Abstract
Co-occurrence of alcohol and nicotine addiction in humans is well documented and there is good evidence that common genes may contribute to both disorders. Although genetic factors contributing to tobacco and alcohol problem use have been well established through adoption, twin and family studies, specific genes remain to be identified and their mode of action elucidated. Recent work from human genetics studies has provided evidence that neuronal nicotinic acetylcholine receptors (nAChR) genes may have a role in mediating early behaviors that are risk factors for alcohol and nicotine dependence, such as age of initiation and early subjective responses to the drugs. Converging evidence suggests that the dopaminergic system is likely to be important in mediating the pleasurable feelings of reward when activated by nicotine and/or alcohol consumption. The nAChRs are important components of the dopaminergic reward system because some of the receptors have been shown to activate the release of dopamine, and mice lacking genes for specific nAChR gene subunits show altered behavioral responses to nicotine and alcohol. Furthermore, complex interactions between other neurotransmitter circuits including GABA, glutamate and serotonin may be modulated by nAChRs, leading researchers to study genes involved in neurobiology shared by different drugs. Future studies aimed at understanding the variation among these genes, and their corresponding functional implications, will help elucidate how natural variants in nicotinic receptor genes contribute to these common co-morbid disorders.
Keywords: nicotine, alcohol, co-morbidity, genetics, nicotinic receptors, linkage, association, behavior, neurotransmitters
INTRODUCTION
Nicotine and alcohol are two of the most widely used addictive drugs and both have hazardous health consequences resulting from their chronic use. Numerous studies have shown that alcohol use and smoking frequently co-occur and that both environmental and genetic factors contribute to the overlap between these two behaviors. A variety of studies using model systems have demonstrated that the neuronal nicotinic receptors (nAChRs) are a common site of action of nicotine and alcohol, in the sense that alcohol may modulate the pharmacological binding properties of nicotine at nAChRs [1–11]. Furthermore, the presence of several nAChR subtypes on dopaminergic nerve terminals implicates their potential involvement in the mesolimbic reward pathway, which may be a common component of many different types of addiction. Here we present a brief overview of the genetic underpinnings of alcohol and tobacco disorders, focusing on several human genetic associations between specific nAChR subunit genes and various alcohol and nicotine-related behaviors. Next, we present a comprehensive discussion of the biological role of nAChRs in mediating alcohol and nicotine responses. We conclude with our thoughts about the challenges still facing genetic studies of substance abuse research, and how those challenges may be overcome in the future.
COMMON GENES CONTRIBUTE TO ALCOHOL AND NICOTINE CO-ADDICTION
Numerous reports have provided evidence for high co-morbidity between smoking tobacco and alcohol use, whereby alcohol dependent individuals are more likely to be dependent on nicotine and vice versa [12–16]. There are several lines of evidence demonstrating that genetic factors are important for predicting long-term tobacco use and that significant genetic effects contribute to smoking, typically accounting for approximately 50% (28–84%) of the total variance [17–19]. Similarly, a variety of twin, adoption, and family studies have shown that genetic components play a role in the development of alcohol dependence [20–27]. More recent studies have provided data to support the theory that common genetic factors may contribute to the concurrent use of these two substances [19, 28–33]. Thus, there is a wealth of information suggesting that as much as half of the risk for nicotine and alcohol disorders may be mediated by genetic factors. Furthermore, the development of new analytical modeling techniques is beginning to help elucidate more detail about the environmental and etiological contributions to these two specific disorders and their co-occurrence.
HUMAN GENETIC STUDIES OF nAChR GENES
Recently, human genetic studies have provided evidence for a role of the nAChRs in the etiology of alcohol and tobacco co-addiction. Among the twelve subunit genes for neuronal nicotinic receptor subunits, those encoding the α4 and β2 nAChR subunits (CHRNA4 and CHRNB2), have been the most widely studied in human populations, since the α4β2 receptors are those most prevalent in the brain. The first line of evidence that natural variations in nAChR genes contributed to phenotypic variation in human disorders, came from a missense mutation in the CHRNA4 gene, that was associated with autosomal dominant nocturnal frontal lobe epilepsy in Australian families [34–38]. Similarly, a missense mutation (Val287Met) in the CHRNB2 gene was found to be associated with autosomal dominant nocturnal frontal lobe epilepsy in a Scottish family [39]. Because of the association between tobacco use and attention problems, the CHRNA4 gene has also been examined for possible association with attention deficit hyperactivity disorder, where one study did not find evidence for association [40], while a later study did find suggestive evidence [41].
Until recently, variations in genes encoding the neuronal nicotinic receptors have not been well studied for potential effects related to nicotine and alcohol abuse and dependence in humans. A partial summary of recent studies examining nicotine and alcohol behavioral association with nAChR human genes is presented in Table 1. Feng et al. [42] used the Fagerstrom Test for Nicotine Dependence (FTND)[43] and the Revised Tolerance Questionnaire (RTQ) [44] and examined six single nucleotide polymorphisms (SNPs) in CHRNA4 and four SNPs in CHRNB2. They reported an association between a haplotype in the CHRNA4 gene and nicotine dependence in Chinese men but no association between these phenotypes and CHRNB2. Similarly, Li et al. [45] detected an association between a different SNP in CHRNA4 and the heaviness of smoking index (HSI) from the Fagerstrom test for nicotine dependence (FTND), but no evidence for association with CHRNB2. In fact, most studies of CHRNB2 variations have not found evidence for association with nicotine or alcohol addictions. [42, 45–47]. Silverman et al. [47] screened for polymorphisms by sequencing pooled DNA samples, and identified five novel SNPs. Four of these, and their estimated haplotypes, were tested for association with smoking initiation and progression to nicotine dependence but no association was found [47]. Six other SNPs in the CHRNB2 gene showed no evidence for association in a separate study of individuals evaluated for smoking history and lifelong nicotine dependence [46]. However, two more recent reports have shown a possible role for this gene in age of initiation of smoking in women [48], and in early subjective response to smoking and alcohol [49].
Table 1.
Human Studies implicating nAChR genes in alcohol and tobacco dependence
| Phenotype | Gene(s) | Sample | Reference |
|---|---|---|---|
| Smoking and nicotine dependence | CHRNB2 no association | non-smokers, smokers, high ND smokers | Silverman, Neale et al. 2000 |
| Smoking behavior and nicotine dependence | CHRNB2 no association | individuals with smoking history available | Lueders, Hu et al. 2002 |
| FTND, RTQ | CHRNA4 | males in families w/multiple nicotine addicted siblings | Feng, Nui et al. 2004 |
| ND, SQ, HIS, FTND | CHRNA4, (no association for CHRNB2) | EA and AA families | Li, Beuten et al. 2005 |
| SQ, HIS, FTND | CHRNB1, CHRM1 (AA only) | Mid-South Tobacco Family sample: EA and AA families | Lou, Ma et al. 2006 |
| Nicotine dependence, smoking initiation | CHRNB2, CHRNA7, CHRNA9, CHRNB3 | Israeli female students | Greenbaum, Kanyas et al. 2006 |
| Subjective Effects, past month use | CHRNA4, CHRNB2 | CADD | Ehringer, Clegg et al. 2007 |
| FTND | CHRNB3, CHRNA5 | Collaborative study on the Genetics of Nicotine Dependence (COGEND) | Bierut, Madden et al. 2007 Saccone, Hinrichs et al. 2007 |
| Age of onset | CHRNA3, CHRNB4 | CADD, National Youth Survey | Schlaepfer, Hoft et al. 2007 |
| Early subjective response to tobacco | CHRNA6, CHRNB3 | Center for Antisocial Drug Dependence (CADD), National Longitudinal Study of Adolescent Health (Add Health) | Zeiger, Haberstick et al. 2008. |
| Cigarettes per day | CHRNA5, CHRNA3 | Three large European samples | Berrettini, Yuan et al. 2008 |
FTND: Fagerstrom Test for Nicotine Dependence, RTQ: Revised Tolerance Questionnaire, SQ: Smoking Quantity, ND: Nicotine dependence, HIS: Heavyness of Smoking Index, EA: European American, AA: African American.
The CHRNA6 and CHRNB3 genes are located next to each other on human chromosome 8 and code for the α6 and β3 nAChR subunits, respectively, which form part of nAChRs abundant in the body of dopamine producing cells. The CHRNB3 gene was found recently to be a top candidate for nicotine dependence (as measured by FTND) in two studies from the Collaborative Genetic Study of Nicotine Dependence (COGEND). Using a whole genome association approach which examined over 2.4 millions SNPs, the SNP rs13277254 emerged as one of the most significant findings [50]. Using a candidate gene approach, whereby 3713 SNPs were genotyped in over 300 candidate genes, a strong association with nicotine dependence was seen with a different SNP in CHRNB3 (rs6474414) [51]. Additional evidence for an important role of variations in CHRNB3 and addiction phenotypes continues to grow. In a recent Colorado-based study using a clinical-based sample from the Center for Antisocial Drug Dependence (CADD), preliminary evidence for association between two SNPs (rs4950 and rs13280604) and early subjective response to nicotine was detected. Moreover, this finding was replicated with these specific SNPs in the separately ascertained National Longitudinal Study of Adolescent Health (Add Health), a community-based sample [52]. In this study, there was also evidence for an association between a SNP in CHRNA6 (rs2304297) and early subjective response to nicotine in both the selected CADD and community-based Add Health samples, suggesting that this genomic region containing CHRNA6 and CHRNB3 may be important in mediating early subjective response to nicotine.
On chromosome 15, a cluster of three phylogenetically conserved nAChR subunit genes includes CHRNA5, CHRNA3, and CHRNB4, which code for the α5, α3 and α4 nAChR subunits respectively. In the candidate gene study of Saccone et al. [51], mentioned above, a non-synonomous SNP in CHRNA5 (rs16969968) was highly significant for association with nicotine dependence. Additionally, nominal significance between a SNP in CHRNA5 and nicotine dependence has also been reported in young Israeli women [48]. More recently, two linked SNPs (rs8023462 and rs1948) of the CHRNA5/A3/B4 gene cluster significantly predicted early age of initiation for tobacco in a clinically-based sample. These findings were then replicated in a separate population-representative sample, showing the same two SNPs to be associated with age of initiation for both tobacco and alcohol use [53]. Furthermore, Berrettini et al. [54] recently reported a significant association between natural variations in the CHRNA5/CHRNA3 genes and number of cigarettes per day in three independent samples of European origin. Thus, variations in CHRNA5/A3/B4 genes may influence behaviors that promote early age of experimentation with drugs and/or nicotine dependence.
Clearly, evidence is accumulating for an important role of nAChR genes in mediating behaviors involved in tobacco and/or alcohol abuse and dependence. Although a variety of pharmacological and animal studies have provided support for involvement of these receptors in nicotine/alcohol action (next section below), few human studies have explored nicotine and/or alcohol phenotypes with the nAChR subunit genes. With greater appreciation of nAChRs diversity on one side and the sophistication of new genetic statistical analysis tools at the other, the elucidation of the molecular genetics of drug addictions is gaining momentum. Despite these advances, however, there remain inconsistent findings in human genetic reports, likely due to differences in study samples, phenotypic measures, and genetic heterogeneity, whereby different genes may be important in different populations.
ROLE OF nAChRs IN ALCOHOL AND TOBACCO EFFECTS
Multiple neurotransmitters appear to orchestrate the reward profile of nicotine and alcohol addiction and co- addiction. Several lines of evidence from model systems using Xenopus oocytes [1, 2], rat electrophysiology [3–7], and mouse behavioral models [8–11] suggest that alcohol may modulate the pharmacological properties of nicotine binding at nAChRs, usually by enhancing receptor function. Since these receptors have been shown to activate release of dopamine, they are likely to be important for mediating the rewarding properties of the mesolimbic dopamine system [55, 56].
Here we first review the role of neuronal nicotinic acetylcholine receptors (nAChRs) and the mesolimbic dopamine system in the mediation of the behavioral and neurochemical effects of ethanol and/or nicotine in ex vivo and in vivo model systems. Subsequently, we highlight some of the main neurotransmitter systems that are modulated by nAChRs in the brain.
Neuronal nicotinic acetylcholine receptors (nAChRs) belong to the large superfamily of ligand-gated ion channels that bind the endogenous neurotransmitter acetylcholine and the alkaloid nicotine, found in tobacco. A broad description of the nAChRs has been published in recent reviews [55, 57–59]. Briefly, nAChRs are formed by the pentameric association of α and β subunits, of which 12 nAChRs subunits (α2-10, β2-4) have been identified in the brain.
Different combinations of subunits generate subtypes of nAChRs with diverse functional and pharmacological properties, which in vivo may have selective roles in specific brain pathways. The main nAChR subtypes found in mammalian brain have been shown to be those containing the α4 and β2 subunits, which bind nicotine with high affinity, and the α-bungarotoxin-sensitive α7 subunits, which form homomeric receptors [55]. The nAChRs found in the autonomic nervous system segregate and assemble into two distinct classes: α7-containing receptors and heteromeric α3 subunit-containing receptors, co-assembling with β2 and β4 with or without α5 subunits [60]. The structure and localization of the nAChRs subtypes have been elucidated using molecular techniques such as in situ hybridization, immunohistochemistry and imaging techniques including positron emission tomography (PET) and single photon emission computed tomography (SPECT) [61]. Despite these complementary techniques, the lack of antibodies specific to the different subtypes of nAChRs and the possibility of false positives due to the use of PCR approaches, limit the verification and/or classification of the nAChRs subtypes. In the future, the development of new technology and methodological approaches may facilitate better characterization and localization of specific subtypes.
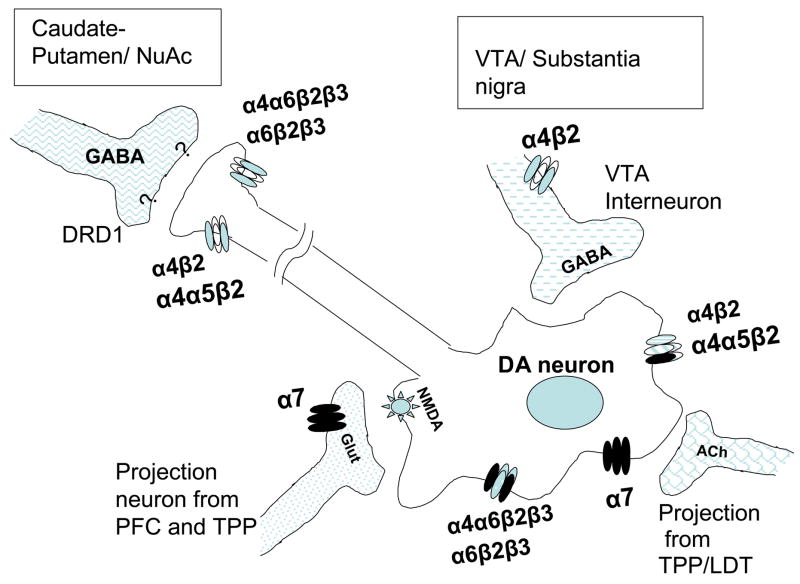
The α4β2 receptor subunits are encoded by the CHRNA4 and CHRNB2 genes (respectively) and expressed throughout the brain and spinal cord. They are principally located on presynaptic nerve terminals where they modulate the release of neurotransmitters including dopamine [56] and γ-aminobutyric acid (GABA) [62] (Figure 1). These α4β2 receptor subtypes, together with the α7 homomeric receptor, are the most prevalent in the brain and the majority of work in the alcohol-nicotine field has focused on them. In fact, results from studies with Xenopus oocytes, mice and cerebral cortex cells grown in culture have confirmed that alcohol is able to stimulate the function of the naturally expressed α4β2* receptors (where * indicates another subunit, often α6 and/or β3) and inhibit the action of the α7 nAChRs [63].
Figure 1. Different combinations of nAChRs subunits modulate dopamine release in the mesolimbic reward pathway.
The modulation of dopamine release in the VTA is mediated through presynaptic and preterminal nAChRs. Binding of nicotine or acetylcholine to the α7 receptors in glutamatergic terminals induce release of glutamate (Glut), which activates NMDA receptors in dopaminergic neurons resulting in activation of the release of dopamine. Cholinergic (ACh) input from mesopontine nuclei (TPP/LDT) also activate the release of dopamine in the VTA. Furthermore, desentizitasion of nAChRs of GABA releasing interneurons, also reduces the inhibition of dopamine release due to GABA, resulting in further induction of dopamine release in the striatum and nucleus accumbens. VTA; ventral tegmental area. PFC; Prefrontal cortex. TPP; tegmental pedunculopontine nucleus. LDP; laterodorsal tegmental nucleus, NuAc; Nucleus accumbens. DRD1; Dopamine receptor 1. NMDA; N-methyl-D-aspartate..
Although not as well-studied, less abundant nAChRs subtypes [64, 65] also appear to modulate some of the rewarding properties of alcohol and nicotine [66]. The main characteristic of these receptors is that they are blocked by a small peptide cone-snail toxin named α-Conotoxin MII. β3 subunits are essential for correct assembly and stability of α6-containing nAChRs in dopamerigic neurons as shown in recent studies using ligand-binding and immuno-purification techniques [67]. The nAChRs containing α6 and β3 subunits are mainly expressed in midbrain dopamine neurons and in the locus coeruleus, which is a nucleus in the brain stem responsible for physiological responses to stress and panic.
Additional nAChR subunits including α5, α3 and β4 have limited distribution in the brain (see below), but can represent a significant population of nAChRs with a critical function. These three subunits (α5, α3 and β4) are encoded by a phylogenetically conserved cluster of nAChR subunit genes (CHRNA5/A3/B4), important in fast cholinergic synaptic transmission. The three subunits are co-expressed in the adrenal medulla, autonomic ganglia and several structures of the brain including, the medial habenula, the interpeduncular nucleus and the inferior colliculus [68]. The significance of the subunit coexpression in these brain regions is not known but previous studies have shown that the habenulo-interpeduncular pathway exerts a tonic inhibitory influence on mesocortical, mesolimbic and mesostriatal dopaminergic neurons [69, 70]. Additionally, the α5 subunit is found in heteromeric receptors of the substantia nigra, ventral tegmental area (VTA), striatum, cortex and hyppocampus, and the α3β4 subunits are found together in hippocampus, medial habenula, pineal gland, cerebellum, locus coreruleus, substantia nigra and VTA [55, 71–73]. Therefore, when multiple α and β subunits are co-expressed, only some of the many possible combinations of nAChRs have been observed, for example the α4β2, α6β3 or α3β4 -containing receptors are very common subunit assemblies. The precise determinants of these restricted assemblies of nAChRs and their preferential locations in the CNS are still not well understood.
NICOTINIC RECEPTORS AND THE DOPAMINERGIC SYSTEM
Neuronal nicotinic acetylcholine receptors (nAChRs) have been shown to activate the release of dopamine, making them important components of the dopaminergic reward system [55, 56]. Furthermore, several nAChR subtypes (α4α6β2β3, α6β2β3, α6β2, α4β2, and α4α5β2) have been shown to be expressed on dopamine nerve terminals [74]. Converging evidence suggests that the dopaminergic system is likely to be important in mediating the pleasurable feelings of reward when activated by nicotine and/or alcohol consumption [71, 75–77]. Drug addiction is believed to involve plastic changes in neuronal systems associated with rewarding behaviors. The mesolimbic dopamine system includes three brain regions: the VTA, the nucleus accumbens (NAcc) and prefrontal cortex (PFC). The VTA contains dopaminergic neurons and extensions from these cells lead into the nucleus accumbens and the prefrontal cortex, where the released dopamine activates other neurons (see Figure 1 for a simplified diagram). The nAChRs located in the soma (body) of dopaminergic neurons of the VTA are able to excite these neurons directly leading to transient responses that are terminated by desensitization of nAChRs. Additionally, the stimulation and consequent desensitization of the gamma-aminobutyric acid (GABA)-containing neurons of the VTA, further contributes to the excitatory effect by eliminating the inhibitory effect of GABA. Another type of indirect modulation of dopamine release by nAChRs, is carried out by activation of α7 homomeric receptors on glutamatergic (Glut ) terminals. This α7 activation triggers release of glutamine, which in turn stimulates ionotropic glutamine receptors (NMDA or N-methyl-D-aspartate) on dopaminergic neurons, leading to the induction of dopamine release [78]. Interestingly, elevated levels of dopamine have also been shown to modulate nAChRs in an area and subtype-dependent manner: Mice deficient in the dopamine transporter gene exhibit constitutively increased dopamine levels that result in significant modifications in nAChRs density and function [79]. This interaction highlights the inter-related modulatory effects that nAChRs have on dopaminergic systems and vice versa. In other words, a localized increase in dopamine levels could account for significant adaptations in the cholinergic/nicotinic neurotransmission pathways
Recent molecular biology results further underscore the importance of the interaction between nAChRs and dopaminergic systems in the etiology of nicotine and alcohol addiction. Inoue et al. [80] have reported synergistic enhancement of gene expression in co-cultures of VTA and NAcc neurons by nicotine and alcohol. Their results suggested that a novel cellular mechanism involving nAChRs, dopamine receptors, adenosine receptors and protein kinase A signaling, results in increased gene activation in cultured neurons exposed to alcohol and nicotine simultaneously. Furthermore, a recent report has demonstrated that simultaneous administration of alcohol and nicotine results in an additive dopamine release in the nucleus accumbens of rats, providing additional support for the importance of the mesolimbic “reward pathway” as a convergent response site for these two drugs [81, 82]. Thus, nicotine/ethanol interaction through co-activation of dopamine and adenosine receptors could contribute to the long term neuro-adaptations (via gene activation) that lead to the development of addiction.
ANIMAL STUDIES OF nAChRs
Animal studies with genetically modified rodents have focused on mimicking specific aspects of human addictions (self-administration, reward, withdrawal, locomotion activation) that can be easily measured in animals. In particular, genetically modified mouse models have been very important for the understanding of complex behaviors, related to addiction, relapse and reinstatement of drug dependence [83–85]. Here we present an overview of some of the results from animal models developed for studying co-addiction of alcohol and tobacco. Highlighting the importance of the dopaminergic reward pathway in mediating these behaviors, we focus on research using mice lacking individual nAChR genes to illustrate how these genes have become a key focus of nicotine and alcohol addiction research.
Drug self-administration or reinforcement is an important component of drug addiction that has been studied in animals. In fact, a recent publication [85] has shown strong experimental evidence for the hypothesis that a shared genetic determinant accounts for the nicotine and alcohol co-morbidity. Le et al.[85] found that naive offspring of alcohol preferring rats self-administered larger amounts of nicotine intravenously and demonstrated more robust nicotine seeking behavior and relapse, than the offspring of alcohol non-preferring rats. These results point to nAChRs and the dopamine reward pathways as potential candidates for the interaction of alcohol and nicotine in the brain. However, alternative pathways or mechanisms that could explain how alcohol increases the reinforcing effects of nicotine and vice-versa need to be proposed and investigated. For example, the vast majority of schizophrenic individuals tend to be avid smokers, even when their treatment involves high doses of dopamine receptor blockers [86–89].
Additional research efforts have also focused on the issue of cross-tolerance between drugs. That is, chronic treatment with one drug results in reduced sensitivity for another. It is known that smokers appear to be less sensitive to the effects of alcohol than non-smokers [29] but this cross-tolerance is difficult to mimic in animal models, mainly due to the differences in pharmacokinetics of both drugs (absorption, distribution, metabolism, etc). Some evidence of changes in α4β2 receptor binding has been observed after chronic ethanol treatment in rats [90] and mice [91], suggesting that nicotinic receptors are involved in mediating cross-tolerance of alcohol and nicotine.
Quantitative trait locus (QTL) mapping analyses using recombinant inbred strains of mice with high (Inbred Long Sleep; ILS) and low (Inbred Short Sleep; ISS) sensitivity to ethanol have been used to identify common regions of the genome contributing to alcohol and nicotine sensitivity [92]. In order to search for candidate genes common to nicotine and alcohol sensitivity, researchers conducted a QTL analysis using the acoustic startle phenotype in these strains. The results led the researchers to a genomic region containing the α4 subunit gene (Chrna4) where a polymorphism within the coding region of the α4 nicotinic receptor subunit gene was identified. This polymorphism encodes for an amino acid substitution at position 529 (threonine in LS mice and alanine in SS mice) [93]. Further testing of a panel of recombinant inbred strains derived from the long sleep and short sleep lines of mice showed an association between this amino acid substitution and the effects of both alcohol and nicotine on the acoustic startle response [94]. Therefore, there is strong evidence from this animal model that the α4 nAChR subunit is likely to be important in modulating response to both substances, and a good candidate for pleiotropic effects contributing to the co-morbidity of alcohol and nicotine behaviors.
Further understanding of the role of the α4β2 nAChRs was obtained using transgenic mice where either the gene coding for the α4 or the β2 subunit of the receptor has been deleted (“knocked-out”) from the genome and is no longer able to produce a protein. Mice with a β2 deletion do not produce any nAChRs containing the β2 subunit. That is, β2 deficient mice do not produce α4β2 receptors. Owens and colleagues [94] found that the mice deficient for the β2 subunit exhibited less sensitivity to the alcohol-induced reduction of the acoustic startle response. Additional studies also indicated that the α4β2 nAChRs are indeed involved in modulation of the effects of alcohol [8, 9].
Studies with α7 knock-out mice (the other nAChR most abundant in brain) have shown that deficiency of α7 homomeric nAChRs results in mice that, when exposed to alcohol, have less anxiety, increased alcohol-induced hypothermic response and longer duration of the alcohol-induced unconsciousness [95]. Interestingly, other measures of the mouse response to alcohol, such as acoustic startle, were not different between the mice lacking the α7 receptors and the control littermates, indicating that different nAChRs may be responsible for different behavioral responses to alcohol effects.
Regarding the α6 and β3 receptor subunits, the α-Conotoxin MII snail toxin can be used to block α6β3-containing receptors and observe behavioral effects. For example, administration of α-Conotoxin MII to the rodent VTA brain region produced a diminished alcohol-induced release of dopamine and reduced stimulation of locomotor activity was observed [66]. More recently, β3 knock-out mice have been developed and studies have shown that this subunit is critical for the correct assembly, trafficking and stability of α6-containing nAChRs in dopaminergic neurons. Further studies by Booker et al. [73] using β3-deficient mice, strongly suggested that the β3-containg nAChRs influence levels of anxiety and may be critical players in the continuation of smoking behaviors. Thus, α6β3-containing receptors emerge as a plausible common site for the interaction between nicotine and alcohol and could represent neurochemical targets for the development of drugs to treat alcoholism.
Animal models indicate that the β4 subunit is important in the behavioral and physiological expression of anxiety in mice [96] and in the resistance to nicotine-induced seizures [97]. In particular, the role of the β4-containing nAChRs in mediating the signs of nicotine withdrawal has been addressed with the use of β4-knock out mice. Results from these studies have shown that the β4-deficient mice show decreased signs of nicotine withdrawal symptoms [97]. These data are of significant importance since β4-containing receptors appear to play a dominant role in the mediation of the negative reinforcement properties of nicotine.
In addition, the β4 subunit is almost always co-expressed with the α3 subunit and both are necessary for normal autonomic function [98] but behavioral and physiological studies related to the α3 subunit have been difficult because mice deficient for this subunit rarely survive the weaning stages of life. However, mice that are heterozygous for the α3 deletion (+/− mice) are partially resistant to nicotine-induced seizures, similarly to the β4 deficient mice [97]. The behavioral effects of ethanol on these subunits has not been addressed, however, some evidence for the modulation of α3β4 -containing nAChRs exists. Studies with rat nAChRs expressed in Xenopus oocytes performed a decade ago [99] concluded that the α3β4 subunit combination may be especially sensitive to modulation by low ethanol concentrations. Furthermore, studies with adrenal PC12 cell lines also have shown that agonist responses in α3β4 -containing nAChRs are sensitive to the effects of low concentrations of ethanol [5]. Thus, the α3β4 -containing receptors could be contributors to the process of mutual reinforcement of alcohol and nicotine co-addiction.
Regarding the α5 subunit, experiments with α5-deficient mice have shown that the presence of the α5 subunit is essential for the development of nicotine-induced seizures and hypolocomotion [96]. In fact, α5-deficient mice show phenotypes very similar to the ones observed in β4 -deficient mice. However, the rate of response to high doses of nicotine appears to be mediated by the α5 subunit, not the β4 subunit [100]. The similar behavioral effects observed for these three subunits perhaps are not surprising since the genes for α5, α3 and β4 subunits are clustered together in the genome and are likely to share regulatory elements [68].
INTERACTIONS OF NICOTINIC RECEPTORS WITH OTHER NEURONAL SYSTEMS
Alcohol and nicotine, at the pharmacological level, do not appear to share many characteristics: Nicotine is pro-convulsant, induces arousal and binds to specific cellular receptors. On the other hand, alcohol is anti-convulsant, induces relaxation and appears to affect multiple receptors [101]. However, as mentioned earlier, the amount of tobacco smoked is positively correlated with the amount of alcohol consumed and the severity of alcohol dependence [30]. In animal models, chronic treatment with either ethanol or nicotine produces cross-tolerance to the other drug [102, 103]. Furthermore, a recent study examining the effects of nicotine and alcohol on fear conditioned learning has shown that these two drugs can each serve to ameliorate the negative effects of the other, which may be a mechanism contributing to their co-addiction [104].
The majority of the nAChRs are expressed on nerve terminals, where they function as modulators of the release of dopamine, GABA, norepinephrine, acetyl-choline and glutamate [92]. Nicotine effects at the nAChRs are complex, because following activation, the nAChRs are quickly inactivated through a mechanism called desensitization. This inactivating mechanism could be the reason why chronic smokers no longer experience some of the effects of nicotine or, alternatively, it may generate a sensation that smokers crave. Since alcohol has been shown to interfere with this inactivation (desensitization) of nAChRs by nicotine [4, 5], this mechanism could contribute to the high prevalence of alcohol and nicotine co-morbidity.
Another mechanism that could explain the high alcohol consumption observed in smokers is based on the nicotinic modulation of GABAA receptors after an ethanol challenge in rodents [105]. In these experiments, sub-chronic modulation of nicotinic receptors (pre-treatment with nicotine) attenuated the decline of dopamine in the nucleus accumbens of rats exposed to ethanol an hour earlier, thereby attenuating the sedative effects of alcohol and prolonging its stimulatory effects. Additional evidence of the ethanol potentiation of nAChRs function comes from recent studies examining the release of GABA and glutamate neurotransmitters from rodent cortical bipolar neurons exposed to acetylcholine and ethanol [106].
The possible involvement of the glutamate (NMDA or N-methyl-D-aspartate) system in the behavioral interaction between nicotine and alcohol has also been recently explored in the rodent cerebellum [107]. In this study, the authors investigated the functional role of cerebellar NMDA in the ethanol and nicotine interaction using ethanol ataxia as the test response. An important observation in this study was the ability of intra-cerebellar nicotine administration to significantly attenuate ethanol ataxia, especially when intra-cerebellar NMDA was microinfused simulateously. These results demonstrate the important function of nAChRs and NMDA receptors in the mediation of nicotine-ethanol motor behavioral interaction.
The relationship between the cholinergic and monoaminergic systems has also been documented [108]. It is of great interest to elucidate the subtypes of nAChRs responsible for the anxiety and depression behaviors related to smoking behaviors in humans. Studies with mutant mice support the role of nAChR subunits in behaviors related to anxiety and depression. For example, mice deficient in either the α4, β3 or α7 subunits show less anxiety-like behaviors [57, 108]. In fact, tricyclic antidepressants, serotonin-selective re-uptake inhibitors and the drug bupropion (atypical antidepressant) are all non-competitive antagonists of nAChRs. Moreover, the expression of nicotine-induced behavioral disinhibition observed in rodents, can be counteracted by selective serotonin re-uptake inhibitors (citalopram) as well as by serotonin receptor (5-HT-1A) agonists that are also known to decrease ethanol consumption in rats and humans [109]. However, despite all the well characterized connections between alcohol, nicotine depression and antidepressants, the role of nAChR as targets for depression-related behaviors is far from being understood.
In summary, a considerable amount of knowledge about the biology and function of nAChRs exists. The understanding of structural and functional subtypes of nAChRs and their specific distribution and pharmacology is rapidly improving, highlighting their role as mediators of addictive behaviors. Despite this expansion on studies of nAChRs, very little is known about human nAChRs at the molecular and pharmacological level.
DISCUSSION AND FUTURE DIRECTIONS
A growing body of research has provided evidence for the important role of genetic components in mediating the co-morbidity of alcohol and nicotine behaviors. Ultimately, the way humans cope with addictive drugs is really a combination of many environmental effects, multiple genes, and a large interaction between these factors. Certain genes may contribute to a greater likelihood of experimenting with and continuing to use drugs in high risk environments. Similarly, genetic constitution is likely to influence differential responses to drugs and whether or not certain individuals go on to continue to use, develop tolerance, and become dependent. Therefore, future behavioral genetic studies need to be aimed at developing approaches which can effectively model and understand potential gene-environment interactions contributing to the developmental stages in the etiology of addiction.
The mesolimbic dopamine system has been studied for many years as a key player in mediating pleasurable responses to drugs. Ongoing work provides evidence that neuronal nicotinic receptors are likely to be important components of this system, and may be a common site of action for nicotine and alcohol. Recent human association studies have provided strong support for an important role of these genes in nicotine behaviors, but few studies have yet examined alcohol phenotypes. Furthermore, the potential molecular functional consequences of specific human variations have not yet been studied, and this will be an important area of research for understanding potential differences in genetic regulatory mechanisms among individuals. One of the most significant advances enabling research of nicotinic receptors has been the development of a collection of mice which are deficient in specific nicotinic subunits and the ability to test these animals for behavioral responses to nicotine and alcohol. Future studies should include the development of double knock-out mice, which lack two or more specific subunits, and the development of mice with specific mutations in certain subunit genes informed by emerging findings from human genetic association studies. Additional results will also be expected from the use of conditional knockout mice, where the deletion of a specific subunit is controlled in a spatiotemporal manner, and the use of antisense oligonucleotide technologies to target specific neurons or areas of the brain. In addition, high resolution imaging techniques are emerging as state-of-the-art methods for studying addiction, and can include integration of genetic data to determine whether certain gene variants are associated with specific patterns of response in the brain [110]. Such translational research approaches are promising in their potential to yield significant insight into the molecular mechanisms of nAChRs and their role in drug-related behaviors.
Box 1. Key Learning Objectives of Paper.
Family, twin, and adoption studies provide substantial evidence of a common genetic vulnerability to alcohol and nicotine addictions.
Candidate gene and whole genome association studies are beginning to identify and replicate findings for nAChR genes that may underlie co-morbidity of these two drugs.
Many pharmacological and animal studies have provided evidence for interactions of nicotine and alcohol within the central nervous system.
Neuronal nicotinic acetylcholine receptors (nAChRs) represent a potential common site of action for nicotine and alcohol.
The variety of subtypes, distribution, and properties of nAChRs are likely to be involved in the complex behavioral effects of alcohol and nicotine.
Nicotine and alcohol also induce changes in many different neuronal pathways with connections to nAChRs, including the dopaminergic system, serotonergic pathways, opioid receptors, γ-aminobutyric acid receptors, and others.
Box 2. Future Research Questions.
Future work needs to focus on the developmental nature of these disorders by recruiting subjects prior to initiation of nicotine and alcohol use and continuing to follow individuals over their lifetime.
New statistical approaches for analyzing possible gene-gene and gene-environment interactions will be essential for interpreting the information
Continued development of mouse behavioral models of nicotine and alcohol phenotypes, including translational approaches that target specific human DNA variations in transgenic mice, will be important in interpreting whether human gene associations lead to changes in behavior.
Biological functional studies will be necessary in order to characterize the underlying molecular mechanisms of specific variations associated with these behaviors, potentially leading to improved prevention approaches and pharmacogenomic-based treatment approaches.
Further development of non-invasive brain imaging techniques will aide in the elucidation of specific ligands and/or receptors in brain regions known to be associated with addiction phenotypes.
Acknowledgments
Ms. Schlaepfer and Dr. Hoft are supported by a T32 NIDA training grant DA017637 and Dr. Ehringer is supported by an NIAAA K01 career award AA015336.
References
- 1.Borghese CM, Wang L, Bleck V, Harris RA. Mutation in neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes blocks ethanol action. Addict Biol. 2003;8(3):313–8. doi: 10.1080/13556210310001602220. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289(2):774–80. [PubMed] [Google Scholar]
- 3.Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol. 1999;55(1):39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- 4.Marszalec W, Aistrup GL, Narahashi T. Ethanol-nicotine interactions at alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors in rat cortical neurons. Alcohol Clin Exp Res. 1999;23(3):439–45. [PubMed] [Google Scholar]
- 5.Nagata K, Aistrup GL, Huang CS, Marszalec W, Song JH, Yeh JZ, et al. Potent modulation of neuronal nicotinic acetylcholine receptor-channel by ethanol. Neurosci Lett. 1996;217(2–3):189–93. [PubMed] [Google Scholar]
- 6.Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, Narahashi T. Alcohol modulation of neuronal nicotinic acetylcholine receptors is alpha subunit dependent. Alcohol Clin Exp Res. 2002;26(6):779–84. [PubMed] [Google Scholar]
- 7.Zuo Y, Nagata K, Yeh JZ, Narahashi T. Single-channel analyses of ethanol modulation of neuronal nicotinic acetylcholine receptors. Alcohol Clin Exp Res. 2004;28(5):688–96. doi: 10.1097/01.alc.0000125349.99823.8a. [DOI] [PubMed] [Google Scholar]
- 8.Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A Polymorphism in the alpha4 Nicotinic Receptor Gene (Chrna4) Modulates Enhancement of Nicotinic Receptor Function by Ethanol. Alcohol Clin Exp Res. 2003;27(5):733–42. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- 9.Butt CM, King NM, Stitzel JA, Collins AC. Interaction of the nicotinic cholinergic system with ethanol withdrawal. J Pharmacol Exp Ther. 2004;308(2):591–9. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- 10.Gould TJ, Collins AC, Wehner JM. Nicotine enhances latent inhibition and ameliorates ethanol-induced deficits in latent inhibition. Nicotine Tob Res. 2001;3(1):17–24. doi: 10.1080/14622200020032060. [DOI] [PubMed] [Google Scholar]
- 11.Tritto T, Marley RJ, Bastidas D, Stitzel JA, Collins AC. Potential regulation of nicotine and ethanol actions by alpha4- containing nicotinic receptors. Alcohol. 2001;24(2):69–78. doi: 10.1016/s0741-8329(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 12.Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90(7):977–80. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- 13.Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever- smokers. J Subst Abuse Treat. 1997;14(6):521–7. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 14.Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Jr, et al. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 2000;35(2):171–5. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- 15.John U, Meyer C, Rumpf HJ, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98(6):805–14. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 16.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Swan GE, Carmelli D. Behavior Genetic Investigations of Cigarette Smoking and Related Issues in Twins. In: Blum K, Noble EP, editors. Handbook of Psychiatric Genetics. Boca Raton, New York, London, Tokyo: CRC Press Inc.; 1997. pp. 387–406. [Google Scholar]
- 18.Heath AC, Madden PA, Martin NG. Statistical methods in genetic research on smoking. Stat Methods Med Res. 1998;7(2):165–86. doi: 10.1177/096228029800700205. [DOI] [PubMed] [Google Scholar]
- 19.Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. J Stud Alcohol. 1997;58(2):182–90. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- 20.Cadoret RJ, Cain CA, Grove WM. Development of alcoholism in adoptees raised apart from alcoholic biologic relatives. Arch Gen Psychiatry. 1980;37(5):561–3. doi: 10.1001/archpsyc.1980.01780180075008. [DOI] [PubMed] [Google Scholar]
- 21.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38(8):861–8. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 22.Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53(8):681–7. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- 23.Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5(2):207–15. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res. 1987;11(4):349–56. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 25.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27(6):1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 26.Cotton NS. The familial incidence of alcoholism: a review. J Stud Alcohol. 1979;40(1):89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- 27.True WR, Heath AC, Scherrer JF, Xian H, Lin N, Eisen SA, et al. Interrelationship of genetic and environmental influences on conduct disorder and alcohol and marijuana dependence symptoms. Am J Med Genet. 1999;88(4):391–7. doi: 10.1002/(sici)1096-8628(19990820)88:4<391::aid-ajmg17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Swan GE, Carmelli D, Cardon LR. The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. J Subst Abuse. 1996;8(1):19–31. doi: 10.1016/s0899-3289(96)90055-3. [DOI] [PubMed] [Google Scholar]
- 29.Madden PA, Heath AC, Martin NG. Smoking and intoxication after alcohol challenge in women and men: genetic influences. Alcohol Clin Exp Res. 1997;21(9):1732–41. [PubMed] [Google Scholar]
- 30.True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, et al. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56(7):655–61. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 31.Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend. 1999;57(1):69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 32.Hopfer CJ, Stallings MC, Hewitt JK. Common genetic and environmental vulnerability for alcohol and tobacco use in a volunteer sample of older female twins. J Stud Alcohol. 2001;62(6):717–23. doi: 10.15288/jsa.2001.62.717. [DOI] [PubMed] [Google Scholar]
- 33.Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36(4):603–15. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 34.Steinlein O, Sander T, Stoodt J, Kretz R, Janz D, Propping P. Possible association of a silent polymorphism in the neuronal nicotinic acetylcholine receptor subunit alpha4 with common idiopathic generalized epilepsies. Am J Med Genet. 1997;74(4):445–9. doi: 10.1002/(sici)1096-8628(19970725)74:4<445::aid-ajmg18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 35.Steinlein O, Weiland S, Stoodt J, Propping P. Exon-intron structure of the human neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) Genomics. 1996;32(2):289–94. doi: 10.1006/geno.1996.0119. [DOI] [PubMed] [Google Scholar]
- 36.Steinlein OK, Deckert J, Nothen MM, Franke P, Maier W, Beckmann H, et al. Neuronal nicotinic acetylcholine receptor alpha 4 subunit (CHRNA4) and panic disorder: an association study. Am J Med Genet. 1997;74(2):199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Steinlein OK, Magnusson A, Stoodt J, Bertrand S, Weiland S, Berkovic SF, et al. An insertion mutation of the CHRNA4 gene in a family with autosomal dominant nocturnal frontal lobe epilepsy. Hum Mol Genet. 1997;6(6):943–7. doi: 10.1093/hmg/6.6.943. [DOI] [PubMed] [Google Scholar]
- 38.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11(2):201–3. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 39.Phillips HA, Favre I, Kirkpatrick M, Zuberi SM, Goudie D, Heron SE, et al. CHRNB2 is the second acetylcholine receptor subunit associated with autosomal dominant nocturnal frontal lobe epilepsy. Am J Hum Genet. 2001;68(1):225–31. doi: 10.1086/316946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent L, Middle F, Hawi Z, Fitzgerald M, Gill M, Feehan C, et al. Nicotinic acetylcholine receptor alpha4 subunit gene polymorphism and attention deficit hyperactivity disorder. Psychiatr Genet. 2001;11(1):37–40. doi: 10.1097/00041444-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol Psychiatry. 2003;8(1):103–8. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- 42.Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, et al. A Common Haplotype of the Nicotine Acetylcholine Receptor alpha 4 Subunit Gene Is Associated with Vulnerability to Nicotine Addiction in Men. Am J Hum Genet. 2004;75(1):112–21. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 44.Tate JC, Schmitz JM. A proposed revision of the Fagerstrom Tolerance Questionnaire. Addict Behav. 1993;18(2):135–43. doi: 10.1016/0306-4603(93)90043-9. [DOI] [PubMed] [Google Scholar]
- 45.Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, et al. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14(9):1211–9. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- 46.Lueders KK, Hu S, McHugh L, Myakishev MV, Sirota LA, Hamer DH. Genetic and functional analysis of single nucleotide polymorphisms in the beta2-neuronal nicotinic acetylcholine receptor gene (CHRNB2) Nicotine Tob Res. 2002;4(1):115–25. doi: 10.1080/14622200110098419. [DOI] [PubMed] [Google Scholar]
- 47.Silverman MA, Neale MC, Sullivan PF, Harris-Kerr C, Wormley B, Sadek H, et al. Haplotypes of four novel single nucleotide polymorphisms in the nicotinic acetylcholine receptor beta2-subunit (CHRNB2) gene show no association with smoking initiation or nicotine dependence. Am J Med Genet. 2000;96(5):646–53. [PubMed] [Google Scholar]
- 48.Greenbaum L, Kanyas K, Karni O, Merbl Y, Olender T, Horowitz A, et al. Why do young women smoke? I. Direct and interactive effects of environment, psychological characteristics and nicotinic cholinergic receptor genes. Mol Psychiatry. 2006;11(3):312–22. 223. doi: 10.1038/sj.mp.4001774. [DOI] [PubMed] [Google Scholar]
- 49.Ehringer MA, Clegg HV, Collins AC, Corley RP, Crowley T, Hewitt JK, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 50.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeiger J, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum Mol Genet. 2008;17(5):724–34. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- 53.Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 Gene Cluster Variability as an Important Determinant of Early Alcohol and Tobacco Initiation in Young Adults. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27(9):482–91. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65(6):1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 57.Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol. 2007 doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3(3):203–23. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- 59.Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31(3):349–52. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 60.Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49(3):166–74. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- 61.Horti AG, Villemagne VL. The quest for Eldorado: development of radioligands for in vivo imaging of nicotinic acetylcholine receptors in human brain. Curr Pharm Des. 2006;12(30):3877–900. doi: 10.2174/138161206778559605. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287(2):648–57. [PubMed] [Google Scholar]
- 63.Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29(3):179–85. [PMC free article] [PubMed] [Google Scholar]
- 64.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71(6):1563–71. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 65.Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48(5):696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34(2–3):239–50. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67(6):2007–15. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- 68.Xu X, Scott MM, Deneris ES. Shared long-range regulatory elements coordinate expression of a gene cluster encoding nicotinic receptor heteromeric subtypes. Mol Cell Biol. 2006;26(15):5636–49. doi: 10.1128/MCB.00456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373(1–2):324–36. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 70.Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, et al. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76(1):258–68. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 71.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22(20):8785–9. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, et al. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat. 2003;25(2):97–113. doi: 10.1016/s0891-0618(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 73.Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87(1):146–57. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74(8):1235–46. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113(1–2):85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 76.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314(3):257–67. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 77.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358(3):189–96. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 78.Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5(1):53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Weiss S, Tzavara ET, Davis RJ, Nomikos GG, Michael McIntosh J, Giros B, et al. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007;52(7):1496–508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Inoue Y, Yao L, Hopf FW, Fan P, Jiang Z, Bonci A, et al. Nicotine and ethanol activate protein kinase A synergistically via G(i) betagamma subunits in nucleus accumbens/ventral tegmental cocultures: the role of dopamine D(1)/D(2) and adenosine A(2A) receptors. J Pharmacol Exp Ther. 2007;322(1):23–9. doi: 10.1124/jpet.107.120675. [DOI] [PubMed] [Google Scholar]
- 81.Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined Effects of Systemic Alcohol and Nicotine on Dopamine Release in the Nucleus Accumbens Shell. Alcohol Alcohol. 2007 doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- 82.Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26(3):394–9. [PubMed] [Google Scholar]
- 83.Crabbe JC, Belknap JK, Buck KJ, Metten P. Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Suppl. 1994;2:67–71. [PubMed] [Google Scholar]
- 84.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 85.Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26(6):1872–9. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal Regulation of High Affinity Nicotinic Receptors in Subjects with Schizophrenia. Neurospsychopharmacology. 2000;23(4):351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 87.Stassen HH, Bridler R, Hagele S, Hergersberg M, Mehmann B, Schinzel A, et al. Schizophrenia and smoking: evidence for a common neurobiological basis? Am J Med Genet. 2000;96(2):173–7. [PubMed] [Google Scholar]
- 88.Ziedonis DM, Kosten TR, Glazer WM, Frances RJ. Nicotine dependence and schizophrenia. Hosp Community Psychiatry. 1994;45(3):204–6. doi: 10.1176/ps.45.3.204. [DOI] [PubMed] [Google Scholar]
- 89.Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida K, Engel J, Liljequist S. The effect of chronic ethanol administration of high affinity 3H-nicotinic binding in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1982;321(1):74–6. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]
- 91.Booker TK, Collins AC. Long-term ethanol treatment elicits changes in nicotinic receptor binding in only a few brain regions. Alcohol. 1997;14(2):131–40. doi: 10.1016/s0741-8329(96)00116-4. [DOI] [PubMed] [Google Scholar]
- 92.Balogh SA, Owens JC, Butt CM, Wehner JM, Collins AC. Animal models as a tool for studying mechanisms of co-abuse of alcohol and tobacco. Alcohol Clin Exp Res. 2002;26(12):1911–4. doi: 10.1097/01.ALC.0000040847.98115.6D. [DOI] [PubMed] [Google Scholar]
- 93.Stitzel JA, Dobelis P, Jimenez M, Collins AC. Long sleep and short sleep mice differ in nicotine-stimulated 86Rb+ efflux and alpha4 nicotinic receptor subunit cDNA sequence. Pharmacogenetics. 2001;11(4):331–9. doi: 10.1097/00008571-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, et al. Alpha4beta2* nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27(12):1867–75. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- 95.Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29(3):295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- 96.Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003;23(15):6255–63. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24(45):10035–9. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Biasi M. Nicotinic receptor mutant mice in the study of autonomic function. Curr Drug Targets CNS Neurol Disord. 2002;1(4):331–6. doi: 10.2174/1568007023339148. [DOI] [PubMed] [Google Scholar]
- 99.Covernton PJ, Connolly JG. Differential modulation of rat neuronal nicotinic receptor subtypes by acute application of ethanol. Br J Pharmacol. 1997;122(8):1661–8. doi: 10.1038/sj.bjp.0701568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kedmi M, Beaudet AL, Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures. Physiol Genomics. 2004;17(2):221–9. doi: 10.1152/physiolgenomics.00202.2003. [DOI] [PubMed] [Google Scholar]
- 101.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 102.Burch JB, de Fiebre CM, Marks MJ, Collins AC. Chronic ethanol or nicotine treatment results in partial cross-tolerance between these agents. Psychopharmacology (Berl) 1988;95(4):452–8. doi: 10.1007/BF00172954. [DOI] [PubMed] [Google Scholar]
- 103.Collins AC, Burch JB, de Fiebre CM, Marks MJ. Tolerance to and cross tolerance between ethanol and nicotine. Pharmacol Biochem Behav. 1988;29(2):365–73. doi: 10.1016/0091-3057(88)90170-0. [DOI] [PubMed] [Google Scholar]
- 104.Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lof E, Ericson M, Stomberg R, Soderpalm B. Characterization of ethanol-induced dopamine elevation in the rat nucleus accumbens. Eur J Pharmacol. 2007;555(2–3):148–55. doi: 10.1016/j.ejphar.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 106.Moriguchi S, Zhao X, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on excitatory and inhibitory synaptic transmission in rat cortical neurons. Alcohol Clin Exp Res. 2007;31(1):89–99. doi: 10.1111/j.1530-0277.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 107.Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006;30(7):1223–33. doi: 10.1111/j.1530-0277.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 108.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13(9):1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 109.Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417(1–2):117–23. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- 110.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]