Abstract
Objective
Longitudinal analysis is crucial in determining the ability of new interventions to successfully reduce negative symptoms in schizophrenia. However, there are still conflicting reports as to whether there are significant treatment effects on these symptoms and the extent of these effects. We examine the possible effects of analysis method on these questions.
Method
We use generalized linear mixed models (GLMM) to assess the change in specific negative symptom items following treatment changes in two separate cohorts of schizophrenia patients, one chronic and one first-episode.
Results
Both data sets indicate that examining the change in prevalence of moderate to severe symptoms provides a useful estimate of the effect size associated with changes in treatment that often differs from that given using analysis of means.
Conclusions
The use of categorical longitudinal methods may be critical to determining the responsiveness of negative symptoms to treatment as well as determining the stability of these symptoms over time.
Keywords: negative symptoms, longitudinal data; ordinal data, symptomatology
1. INTRODUCTION
Negative symptoms are a primary focus of treatment and rehabilitation in schizophrenia patients (Stahl and Buckley, 2007), and longitudinal studies of these symptoms are paramount to establishing treatment efficacy. The early studies of schizophrenia often considered negative symptoms to be more stable and enduring than psychotic symptoms (Johnstone et al., 1987; McGlashan and Fenton, 1992; Pfohl and Winokur, 1983; Pogue-Geile and Harrow, 1985; Venables and Wing, 1962), as well as refractory to pharmacologic intervention. Crow (1980) further proposed to divide schizophrenia into two subtypes, each characterized by the predominance of one type of symptoms over the other. Type 1 patients exhibited mainly positive symptoms, while Type 2 patients showed mainly negative symptoms. Others proposed a third, disorganized subtype (Andreasen and Olsen, 1982; Bilder et al., 1985; Liddle, 1987).
Later observations, however, revealed significant changes in negative symptoms over time (Kay, 1990; McGlashan and Fenton, 1993; Mueser et al., 1991) that were related to changes in psychosis (Addington and Addington, 1991; Miller et al., 1994; van Kammen et al., 1990) and medication treatment (Goldberg, 1985; Rosen et al., 1984; Tandon et al., 1990, 1993). In addition, the subtype classifications were also shown to fluctuate over time (Andreasen et al., 1990; Arndt et al., 1995; Eaton et al., 1995; Fenton and McGlashan, 1992; Marneros et al., 1992). These conflicting observations led to the idea that the persistence, rather than the presence, of negative symptoms may more reliably identify a clinically relevant subgroup of patients. This then led to classifications based on longitudinal presentation, such as the deficit syndrome (Carpenter et al., 1988) and a revisiting of the Kraepelinian subtype (Keefe et al., 1987,1996). The deficit syndrome has been a useful construct for identifying a group of patients with a more severe form of the illness that may require more aggressive or entirely new modalities of treatment (Buchanan and Carpenter, 2001; Carpenter et al., 1999).
More recent evaluation of the benefits of pharmacological treatment on negative symptoms has demonstrated either limited effects or inconsistent results (Alphs, 2006; Buchanan et al., 2007; Buckley and Stahl, 2007; Erhart et al., 2006; Lieberman et al., 2003). While much of this is due to design concerns such as measurement and control of possible secondary effects, a primary concern is clinical relevance or effect size (Alphs, 2006; Buckley and Stahl, 2007; Kirkpatrick et al., 2006). For the quantification of longitudinal changes, most recent assessments have used linear mixed models that provide substantial improvement over standard linear models such as ANOVA and MANOVA (Gibbons et al., 1993; Gueorguieva and Krystal, 2004). This is partly due to the fact that mixed models provide more flexibility by allowing the inclusion of subjects that have some missing data without introducing the bias found with approaches such as last observation carried forward (LOCF) (Elliott and Hawthorne, 2005). More importantly, however, these models explicitly incorporate terms that reflect subject-level heterogeneity; i.e., they can take into account the fact that subjects differ in both their severity of illness at baseline as well as their rate of response. These models are well suited to the analysis of summary measures such as total scores and have been used for the assessment of treatment effects on negative symptoms (Buchanan et al., 2007; Lieberman et al., 2003).
However, it would also be of interest to assess the effects of treatments on the individual symptoms, such as affective flattening, that are measured on an ordinal scale. Unfortunately these data do not meet the assumptions of these linear methods that require continuous data. Recently, the development of generalized linear mixed models (GLMM) has provided analysts with analogues to these linear mixed models for use with categorical (e.g. ordinal and binary) data (Rabe-Hesketh et al., 2002). However, most assessments of longitudinal change in negative symptoms either have not analyzed individual symptoms or have not employed these methods because they were not readily available until more recently. However, it may be important to make use of these methods because using linear methods on categorical data results in biased estimates (Lipsitz, 1992), which may subsequently alter the significance of effects. More importantly, continuous data methods are especially biased when many subjects have no evidence of a symptom (Hastie et al., 1989). In that case, the variance is even further reduced and we are essentially measuring prevalence and not average severity across subjects. For these reasons, the use of categorical methods for the analysis of longitudinal symptom data may provide both an opportunity to further explore treatment effects as well as give more meaningful estimates of the mutability of symptoms than would a continuous analytic approach.
For the current study, the aim was to propose the analysis of prevalence as an alternative measure to quantify and visualize the patterns of change in negative symptoms over time. Two different experimental paradigms were used to evaluate the effects of treatment changes on negative symptoms: 1) following drug discontinuation in chronic schizophrenia inpatients and 2) after initiation of treatment in a group of first-episode schizophrenia patients. As an example of the possible effects of analysis choice on interpretation, we produce and compare the significance of the changes over time (slopes) with those of similar linear models run on the same data.
2. METHODS
2.1 Subjects
2.1.1 Chronic schizophrenia
This cohort consisted of 100 male veterans admitted to the Schizophrenia Research and Treatment Unit (SRTU) at the VA Healthcare System in Pittsburgh, Pennsylvania. Admission to the study followed completion of oral and written informed consent, in compliance with the policies of the Highland Drive VA Medical Center’s Institutional Review Board (IRB). All subjects were fully informed about the risks of participation and alternative treatment options. Subjects were recruited for the same experimental protocol from 1982 through 1997 and interviewed to determine DSM-III-R (APA, 1987) diagnosis using the Schedule for Affective Disorders and Schizophrenia (SADS) (Spitzer and Endicott, 1979) or the Structured Clinical Interview for DSM-III-R (SCID) (Spitzer et al., 1989). Subjects were included who had a DSM-III-R diagnosis of either schizophrenia or schizoaffective disorder. All diagnostic information obtained using the SADS or SCID was reviewed in a multidisciplinary case conference to confirm diagnosis. Subjects who met the DSM-III-R criteria for alcohol or substance abuse or dependence were excluded at the time of the study unless they had been in remission for at least 6 months. All subjects participated in a double-blind protocol that consisted of the replacement of long-term antipsychotic drug treatment with placebo following a minimum 4-week stabilization period on the minimum dose of haloperidol (10.5 ± 6.4 mg/day, range 0.5 – 40 mg/day). All subjects remained in the hospital for the duration of the study. Benztropine was administered in doses of 1–4 mg/day as clinically indicated for extrapyramidal symptoms during the stabilization period; no other medications were given. After the stabilization period (week 0), medication was replaced with placebo capsules for up to 6 weeks or until the subject experienced an increase in psychosis, according to previously established criteria (van Kammen et al., 1990). Behavioral ratings were performed daily for assessment of the subject’s clinical status (Bunney and Hamburg, 1963) and weekly throughout the study (see below). Exacerbation was defined empirically as an increase of 3 points or more on the psychosis item of the Bunney-Hamburg scale, sustained over a period of at least 3 days (van Kammen et al., 1990). Those patients who did not meet this criterion were considered “clinically stable” for the purposes of the analysis. For patients who experienced a worsening of psychotic symptoms (n=44), as evidenced by their daily ratings, we used their behavioral data until the time they met criteria, as they were returned to medication treatment at that time. Those who remained clinically stable (n=56) had six weeks of drug-free ratings. Discussion of the costs and benefits of this methodology are cited by Carpenter (2006).
2.1.2 First episode schizophrenia
A consecutive series of in- and out-patient first-episode psychotic patients who met criteria for DSM-III-R Schizophrenia or Schizoaffective Disorder (n=62) were recruited to participate in the Pittsburgh First Episode Study at the Western Psychiatric Institute and Clinic of the University of Pittsburgh Medical Center. Subjects were recruited between 1990 and 1999. Admission to the study followed completion of oral and written informed consent, in compliance with procedures approved by the IRB of the University of Pittsburgh. Diagnoses were determined based on SCID interview with subjects, report from collateral sources (e.g. family) and a consensus conference review of all available clinical data collected at the time of admission to treatment. Because accuracy of differential diagnosis of the first episode of a psychotic disorder is highly dependent on a longitudinal view of the early course of illness, diagnoses were re-reviewed at 6- and 12 months using follow-up SCID interview information and information from treatment providers obtained from review of medical records. Subjects were included in this study if they met criteria for schizophrenia or schizoaffective disorder at the one year follow-up assessment. All subjects were followed by the treatment team over a period of one year from consent. Individuals who met DSM-III-R criteria for alcohol or substance dependence in the past 6 months (at baseline assessment) were excluded from study, as were individuals who met criteria for alcohol or substance abuse within the past four weeks, had a history of head injury with significant unconsciousness, or current neurologic or medical condition that might confound clinical presentation. All subjects were maintained on an open-label antipsychotic regimen as per their treating physician using the low-dose strategy developed by McEvoy and colleagues (McEvoy et al., 1991). Anticholinergic medications were used in low doses (1 to 4 mg/day), as needed. Behavioral assessments were conducted within the first week of admission to hospital (baseline) and prior to initiation of medication, as well as at 1 or 2 months, 6 months and 12 months following the baseline assessment. Given that each patient’s medications were adjusted continuously throughout the study to provide the maximum clinical benefit, all patients were considered to be successfully treated, i.e., there were no subgroups used for this analysis.
2.2 Behavioral ratings
Negative symptoms were measured with the global items of the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982). All symptom ratings were conducted by experienced clinical interviewers. Inter-rater reliability was maintained at an adequate level (ICC ≥ .70) through monthly inter-rater reliability assessment meetings. Following the recommendations of Buchanan and Carpenter (Buchanan and Carpenter, 2001), this study focused on those negative symptoms that are distinct and consistent across all definitions of the negative syndrome: affective flattening, poverty of speech, avolition, and anhedonia.
2.3 Statistics
For the analysis of progression of symptoms over time, the ideal approach would be a generalized linear mixed model (GLMM) for the analysis of repeated measures ordinal data (Hedeker and Gibbons, 1994). However, we did not pursue ordinal models for three reasons: 1) from a clinical point of view, it is the more severe levels of negative symptoms that are of most interest, especially when defining the negative syndrome; 2) the sample sizes would need to be larger in order to distinguish these ordinal models from binary models with similar predictors, and 3) the fact that several of the symptoms examined were rare (e.g., poverty of speech was absent in 44.1% of the repeated measures in the chronic sample) makes the observed data behave in a binary fashion even though the scale itself is based on an underlying continuum. Thus for the purposes of the current analysis, we dichotomized each time-specific symptom rating as either being moderate to severe (3–5) or not. More specifically, we modeled the prevalence of moderate to severe symptoms as a function of time using random effects logistic regression using the generalized linear latent and mixed models (gllamm) procedure in Stata (Rabe-Hesketh et al., 2002). For all models, we used patient-specific adjustments to the estimates of the symptom level (intercept) as well as progression over time (slope), to allow for subject heterogeneity. To demonstrate the benefits associated with an analysis of prevalence, we compared significance values and effect sizes to those for linear mixed models with the same structure.
2.3.1 Missing data
The GLMM assumes that any missing data be missing at random (MAR), i.e., independent of the outcome, conditional on any covariates in the model. In other words, the model will remain valid even if the pattern of missingness is related to any of the predictors or to any previous measures of the symptom (Little and Rubin, 2002). Thus, even if missingness is due to exacerbation, as is clearly the case for the drug discontinuation protocol, the models will be valid as long as exacerbation status is included.
2.3.2
Given that the analyses were of negative symptoms which are all highly correlated, we did not adjust for multiple comparisons as the majority of these adjustments assume independence across multiple variables. Instead, we took the approach of interpreting with caution those tests with significance near 0.05.
3. RESULTS
Basic descriptives for each sample are shown in Table 1. We have plotted the relevant changes over time for the chronic schizophrenia subjects [Figure 1,2], as well as the first-episode sample [Figure 3]. The first episode data indicate that a large change was seen in the first month, as expected due to treatment initiation. Thus we fit a “piecewise” time course assessing the slope and significance of the change in the first month, then the progression from 1 month to 1 year. This allowed us to test specifically whether the initial change was significantly different from zero, and whether or not there was a significant change in progression after the initial treatment effect.
Table 1.
Demographic characteristics
| Chronic schizophrenia (n=100) | First-episode schizophrenia (n=62) | |||
|---|---|---|---|---|
| Variable | mean (sd) | range | mean(sd) | range |
| Age (yrs) | 37.4 (8.3) | 20–63 | 26.3 (8.2) | 14–45 |
| Age of onset (yrs) | 24.2 (5.8) | 13–56 | NA | |
| Duration of illness (yrs) | 13.2 (7.4) | 0.5 – 30 | NA | |
| % male | 100 | 60 | ||
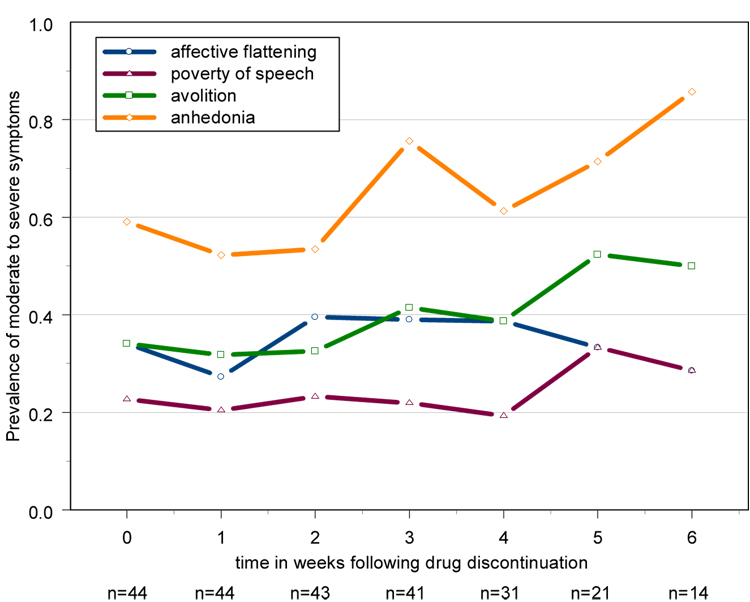
Figure 1. Prevalence of moderate to severe symptoms over time in chronic schizophrenia patients who experienced measurable increases in psychosis following inpatient drug discontinuation(n=44) – raw data.
Exacerbated subjects returned to medication after meeting criteria, thus the sample sizes are different across time. Increases in psychosis were defined as a 3 point increase on the Bunney-Hamburg (Bunney and Hamburg, 1963) psychosis scale from the baseline stabilization phase on haloperidol. Baseline (week 0) is the last rating on haloperidol treatment.
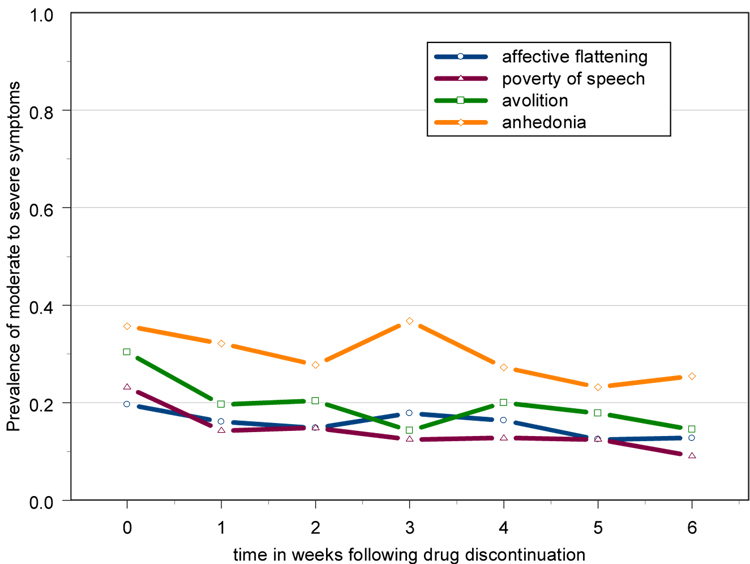
Figure 2. Prevalence of moderate to severe symptoms over time in chronic schizophrenia patients who had no measurable increases in psychosis (n=56) – raw data.
Baseline (week 0) is the last rating on haloperidol treatment.
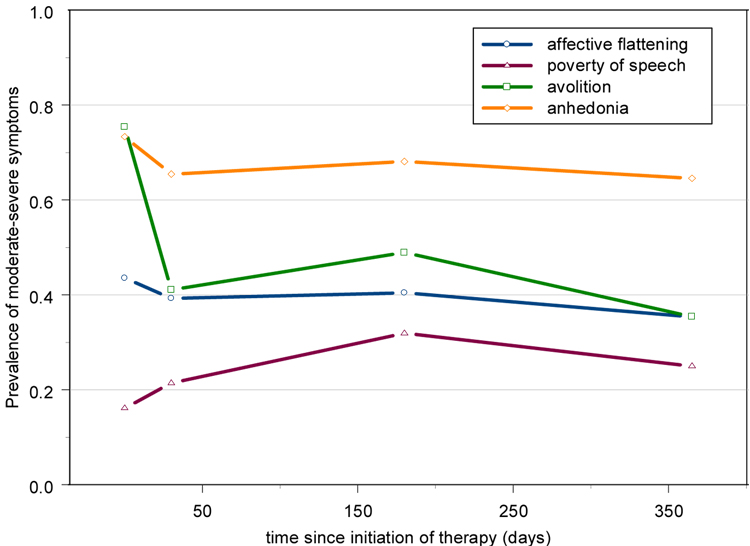
Figure 3. Prevalence of moderate to severe symptoms over time in first episode schizophrenia patients after initiation of treatment (n=62)– raw data.
3.1 Chronic schizophrenia sample
For both clinical (effects of increases in psychosis/relapse) and methodological (missing data) reasons, patients were categorized into those who experienced an exacerbation following drug discontinuation and those who did not. Almost all symptoms exhibited significant evidence of individual level variation in both the level (intercept) and progression (slope) over time, as evidenced by the significance of the random effects [Table 2]. After adjusting for individual-level variation, both avolition and anhedonia showed significant differences in progression over time between exacerbated and non-exacerbated subjects, as indicated by the exacerbation-by-time interaction term in each model [Table 2]. Further examination of the model estimates indicated that for avolition, there was a significant decrease in the severity of this symptom in the “clinically stable” subjects, while exacerbated subjects had a non-significant increase in symptom severity. For anhedonia, the increase in symptom severity among exacerbated subjects was significant, while the decrease in clinically stable subjects was not. In contrast, if we mistakenly use linear models and treat the data as continuous, the significance levels change substantially, and in some cases, give different interpretations. Most notably, the apparent reduction in avolition in the clinically stable subjects (p=0.04) from a baseline prevalence of moderate to severe symptoms of 30% to a 6 week prevalence of 15% is not detected by the linear analysis (p=0.18), in which there was a decrease in mean level from 1.8 to 1.5 [Table 2]. This is most likely due to the fact that 48% of all measures of avolition in this subgroup were absent or questionable (scores of 0 or 1) which biased the mean downwards.
Table 2.
Chronic schizophrenia patients: Progression of moderate to severe negative symptom levels over time
| affective flattening | poverty of speech | avolition | anhedonia | |||||
|---|---|---|---|---|---|---|---|---|
| Fixed effects: | est (se) | p-value* | est (se) | p-value* | est (se) | p-value* | est (se) | p-value* |
| Intercept: CS | −2.811 (0.72) | −2.923 (0.85) | −1.894 (0.62) | −1.157 (0.49) | ||||
| slope (wks) : CS | −0.248 (0.21) | 0.228 0.389# |
−0.540 (0.28) | 0.055 0.146# |
−0.444 (0.21) |
0.037 0.180# |
−0.231 (0.13) | 0.086 0.084# |
| Intercept: E | −1.557 (0.66) | −2.912 (0.88) | −1.776 (0.65) | 0.199 (0.52) | ||||
| slope(wks): E | 0.136 (0.18) | 0.447 0.360# |
0.011 (0.28) | 0.969 0.365# |
0.332 (0.20) | 0.099 0.009# |
0.365 (0.16) |
0.023 0.006# |
| exac * time int^ | 0.132 | 0.096 | 0.007 | 0.008 | ||||
| Random effects: | ||||||||
| σint | 3.016 (0.62) | < 0.0005 | 3.514 (0.70) | < 0.0005 | 2.985 (0.60) | < 0.0005 | 2.549 (0.50) | < 0.0005 |
| σslope | 0.596 (0.15) | < 0.0005 | 0.805 (0.18) | < 0.0005 | 0.726 (0.15) | < 0.0005 | 0.492 (0.12) | < 0.0005 |
| σintslope | −0.267 (0.18) | 0.142 | −0.490 (0.20) | 0.012 | −0.354 (0.18) | 0.055 | −0.215 (0.15) | 0.161 |
Significance of the random effects estimates is only approximate; estimates (σ) are the components of the variance attributed to subject level differences – CS=clinically stable subjects; E=exacerbated subjects
Significance of group by time interaction, i.e., model test of the slope difference in E vs CS subjects
for comparison, p-values of a continuous data linear mixed model with the same structure are given
3.2 First episode sample
As with the chronic schizophrenia sample, there was significant evidence of individual-level variation indicated by the significance of the random effects [Table 3]. The only negative symptom that showed change over the course of treatment was avolition, which decreased initially and then leveled off [Figure 3]. When comparing analysis methods, the most striking difference is that the linear analysis showed a significant change in anhedonia for both slope estimates, while the categorical analysis did not. The effect sizes for the change in the first month revealed a decrease in prevalence of 8% that corresponded to a mean decrease of 0.35 scale points. It is most likely that this would not be considered a clinically significant change. Even more illustrative is the examination of the change from one month to one year which resulted in a decrease of only 0.8% and a mean decrease of 0.05 scale points, but was still significant in the linear analysis due to the decreased variance of the mean for this sample.
Table 3.
First-episode schizophrenia patients: Progression of moderate to severe negative symptom levels over time
| affective flattening | poverty of speech | avolition | anhedonia | |||||
|---|---|---|---|---|---|---|---|---|
| Fixed effects: | est (se) | p-value* | est (se) | p-value* | est (se) | p-value* | est (se) | p-value* |
| Intercept | −0.441 (0.44) | −2.827 (0.77) | 1.317 (0.40) | 1.434 (0.48) | ||||
| slope (0–30 days) | −0.012 (0.02) | 0.453 0.696# |
0.029 (0.02) | 0.149 0.148# |
−0.053 (0.02) |
0.001 <0.0005# |
−0.016 (0.02) | 0.331 0.007# |
| slope (30-365 days) | 0.008 (0.02) | 0.634 0.724# |
−0.030 (0.02) | 0.154 0.181# |
0.052 (0.02) |
0.002 < 0.0005# |
0.018 (0.02) | 0.297 0.011# |
| Random effects: | ||||||||
| σint | 2.033 (0.68) | 0.003 | 2.249 (0.73) | 0.002 | 0.962 (0.56) | 0.088 | 1.591 (0.62) | 0.011 |
| σslope | −0.010 (0.007) | 0.094 | −0.010 (0.005) | 0.027 | 0.005 (0.004) | 0.107 | 0.010 (0.005) | 0.045 |
| σintslope | 0.006 (0.006) | 0.340 | −0.005 (0.004) | 0.150 | −0.001 (0.004) | 0.742 | 0.0004 (0.004) | 0.999 |
Significance of the random effects estimates is only approximate; estimates (σ) are the components of the variance attributed to subject level differences
for comparison, p-values of a continuous data linear mixed model with the same structure are given
4. CONCLUSIONS
The current data indicate that previous hypotheses that certain negative symptoms such as affective flattening may be less prone to medication effects (Crow, 1985; Kelley et al., 1999) than other negative symptoms may still hold true The lack of change observed in affective flattening is intriguing, due to the current emphasis on classification of patients through evidence of persistent symptoms such as the deficit syndrome (Buchanan and Carpenter, 2001). It may be that in these patient groups, the levels of affective flattening and poverty of speech were too low to determine reasonable changes over time. However, it has been consistently noted, both statistically (Johnstone et al., 1987; McGlashan and Fenton, 1993; Mueser et al., 1991) and heuristically (Crow, 1985; Liddle, 1987; McGlashan and Fenton, 1992), that these symptoms tend to be more stable. Given that the rates of moderate to severe ratings of affective flattening were similar in a first episode sample and a sample with a mean duration of illness of 13 years, it might be argued that the rates of these symptoms in the two samples included in this report are similar to those characteristic of the larger population of individuals with schizophrenia.
The current study did not attempt to control for possible secondary sources of negative symptoms other than the possible effects of psychosis which were controlled by design. The focus of the current paper was to address the effects of analysis choice on the assessment of the size of the treatment effects, and not weigh in on the proper method of determining primary negative symptoms (e.g., design or statistical adjustment). However, it should be noted that if statistical control of secondary sources is considered viable, the treatment effects observed here could be adjusted for other measures such as side effects by simply including them as repeated covariates.
We postulate that some of the conflicting reports on the mutability of negative symptoms over time may be due to the fact that continuous data methods were used to analyze the data. This is not necessarily because the appropriate techniques were not used but because the concept of slope as an estimate of effect size has little meaning for categorical data (Blalock, 1976). As an example, for ratings of (1,2,2,2) the computed change in affective flattening is 0.3 points per unit of time (0,1,2,3). From a clinical point of view, this pattern of data would most likely be judged as no effective change in this symptom; however, significant changes over time of a similar magnitude (range 0.3 – 0.7) have been reported (Addington and Addington, 1991; Arndt et al., 1995; Tandon et al., 1993). Considering the current data, it would seem a 15% decrease in the prevalence of moderate to severe symptoms post-treatment might be more clinically interpretable than a decrease in 0.3 scale points regardless of whether this difference was found to be statistically significant. Thus, we propose that the examination of average prevalence may give a more clinically relevant estimate of overall change than a comparison of mean symptom levels over time when examining individual items or subscales with low variability.
Another of the benefits of the use of the categorical approach is that we do not have to assume that the anchors of the ordinal scale represent a true scaled measurement, i.e., that a one point difference between a score of 2 and 3 is exactly equivalent to the difference between scores of 4 and 5. As clinicians, we inherently know this is not the case, and yet we often overlook this fact when analyzing data, because we are limited to the methods with which we are familiar. We can possibly avoid using categorical approaches by analyzing subscales or sums of the relevant items. However, in many cases even this approach does not sufficiently increase the variation to justify the use of continuous data analytic methods. One example of this would be side effect scales such as the Simpson-Angus wherein most of the items are not endorsed, and therefore the subscale scores and even the total scores can resemble categorical rather than continuous data.
Finally, the examination of average prevalence over time gives us another tool to examine treatment effects on specific symptoms in addition to the effects on overall total negative symptom scores. The recent NIMH-MATRICS consensus statement on the treatment of negative symptoms stressed the need for new definitions of clinically meaningful effect size (Alphs, 2006; Kirkpatrick et al., 2006) and that analysis of subdomains or item level data, rather than a combined negative symptom score, may be needed to distinguish different neuropharmacological substrates (Kirkpatrick and Fischer, 2006). The current study shows that the examination of the prevalence of a specified level of symptoms over time may provide clinically meaningful estimates of treatment effects on item level data as well as any subdomain that exhibits low incidence. Thus, this approach to analysis could be beneficial when evaluating the effect of new treatments on negative symptoms in schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Addington D. Positive and negative symptoms of schizophrenia: Their course and relationship over time. Schizophr. Res. 1991;5(1):51–59. doi: 10.1016/0920-9964(91)90053-t. [DOI] [PubMed] [Google Scholar]
- Alphs L. An industry perspective on the NIMH consensus statement on negative symptoms. Schizophr. Bull. 2006;32(2):225–230. doi: 10.1093/schbul/sbj056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia: Definition and reliability. Arch. Gen. Psychiatry. 1982;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW, Tyrrell G, Arndt S. Positive and negative symptoms in schizophrenia. A critical reappraisal. Arch. Gen. Psychiatry. 1990;47(7):615–621. doi: 10.1001/archpsyc.1990.01810190015002. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch. Gen. Psychiatry. 1982;39(7):789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- APA. Washington, DC: American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders. 1987
- Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P. A longitudinal study of symptom dimensions in schizophrenia. Prediction and patterns of change. Arch. Gen. Psychiatry. 1995;52(5):352–360. doi: 10.1001/archpsyc.1995.03950170026004. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK. Symptomatic and neuropsychological components of defect states. Schizophr. Bull. 1985;11(3):409–419. doi: 10.1093/schbul/11.3.409. [DOI] [PubMed] [Google Scholar]
- Blalock HM., Jr Can We Find a Genuine Ordinal Slope Analogue? Sociological Methodology. 1976;7:195–229. [Google Scholar]
- Buchanan RW, Carpenter WT. Evaluating negative symptom treatment efficacy. In: Keefe RSE, McEvoy JP, editors. Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, Inc.; 2001. pp. 1–18. [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry. 2007;164(10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr. Scand. 2007;115(2):93–100. doi: 10.1111/j.1600-0447.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Hamburg DA. Methods for reliable longitudinal observation of behavior: Development of a method for systematic observation of emotional behavior on psychiatric wards. Arch. Gen. Psychiatry. 1963;9(3):280–294. doi: 10.1001/archpsyc.1963.01720150090010. [DOI] [PubMed] [Google Scholar]
- Carpenter WT. Traditional ethics and new sensitivities. Schizophr. Bull. 2006;32(1):1–2. doi: 10.1093/schbul/sbj029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: The concept. Am. J. Psychiatry. 1988;145(5):578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Arango C, Buchanan RW, Kirkpatrick B. Deficit psychopathology and a paradigm shift in schizophrenia research. Biol. Psychiatry. 1999;46(3):352–360. doi: 10.1016/s0006-3223(99)00088-8. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br. Med. J. 1980;280(6207):66–68. doi: 10.1136/bmj.280.6207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. The Two-Syndrome Concept: Origins and Current Status. Schizophr. Bull. 1985;11(3):471–486. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Thara R, Federman B, Melton B, Liang KY. Structure and course of positive and negative symptoms in schizophrenia. Arch. Gen. Psychiatry. 1995;52(2):127–134. doi: 10.1001/archpsyc.1995.03950140045005. [DOI] [PubMed] [Google Scholar]
- Elliott P, Hawthorne G. Imputing missing repeated measures data: How should we proceed? Aust. N. Z. J. Psychiatry. 2005;39(7):575–582. doi: 10.1080/j.1440-1614.2005.01629.x. [DOI] [PubMed] [Google Scholar]
- Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr. Bull. 2006;32(2):234–237. doi: 10.1093/schbul/sbj055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH. Testing systems for assessment of negative symptoms in schizophrenia. Arch. Gen. Psychiatry. 1992;49(3):179–184. doi: 10.1001/archpsyc.1992.01820030011002. [see comment] [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch. Gen. Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Goldberg SC. Negative and deficit symptoms in schizophrenia do respond to neuroleptics. Schizophr. Bull. 1985;11(3):453–456. doi: 10.1093/schbul/11.3.453. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move Over ANOVA Progress in Analyzing Repeated-Measures Data and Its Reflection in Papers Published in the Archives of General Psychiatry. Arch. Gen. Psychiatry. 2004;61(3):310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Botha JL, Schnitzler CM. Regression with an ordered categorical response. Stat. Med. 1989;8(7):785–794. doi: 10.1002/sim.4780080703. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50(4):933–944. [PubMed] [Google Scholar]
- Johnstone EC, Owens DG, Frith CD, Crow TJ. The relative stability of positive and negative features in chronic schizophrenia. Br. J. Psychiatry. 1987;150:60–64. doi: 10.1192/bjp.150.1.60. [DOI] [PubMed] [Google Scholar]
- Kay SR. Significance of the positive-negative distinction in schizophrenia. Schizophr. Bull. 1990;16(4):635–652. doi: 10.1093/schbul/16.4.635. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Frescka E, Apter SH, Davidson M, Macaluso JM, Hirschowitz J, Davis KL. Clinical characteristics of Kraepelinian schizophrenia: Replication and extension of previous findings. Am. J. Psychiatry. 1996;153(6):806–811. doi: 10.1176/ajp.153.6.806. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, Horvath TB, Nora R, Davis KL. Characteristics of very poor outcome schizophrenia. Am. J. Psychiatry. 1987;144(7):889–895. doi: 10.1176/ajp.144.7.889. [DOI] [PubMed] [Google Scholar]
- Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am. J. Psychiatry. 1999;156(3):406–411. doi: 10.1176/ajp.156.3.406. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr. Bull. 2006;32(2):246–249. doi: 10.1093/schbul/sbj054. [comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br. J. Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [see comment] [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, McEvoy J, Perkins D, Sharma T, Zipursky R, Wei H, Hamer RM, Group HS. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am. J. Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- Lipsitz SR. Methods for estimating the parameters of a linear model for ordered categorical data. Biometrics. 1992;48(1):271–281. [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, N.J.: Wiley; 2002. [Google Scholar]
- Marneros A, Deister A, Rohde A. Validity of the negative/positive dichotomy for schizophrenic disorders under long-term conditions. Schizophr. Res. 1992;7(2):117–123. doi: 10.1016/0920-9964(92)90041-3. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch. Gen. Psychiatry. 1991;48(8):739–745. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia: Review of natural history validators. Arch. Gen. Psychiatry. 1992;49(1):63–72. doi: 10.1001/archpsyc.1992.01820010063008. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Fenton WS. Subtype Progression and Pathophysiologic Deterioration in Early Schizophrenia. Schizophr. Bull. 1993;19(1):71–84. doi: 10.1093/schbul/19.1.71. [DOI] [PubMed] [Google Scholar]
- Miller DD, Flaum M, Arndt S, Fleming F, Andreasen NC. Effect of antipsychotic withdrawal on negative symptoms in schizophrenia. Neuropsychopharmacology. 1994;11(1):11–20. doi: 10.1038/npp.1994.31. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Douglas MS, Bellack AS, Morrison RL. Assessment of Enduring Deficit and Negative Symptom Subtypes in Schizophrenia. Schizophr. Bull. 1991;17(4):565–582. doi: 10.1093/schbul/17.4.565. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J. Nerv. Ment. Dis. 1983;171(5):296–300. doi: 10.1097/00005053-198305000-00006. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile MF, Harrow M. Negative Symptoms in Schizophrenia: Their Longitudinal Course and Prognostic Importance. Schizophr. Bull. 1985;11(3):427–439. doi: 10.1093/schbul/11.3.427. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. Stata Journal. 2002;2(1):1–21. [Google Scholar]
- Rosen WG, Mohs RC, Johns CA, Small NS, Kendler KS, Horvath TB, Davis KL. Positive and negative symptoms in schizophrenia. Psychiatry Res. 1984;13(4):277–284. doi: 10.1016/0165-1781(84)90075-1. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia - Lifetime Version (SADS-L) 3rd edition. New York: New York State Psychiatric Institute; 1979. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction manual for the Structured Clinical Interview for DSM-III-R (SCID) New York: New York State Psychiatric Institute; 1989. [Google Scholar]
- Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr. Scand. 2007;115(1):4–11. doi: 10.1111/j.1600-0447.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- Tandon R, Goldman RS, Goodson J, Greden JF. Mutability and relationship between positive and negative symptoms during neuroleptic treatment in schizophrenia. Biol. Psychiatry. 1990;27(12):1323–1326. doi: 10.1016/0006-3223(90)90502-s. [see comment] [DOI] [PubMed] [Google Scholar]
- Tandon R, Ribeiro SC, DeQuardo JR, Goldman RS, Goodson J, Greden JF. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol. Psychiatry. 1993;34(7):495–497. doi: 10.1016/0006-3223(93)90242-6. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Peters J, Yao J, Van Kammen WB, Neylan T, Shaw D, Linnoila M. Norepinephrine in acute exacerbations of chronic schizophrenia: Negative symptoms revisited. Arch. Gen. Psychiatry. 1990;47(2):161–168. doi: 10.1001/archpsyc.1990.01810140061009. [DOI] [PubMed] [Google Scholar]
- Venables P, Wing J. Level of arousal and the subclassification of schizophrenia. Arch. Gen. Psychiatry. 1962;7(8):114–119. doi: 10.1001/archpsyc.1962.01720020038006. [DOI] [PubMed] [Google Scholar]