Abstract
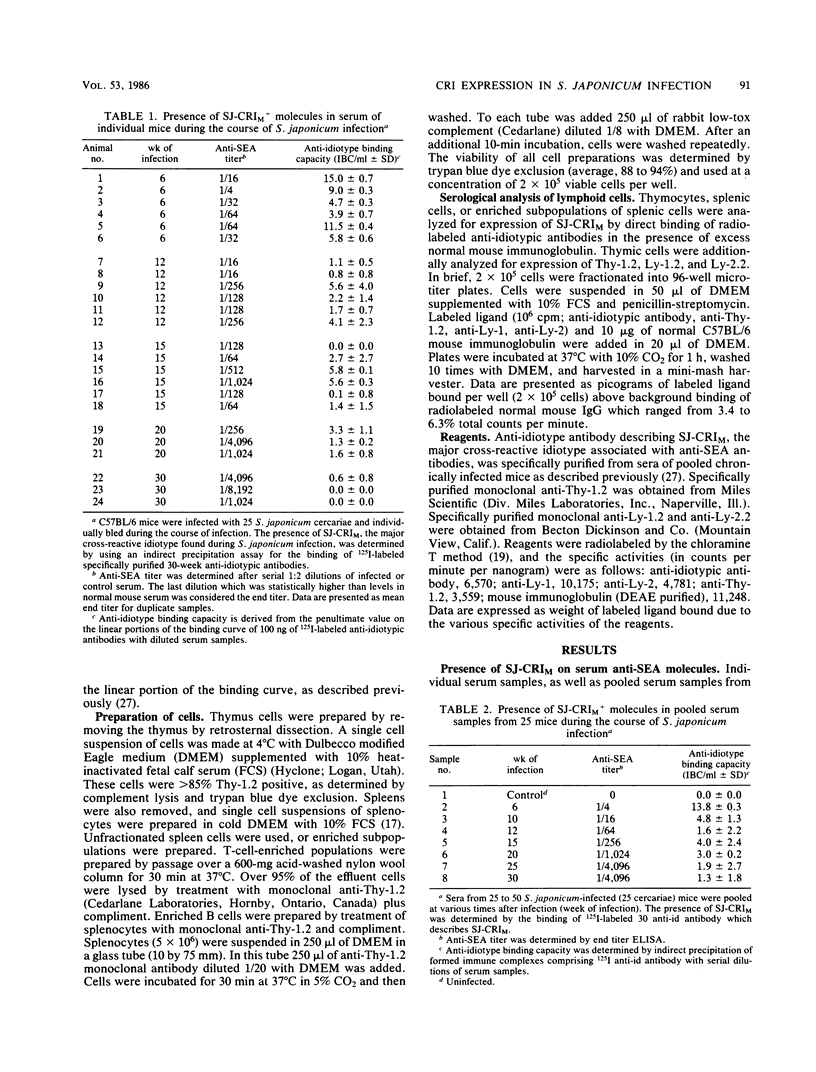
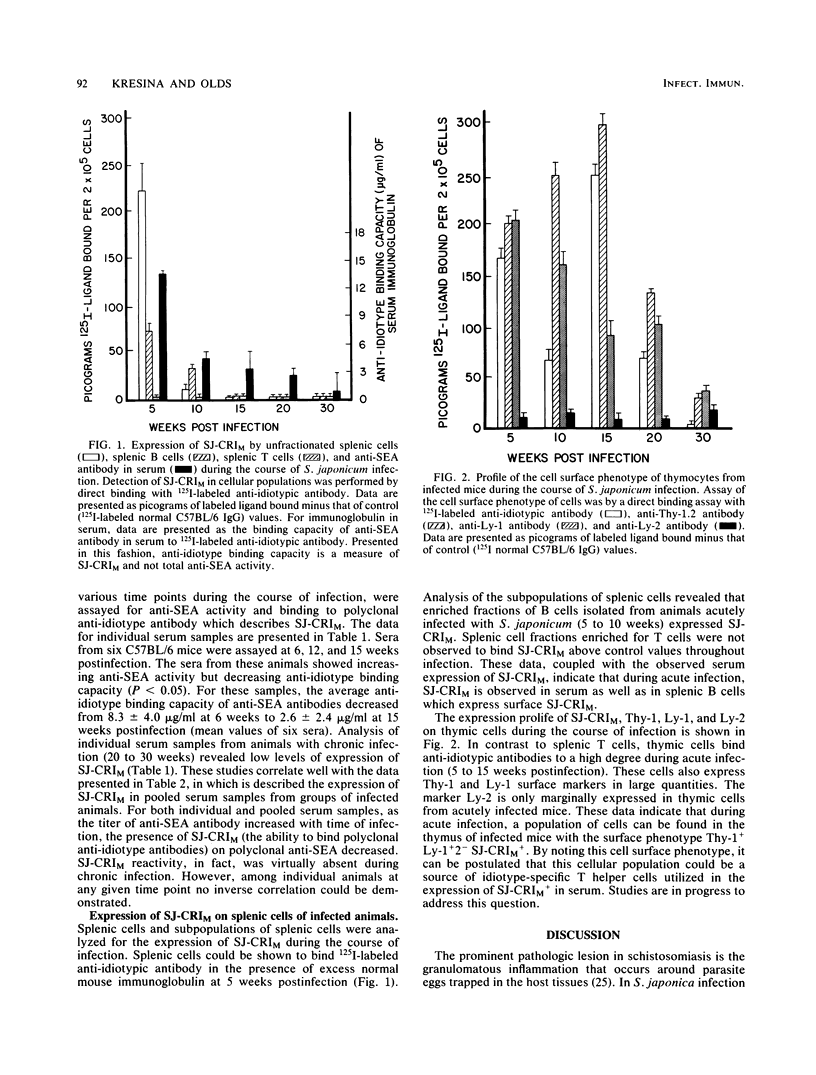
In this study the expression of a regulatory cross-reactive idiotype (SJ-CRIM), which is associated with anti-soluble egg antigen (SEA) molecules in murine Schistosoma japonicum infection, is described. Both humoral and cellular components of the immune response were analyzed during the course of infection with S. japonicum. In the humoral immune response, the content of SJ-CRIM decreases as the titer of anti-SEA antibody increases throughout infection. Quantitatively, values for serum ranged from 13.8 +/- 0.3 micrograms of SJ-CRIM, which binds anti-idiotypic antibody per ml of serum at 6 weeks postinfection, to 1.3 +/- 1.8 micrograms/ml at 30 weeks postinfection. Analysis of splenic cell subpopulations for expression of SJ-CRIM revealed that only splenic B cells expressed SJ-CRIM during acute infection (5 to 10 weeks postinfection). On the other hand, thymic cells with a high expression of the SJ-CRIM and Ly-1 marker were observed in acute infections up to 15 weeks postinfection. These data indicate that SJ-CRIM-bearing T cells are selectively localized in acute infection. In addition, the disappearance of expression of SJ-CRIM in serum and cells of chronically infected animals parallels the modulation of granulomatous inflammation and portal hypertension. Results of this study suggest that expression of SJ-CRIM on anti-SEA molecules could represent a marker for acute infection, while its disappearance from serum serves as a marker for modulation of disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., Hieny S., von Lichtenberg F., Lunde M. N., Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985 Jul;7(4):399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., von Lichtenberg F. Immunopathology of Schistosoma japonicum infection in athymic mice. Parasite Immunol. 1985 Jul;7(4):387–398. doi: 10.1111/j.1365-3024.1985.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi V., Giuntini M., Garzelli C., Campa M., Falcone G. Auto-anti-idiotypic antibodies inhibit T-cell-mediated hypersensitivity in BCG-infected mice. Cell Immunol. 1983 Aug;80(1):205–210. doi: 10.1016/0008-8749(83)90107-7. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Freeman G. L., Jr Differences in adult Schistosoma mansoni worm burden requirements for the establishment of resistance to reinfection in inbred mice. II. C57BL/KsJ, SWR/J, SJL/J, BALB/cAnN, DBA/2N, A/J, B10.A(3R), and B10.A(5R) mice. Am J Trop Med Hyg. 1983 May;32(3):543–549. doi: 10.4269/ajtmh.1983.32.543. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Dreesman G. R., Kennedy R. C. Anti-idiotypic antibodies: implications of internal image-based vaccines for infectious diseases. J Infect Dis. 1985 May;151(5):761–765. doi: 10.1093/infdis/151.5.761. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Murphy D. B., Kemp J. D., Shen F. W., Cantor H., Gershon R. K. Ly-1 inducer and Ly-1,2 acceptor T cells in the feedback suppression circuit bear an I-J-subregion controlled determinant. Immunogenetics. 1980;11(6):549–557. doi: 10.1007/BF01567824. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975 Oct;5(10):661–666. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Chen P., Carson D. A., Fong S. Expression of a cross-reactive idiotype on rheumatoid factor in patients with Sjogren's syndrome. J Immunol. 1986 Jan;136(2):477–483. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Grzych J. M., Capron M., Lambert P. H., Dissous C., Torres S., Capron A. An anti-idiotype vaccine against experimental schistosomiasis. Nature. 1985 Jul 4;316(6023):74–76. doi: 10.1038/316074a0. [DOI] [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Krolick K. A., Isakson P. C., Uhr J. W., Vitetta E. S. BCL1, a murine model for chronic lymphocytic leukemia: use of the surface immunoglobulin idiotype for the detection and treatment of tumor. Immunol Rev. 1979;48:81–106. doi: 10.1111/j.1600-065x.1979.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Vogler L. B., Capra J. D., Conrad M. E., Lawton A. R., Cooper M. D. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979 Oct 1;150(4):792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Kresina T. F. Network interactions in Schistosoma japonicum infection. Identification and characterization of a serologically distinct immunoregulatory auto-antiidiotypic antibody population. J Clin Invest. 1985 Dec;76(6):2338–2347. doi: 10.1172/JCI112245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981 May 15;60(2):251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Olds G. R., Stavitsky A. B. Mechanisms of in vivo modulation of granulomatous inflammation in murine schistosomiasis japonicum. Infect Immun. 1986 May;52(2):513–518. doi: 10.1128/iai.52.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell C. A., Arnold L. W., Lutz P. M., LoCascio N. J., Willoughby P. B., Haughton G. Cross-reactive idiotypes and common antigen binding specificities expressed by a series of murine B-cell lymphomas: etiological implications. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3799–3803. doi: 10.1073/pnas.82.11.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. R., Colley D. G. Demonstration of splenic auto-anti-idiotypic plaque-forming cells in mice infected with Schistosoma mansoni. J Immunol. 1985 Jun;134(6):4140–4145. [PubMed] [Google Scholar]
- Sacks D. L., Sher A. Evidence that anti-idiotype induced immunity to experimental African trypanosomiasis is genetically restricted and requires recognition of combining site-related idiotopes. J Immunol. 1983 Sep;131(3):1511–1515. [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Olds G. R., Peterson L. B. Regulation of egg antigen-induced in vitro proliferative response by splenic suppressor T cells in murine Schistosoma japonicum infection. Infect Immun. 1985 Sep;49(3):635–640. doi: 10.1128/iai.49.3.635-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


