 62.Dhainaut A, Regnier G, Atassi G, Pierre A, Leonce S, Kraus-Berthier L, Prost JF. New triazine derivatives as potent modulators of multidrug resistance. J Med Chem. 1992;35:2481–2496. doi: 10.1021/jm00091a017. [DOI] [PubMed] [Google Scholar]
- 63.Twentyman PR, Bleehen NM. Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporine [corrected] Eur J Cancer. 1991;27:1639–1642. doi: 10.1016/0277-5379(91)90435-g. [DOI] [PubMed] [Google Scholar]
- 64.Boesch D, Gaveriaux C, Jachez B, Pourtier-Manzanedo A, Bollinger P, Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991;51:4226–4233. [PubMed] [Google Scholar]
- 65.Boote DJ, Dennis IF, Twentyman PR, Osborne RJ, Laburte C, Hensel S, Smyth JF, Brampton MH, Bleehen NM. Phase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancer. J Clin Oncol. 1996;14:610–618. doi: 10.1200/JCO.1996.14.2.610. [DOI] [PubMed] [Google Scholar]
- 66.Giaccone G, Linn SC, Welink J, Catimel G, Stieltjes H, van der Vijgh WJ, Eeltink C, Vermorken JB, Pinedo HM. A dose-finding and pharmacokinetic study of reversal of multidrug resistance with SDZ PSC 833 in combination with doxorubicin in patients with solid tumors. Clin Cancer Res. 1997;3:2005–2015. [PubMed] [Google Scholar]
- 67.Friedenberg WR, Rue M, Blood EA, Dalton WS, Shustik C, Larson RA, Sonneveld P, Greipp PR. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106:830–838. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]
- 68.Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–4602. [PubMed] [Google Scholar]
- 69.Newman MJ, Rodarte JC, Benbatoul KD, Romano SJ, Zhang C, Krane S, Moran EJ, Uyeda RT, Dixon R, Guns ES, Mayer LD. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60:2964–2972. [PubMed] [Google Scholar]
- 70.Roe M, Folkes A, Ashworth P, Brumwell J, Chima L, Hunjan S, Pretswell I, Dangerfield W, Ryder H, Charlton P. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett. 1999;9:595–600. doi: 10.1016/s0960-894x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 71.Slate DL, Bruno NA, Casey SM, Zutshi N, Garvin LJ, Wu H, Pfister JR. RS-33295-198: a novel, potent modulator of P-glycoprotein-mediated multidrug resistance. Anticancer Res. 1995;15:811–814. [PubMed] [Google Scholar]
- 72.Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128:403–411. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61:749–758. [PubMed] [Google Scholar]
- 74.Walker J, Martin C, Callaghan R. Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer. 2004;40:594–605. doi: 10.1016/j.ejca.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 75.Dantzig AH, Law KL, Cao J, Starling JJ. Reversal of multidrug resistance by the P-glycoprotein modulator, LY335979, from the bench to the clinic. Curr Med Chem. 2001;8:39–50. doi: 10.2174/0929867013373903. [DOI] [PubMed] [Google Scholar]
- 76.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 77.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 78.Chi KN, Chia SK, Dixon R, Newman MJ, Wacher VJ, Sikic B, Gelmon KA. A phase I pharmacokinetic study of the P-glycoprotein inhibitor, ONT-093, in combination with paclitaxel in patients with advanced cancer. Invest New Drugs. 2005;23:311–315. doi: 10.1007/s10637-005-1439-x. [DOI] [PubMed] [Google Scholar]
- 79.Fracasso PM, Goldstein LJ, de Alwis DP, Rader JS, Arquette MA, Goodner SA, Wright LP, Fears CL, Gazak RJ, Andre VA, Burgess MF, Slapak CA, Schellens JH. Phase I study of docetaxel in combination with the P-glycoprotein inhibitor, zosuquidar, in resistant malignancies. Clin Cancer Res. 2004;10:7220–7228. doi: 10.1158/1078-0432.CCR-04-0452. [DOI] [PubMed] [Google Scholar]
- 80.Pusztai L, Wagner P, Ibrahim N, Rivera E, Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming DR, Rouzier R, Boniface G, Hortobagyi GN. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104:682–691. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 81.Stewart A, Steiner J, Mellows G, Laguda B, Norris D, Bevan P. Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin Cancer Res. 2000;6:4186–4191. [PubMed] [Google Scholar]
- 82.Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 83.Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–3285. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 84.Morschhauser F, Zinzani PL, Burgess M, Sloots L, Bouafia F, Dumontet C. Phase I/II trial of a P-glycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride ( LY335979), given orally in combination with the CHOP regimen in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48:708–715. doi: 10.1080/10428190701190169. [DOI] [PubMed] [Google Scholar]
- 85.Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- 86.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 87.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 88.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 89.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59:1171–1180. doi: 10.1124/mol.59.5.1171. [DOI] [PubMed] [Google Scholar]
- 90.Mei Y, Qian F, Wei D, Liu J. Reversal of cancer multidrug resistance by green tea polyphenols. J Pharm Pharmacol. 2004;56:1307–1314. doi: 10.1211/0022357044364. [DOI] [PubMed] [Google Scholar]
- 91.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68:2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 93.Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1) Cancer Chemother Pharmacol. 2006;57:376–388. doi: 10.1007/s00280-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 94.Crooke ST, Bennett CF. Progress in antisense oligonucleotide therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:107–129. doi: 10.1146/annurev.pa.36.040196.000543. [DOI] [PubMed] [Google Scholar]
- 95.Alahari SK, Dean NM, Fisher MH, Delong R, Manoharan M, Tivel KL, Juliano RL. Inhibition of expression of the multidrug resistance-associated P-glycoprotein of by phosphorothioate and 5′ cholesterol-conjugated phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1996;50:808–819. [PubMed] [Google Scholar]
- 96.Quattrone A, Papucci L, Morganti M, Coronnello M, Mini E, Mazzei T, Colonna FP, Garbesi A, Capaccioli S. Inhibition of MDR1 gene expression by antimessenger oligonucleotides lowers multiple drug resistance. Oncol Res. 1994;6:311–320. [PubMed] [Google Scholar]
- 97.Kang H, Fisher MH, Xu D, Miyamoto YJ, Marchand A, Van Aerschot A, Herdewijn P, Juliano RL. Inhibition of MDR1 gene expression by chimeric HNA antisense oligonucleotides. Nucleic Acids Res. 2004;32:4411–4419. doi: 10.1093/nar/gkh775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobayashi H, Dorai T, Holland JF, Ohnuma T. Cleavage of human MDR1 mRNA by a hammerhead ribozyme. FEBS Lett. 1993;319:71–74. doi: 10.1016/0014-5793(93)80039-w. [DOI] [PubMed] [Google Scholar]
- 99.Kowalski P, Stein U, Scheffer GL, Lage H. Modulation of the atypical multidrug-resistant phenotype by a hammerhead ribozyme directed against the ABC transporter BCRP/MXR/ABCG2. Cancer Gene Ther. 2002;9:579–586. doi: 10.1038/sj.cgt.7700471. [DOI] [PubMed] [Google Scholar]
- 100.Materna V, Liedert B, Thomale J, Lage H. Protection of platinum-DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 2005;115:393–402. doi: 10.1002/ijc.20899. [DOI] [PubMed] [Google Scholar]
- 101.Kowalski P, Surowiak P, Lage H. Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol Ther. 2005;11:508–522. doi: 10.1016/j.ymthe.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 102.Eckstein F. The versatility of oligonucleotides as potential therapeutics. Expert Opinion on Biological Therapy. 2007;7:1021–1034. doi: 10.1517/14712598.7.7.1021. [DOI] [PubMed] [Google Scholar]
- 103.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–838. [PubMed] [Google Scholar]
- 104.Tian X, Zamek-Gliszczynski MJ, Zhang P, Brouwer KL. Modulation of multidrug resistance-associated protein 2 (Mrp2) and Mrp3 expression and function with small interfering RNA in sandwich-cultured rat hepatocytes. Mol Pharmacol. 2004;66:1004–1010. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- 105.Ee PL, He X, Ross DD, Beck WT. Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther. 2004;3:1577–1583. [PubMed] [Google Scholar]
- 106.Stierle V, Laigle A, Jolles B. Modulation of MDR1 gene expression in multidrug resistant MCF7 cells by low concentrations of small interfering RNAs. Biochem Pharmacol. 2005;70:1424–1430. doi: 10.1016/j.bcp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 107.Aleman C, Annereau JP, Liang XJ, Cardarelli CO, Taylor B, Yin JJ, Aszalos A, Gottesman MM. P-glycoprotein, expressed in multidrug resistant cells, is not responsible for alterations in membrane fluidity or membrane potential. Cancer Res. 2003;63:3084–3091. [PubMed] [Google Scholar]
- 108.Stege A, Priebsch A, Nieth C, Lage H. Stable and complete overcoming of MDR1/P-glycoprotein-mediated multidrug resistance in human gastric carcinoma cells by RNA interference. Cancer Gene Ther. 2004;11:699–706. doi: 10.1038/sj.cgt.7700751. [DOI] [PubMed] [Google Scholar]
- 109.Materna V, Stege A, Surowiak P, Priebsch A, Lage H. RNA interference-triggered reversal of ABCC2-dependent cisplatin resistance in human cancer cells. Biochem Biophys Res Commun. 2006;348:153–157. doi: 10.1016/j.bbrc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 110.Priebsch A, Rompe F, Tonnies H, Kowalski P, Surowiak P, Stege A, Materna V, Lage H. Complete reversal of ABCG2-depending atypical multidrug resistance by RNA interference in human carcinoma cells. Oligonucleotides. 2006;16:263–274. doi: 10.1089/oli.2006.16.263. [DOI] [PubMed] [Google Scholar]
- 111.Kaszubiak A, Holm PS, Lage H. Overcoming the classical multidrug resistance phenotype by adenoviral delivery of anti-MDR1 short hairpin RNAs and ribozymes. Int J Oncol. 2007;31:419–430. [PubMed] [Google Scholar]
- 112.Jiang Z, Zhao P, Zhou Z, Liu J, Qin L, Wang H. Using Attenuated Salmonella Typhi as Tumor Targeting Vector for MDR1 siRNA Delivery: An Experimental Study. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.4.3850. [DOI] [PubMed] [Google Scholar]
- 113.Rumpold H, Wolf AM, Gruenewald K, Gastl G, Gunsilius E, Wolf D. RNAi-mediated knockdown of P-glycoprotein using a transposon-based vector system durably restores imatinib sensitivity in imatinib-resistant CML cell lines. Exp Hematol. 2005;33:767–775. doi: 10.1016/j.exphem.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 114.Widmer N, Rumpold H, Untergasser G, Fayet A, Buclin T, Decosterd LA. Resistance reversal by RNAi silencing of MDR1 in CML cells associated with increase in imatinib intracellular levels. Leukemia. 2007;21:1561–1562. doi: 10.1038/sj.leu.2404671. author reply 1562–1564. [DOI] [PubMed] [Google Scholar]
- 115.Rumpold H, Salvador C, Wolf AM, Tilg H, Gastl G, Wolf D. Knockdown of PgP resensitizes leukemic cells to proteasome inhibitors. Biochem Biophys Res Commun. 2007;361:549–554. doi: 10.1016/j.bbrc.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 116.Pichler A, Zelcer N, Prior JL, Kuil AJ, Piwnica-Worms D. In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein. Clin Cancer Res. 2005;11:4487–4494. doi: 10.1158/1078-0432.CCR-05-0038. [DOI] [PubMed] [Google Scholar]
- 117.Stein U, Walther W, Stege A, Kaszubiak A, Fichtner I, Lage H. Complete In Vivo Reversal of the Multidrug Resistance Phenotype by Jet-injection of Anti-MDR1 Short Hairpin RNA-encoding Plasmid DNA. Mol Ther. 2007:1–9. doi: 10.1038/sj.mt.6300304. [DOI] [PubMed] [Google Scholar]
- 118.Marthinet E, Divita G, Bernaud J, Rigal D, Baggetto LG. Modulation of the typical multidrug resistance phenotype by targeting the MED-1 region of human MDR1 promoter. Gene Ther. 2000;7:1224–1233. doi: 10.1038/sj.gt.3301231. [DOI] [PubMed] [Google Scholar]
- 119.Park S, James CD. Lanthionine synthetase components C-like 2 increases cellular sensitivity to adriamycin by decreasing the expression of P-glycoprotein through a transcription-mediated mechanism. Cancer Res. 2003;63:723–727. [PubMed] [Google Scholar]
- 120.Bartsevich VV, Juliano RL. Regulation of the MDR1 gene by transcriptional repressors selected using peptide combinatorial libraries. Mol Pharmacol. 2000;58:1–10. doi: 10.1124/mol.58.1.1. [DOI] [PubMed] [Google Scholar]
- 121.Xu D, Ye D, Fisher M, Juliano RL. Selective inhibition of P-glycoprotein expression in multidrug-resistant tumor cells by a designed transcriptional regulator. J Pharmacol Exp Ther. 2002;302:963–971. doi: 10.1124/jpet.102.033639. [DOI] [PubMed] [Google Scholar]
- 122.Scala S, Budillon A, Zhan Z, Cho-Chung YS, Jefferson J, Tsokos M, Bates SE. Downregulation of mdr-1 expression by 8-Cl-cAMP in multidrug resistant MCF-7 human breast cancer cells. J Clin Invest. 1995;96:1026–1034. doi: 10.1172/JCI118088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaudhary PM, Roninson IB. Activation of MDR1 (P-glycoprotein) gene expression in human cells by protein kinase C agonists. Oncol Res. 1992;4:281–290. [PubMed] [Google Scholar]
- 124.Jin S, Gorfajn B, Faircloth G, Scotto KW. Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc Natl Acad Sci U S A. 2000;97:6775–6779. doi: 10.1073/pnas.97.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ueda K, Pastan I, Gottesman MM. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J Biol Chem. 1987;262:17432–17436. [PubMed] [Google Scholar]
- 126.Wang Q, Beck WT. Transcriptional suppression of multidrug resistance-associated protein (MRP) gene expression by wild-type p53. Cancer Res. 1998;58:5762–5769. [PubMed] [Google Scholar]
- 127.Kurz EU, Cole SP, Deeley RG. Identification of DNA-protein interactions in the 5′ flanking and 5′ untranslated regions of the human multidrug resistance protein (MRP1) gene: evaluation of a putative antioxidant response element/AP-1 binding site. Biochem Biophys Res Commun. 2001;285:981–990. doi: 10.1006/bbrc.2001.5262. [DOI] [PubMed] [Google Scholar]
- 128.Komarov PG, Shtil AA, Buckingham LE, Balasubramanian M, Piraner O, Emanuele RM, Roninson IB, Coon JS. Inhibition of cytarabine-induced MDR1 (P-glycoprotein) gene activation in human tumor cells by fatty acid-polyethylene glycol-fatty acid diesters, novel inhibitors of P-glycoprotein function. Int J Cancer. 1996;68:245–250. doi: 10.1002/(SICI)1097-0215(19961009)68:2<245::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 129.van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 130.Krishna R, Mayer LD. Liposomal Doxorubicin Circumvents PSC 833-Free Drug Interactions, Resulting in Effective Therapy of Multidrug-resistant Solid Tumors. Cancer Res. 1997;57:5246–5253. [PubMed] [Google Scholar]
- 131.Pawlak-Roblin C, Tosi PF, Perrin L, Devy J, Venteo L, Albert P, Nicolau C, Madoulet C. Inhibition of multidrug resistance by immunisation with synthetic P-glycoprotein-derived peptides. Eur J Cancer. 2004;40:606–613. doi: 10.1016/j.ejca.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 132.Yoshikawa M, Ikegami Y, Hayasaka S, Ishii K, Ito A, Sano K, Suzuki T, Togawa T, Yoshida H, Soda H, Oka M, Kohno S, Sawada S, Ishikawa T, Tanabe S. Novel camptothecin analogues that circumvent ABCG2-associated drug resistance in human tumor cells. Int J Cancer. 2004;110:921–927. doi: 10.1002/ijc.20216. [DOI] [PubMed] [Google Scholar]
- 133.Huang Y, Sadee W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239:168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 134.Cano-Gauci DF, Riordan JR. Action of calcium antagonists on multidrug resistant cells. Specific cytotoxicity independent of increased cancer drug accumulation. Biochem Pharmacol. 1987;36:2115–2123. doi: 10.1016/0006-2952(87)90139-0. [DOI] [PubMed] [Google Scholar]
- 135.Warr JR, Anderson M, Fergusson J. Properties of verapamil-hypersensitive multidrug-resistant Chinese hamster ovary cells. Cancer Res. 1988;48:4477–4483. [PubMed] [Google Scholar]
- 136.Karwatsky J, Lincoln MC, Georges E. A mechanism for P-glycoprotein-mediated apoptosis as revealed by verapamil hypersensitivity. Biochemistry. 2003;42:12163–12173. doi: 10.1021/bi034149+. [DOI] [PubMed] [Google Scholar]
- 137.Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, Caplen NJ, Fales HM, Ambudkar SV, Weinstein JN, Gottesman MM. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heffeter P, Jakupec MA, Korner W, Wild S, von Keyserlingk NG, Elbling L, Zorbas H, Korynevska A, Knasmuller S, Sutterluty H, Micksche M, Keppler BK, Berger W. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)]trithiocyanate (KP772; FFC24) Biochem Pharmacol. 2006;71:426–440. doi: 10.1016/j.bcp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 139.Heffeter P, Jakupec MA, Korner W, Chiba P, Pirker C, Dornetshuber R, Elbling L, Sutterluty H, Micksche M, Keppler BK, Berger W. Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24) Biochem Pharmacol. 2007;73:1873–1886. doi: 10.1016/j.bcp.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nicholson KM, Quinn DM, Kellett GL, Warr JR. LY294002, an inhibitor of phosphatidylinositol-3-kinase, causes preferential induction of apoptosis in human multidrug resistant cells. Cancer Lett. 2003;190:31–36. doi: 10.1016/s0304-3835(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 141.Bergman AM, Pinedo HM, Talianidis I, Veerman G, Loves WJ, van der Wilt CL, Peters GJ. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer. 2003;88:1963–1970. doi: 10.1038/sj.bjc.6601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Warr JR, Bamford A, Quinn DM. The preferential induction of apoptosis in multidrug-resistant KB cells by 5-fluorouracil. Cancer Lett. 2002;175:39–44. doi: 10.1016/s0304-3835(01)00721-2. [DOI] [PubMed] [Google Scholar]
- 143.Fantappie O, Solazzo M, Lasagna N, Platini F, Tessitore L, Mazzanti R. P-glycoprotein mediates celecoxib-induced apoptosis in multiple drug-resistant cell lines. Cancer Res. 2007;67:4915–4923. doi: 10.1158/0008-5472.CAN-06-3952. [DOI] [PubMed] [Google Scholar]
- 144.Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, Weinstein JN, Gottesman MM. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–137. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 145.Germann UA, Ford PJ, Shlyakhter D, Mason VS, Harding MW. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anticancer Drugs. 1997;8:141–155. doi: 10.1097/00001813-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 146.Naito M, Tsuruo T. New multidrug-resistance-reversing drugs, MS-209 and SDZ PSC 833. Cancer Chemother Pharmacol. 1997;40(Suppl):S20–24. doi: 10.1007/s002800051056. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura T, Oka M, Aizawa K, Soda H, Fukuda M, Terashi K, Ikeda K, Mizuta Y, Noguchi Y, Kimura Y, Tsuruo T, Kohno S. Direct interaction between a quinoline derivative, MS-209, and multidrug resistance protein (MRP) in human gastric cancer cells. Biochem Biophys Res Commun. 1999;255:618–624. doi: 10.1006/bbrc.1999.0245. [DOI] [PubMed] [Google Scholar]
- 148.Gandhi L, Harding MW, Neubauer M, Langer CJ, Moore M, Ross HJ, Johnson BE, Lynch TJ. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer. 2007;109:924–932. doi: 10.1002/cncr.22492. [DOI] [PubMed] [Google Scholar]
- 149.Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–1834. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 150.Shepard RL, Cao J, Starling JJ, Dantzig AH. Modulation of P-glycoprotein but not MRP1- or BCRP-mediated drug resistance by LY335979. Int J Cancer. 2003;103:121–125. doi: 10.1002/ijc.10792. [DOI] [PubMed] [Google Scholar]
- 151.van Zuylen L, Verweij J, Nooter K, Brouwer E, Stoter G, Sparreboom A. Role of intestinal P-glycoprotein in the plasma and fecal disposition of docetaxel in humans. Clin Cancer Res. 2000;6:2598–2603. [PubMed] [Google Scholar]
- 152.Bates SE, Shieh CY, Mickley LA, Dichek HL, Gazdar A, Loriaux DL, Fojo AT. Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73:18–29. doi: 10.1210/jcem-73-1-18. [DOI] [PubMed] [Google Scholar]
- 153.Jekerle V, Wang JH, Scollard DA, Reilly RM, Wiese M, Piquette-Miller M. 99mTc-Sestamibi, a sensitive probe for in vivo imaging of P-glycoprotein inhibition by modulators and mdr1 antisense oligodeoxynucleotides. Mol Imaging Biol. 2006;8:333–339. doi: 10.1007/s11307-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 154.Jekerle V, Klinkhammer W, Scollard DA, Breitbach K, Reilly RM, Piquette-Miller M, Wiese M. In vitro and in vivo evaluation of WK-X-34, a novel inhibitor of P-glycoprotein and BCRP, using radio imaging techniques. Int J Cancer. 2006;119:414–422. doi: 10.1002/ijc.21827. [DOI] [PubMed] [Google Scholar]
- 155.Consoli U, Priebe W, Ling YH, Mahadevia R, Griffin M, Zhao S, Perez-Soler R, Andreeff M. The novel anthracycline annamycin is not affected by P-glycoprotein-related multidrug resistance: comparison with idarubicin and doxorubicin in HL-60 leukemia cell lines. Blood. 1996;88:633–644. [PubMed] [Google Scholar]
- 156.Perez-Soler R, Neamati N, Zou Y, Schneider E, Doyle LA, Andreeff M, Priebe W, Ling YH. Annamycin circumvents resistance mediated by the multidrug resistance-associated protein (MRP) in breast MCF-7 and small-cell lung UMCC-1 cancer cell lines selected for resistance to etoposide. Int J Cancer. 1997;71:35–41. doi: 10.1002/(sici)1097-0215(19970328)71:1<35::aid-ijc8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 157.Walter RB, Pirga JL, Cronk MR, Mayer S, Appelbaum FR, Banker DE. PK11195, a peripheral benzodiazepine receptor (pBR) ligand, broadly blocks drug efflux to chemosensitize leukemia and myeloma cells by a pBR-independent, direct transporter-modulating mechanism. Blood. 2005;106:3584–3593. doi: 10.1182/blood-2005-02-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sauna ZE, Peng XH, Nandigama K, Tekle S, Ambudkar SV. The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1. Mol Pharmacol. 2004;65:675–684. doi: 10.1124/mol.65.3.675. [DOI] [PubMed] [Google Scholar]
- 159.Qi J, Yang CZ, Wang CY, Wang SB, Yang M, Wang JH. Function and mechanism of pyronaridine: a new inhibitor of P-glycoprotein-mediated multidrug resistance. Acta Pharmacol Sin. 2002;23:544–550. [PubMed] [Google Scholar]
- 160.Riffkin CD, Chung R, Wall DM, Zalcberg JR, Cowman AF, Foley M, Tilley L. Modulation of the function of human MDR1 P-glycoprotein by the antimalarial drug mefloquine. Biochem Pharmacol. 1996;52:1545–1552. doi: 10.1016/s0006-2952(96)00556-4. [DOI] [PubMed] [Google Scholar]
- 161.Tsuruo T, Iida H, Kitatani Y, Yokota K, Tsukagoshi S, Sakurai Y. Effects of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and adriamycin in drug-resistant tumor cells. Cancer Res. 1984;44:4303–4307. [PubMed] [Google Scholar]
- 162.Vezmar M, Georges E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem Pharmacol. 2000;59:1245–1252. doi: 10.1016/s0006-2952(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 163.Wu CP, Klokouzas A, Hladky SB, Ambudkar SV, Barrand MA. Interactions of mefloquine with ABC proteins, MRP1 (ABCC1) and MRP4 (ABCC4) that are present in human red cell membranes. Biochem Pharmacol. 2005;70:500–510. doi: 10.1016/j.bcp.2005.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317:269–275. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 166.Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3) Biochem Pharmacol. 2003;65:75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- 167.Jin J, Shahi S, Kang HK, van Veen HW, Fan TP. Metabolites of ginsenosides as novel BCRP inhibitors. Biochem Biophys Res Commun. 2006;345:1308–1314. doi: 10.1016/j.bbrc.2006.04.152. [DOI] [PubMed] [Google Scholar]
- 168.Perrotton T, Trompier D, Chang XB, Di Pietro A. Baubichon-Cortay, H. S- and R-verapamil differentially modulate the multidrug resistance protein MRP1. J Biol Chem. 2007 doi: 10.1074/jbc.M703964200. [DOI] [PubMed] [Google Scholar]
- 169.Trompier D, Chang XB, Barattin R, du Moulinet D’Hardemare A, Di Pietro A, Baubichon-Cortay H. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res. 2004;64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]
- 170.Fattman CL, Allan WP, Hasinoff BB, Yalowich JC. Collateral sensitivity to the bisdioxopiperazine dexrazoxane (ICRF-187) in etoposide (VP-16)-resistant human leukemia K562 cells. Biochem Pharmacol. 1996;52:635–642. doi: 10.1016/0006-2952(96)00338-3. [DOI] [PubMed] [Google Scholar]
- 171.Bentley J, Quinn DM, Pitman RS, Warr JR, Kellett GL. The human KB multidrug-resistant cell line KB-C1 is hypersensitive to inhibitors of glycosylation. Cancer Lett. 1997;115:221–227. doi: 10.1016/s0304-3835(97)04739-3. [DOI] [PubMed] [Google Scholar]
- 172.Bergman AM, Munch-Petersen B, Jensen PB, Sehested M, Veerman G, Voorn DA, Smid K, Pinedo HM, Peters GJ. Collateral sensitivity to gemcitabine (2′,2′-difluorodeoxycytidine) and cytosine arabinoside of daunorubicin- and VM-26-resistant variants of human small cell lung cancer cell lines. Biochem Pharmacol. 2001;61:1401–1408. doi: 10.1016/s0006-2952(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 173.Turella P, Filomeni G, Dupuis ML, Ciriolo MR, Molinari A, De Maria F, Tombesi M, Cianfriglia M, Federici G, Ricci G, Caccuri AM. A strong glutathione S-transferase inhibitor overcomes the P-glycoprotein-mediated resistance in tumor cells. 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) triggers a caspase-dependent apoptosis in MDR1-expressing leukemia cells. J Biol Chem. 2006;281:23725–23732. doi: 10.1074/jbc.M604372200. [DOI] [PubMed] [Google Scholar]