SUMMARY
Recent studies suggest that subtype specific activators of metabotropic glutamate receptors (mGluRs) have exciting potential for the development of novel treatment strategies for numerous psychiatric and neurological disorders. A number of positive allosteric modulators (PAMs) have been identified that are highly selective for mGluR1, including the compounds Ro 01-6128, Ro 67-4853, and Ro 67-7476. These PAMs have been previously found to interact with a site distinct from that of negative allosteric modulators (NAMs), typified by R214127. These mGluR1 PAMs do not have an effect on baseline calcium levels but induce leftward shifts in the concentration response of mGluR1 to agonists. However, their effects on a variety of signaling pathways and their mechanism of action have not been fully explored and are of critical importance for further development of mGluR1 allosteric modulators as novel drugs. In baby hamster kidney (BHK) cells, mGluR1 activates calcium mobilization, cAMP production, and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation; signaling cascades which are distinct and differentially regulated. In contrast to their effects on calcium mobilization, these compounds were found to activate ERK1/2 phosphorylation in the absence of exogenously added agonist, an effect that was fully blocked by both orthosteric (LY341495) and allosteric (R214127) mGluR1 antagonists. The mGluR1 PAMs were also found to activate cAMP production in the absence of agonist. Thus, these mGluR1 PAMs have qualitatively different effects on a variety of mGluR1-mediated signal transduction cascades. Together, these data provide further evidence that allosteric compounds can differentially modulate the coupling of a single receptor to independent signaling pathways or act in a system-dependent manner.
Keywords: mGluR1, allosteric potentiator, glutamate, ligand-induced differential signaling
INTRODUCTION
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS) and is responsible for the generation of fast excitatory synaptic responses at the vast majority of CNS synapses (Dingledine et al., 1999). Fast excitatory synaptic responses at glutamatergic synapses are mediated by activation of the ionotropic glutamate receptors (iGluRs), comprised of the α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate receptor subtypes. In addition, glutamate activates metabotropic glutamate receptors (mGluRs), which are G protein-coupled receptors (GPCRs) (Pin and Duvoisin, 1995). The mGluRs provide a mechanism by which glutamate can modulate activity at the same synapses at which it elicits fast synaptic responses via the iGluRs (Anwyl, 1999). Because of the ubiquitous distribution of glutamatergic synapses, mGluRs participate in a wide variety of functions of the CNS (Anwyl, 1999; Conn and Pin, 1997; Coutinho and Knopfel, 2002).
Eight receptor subtypes of mGluRs have been cloned, which are delineated based upon G protein-coupling specificity, pharmacology, and sequence homology (Nakanishi, 1994). Group I mGluRs (mGluR1 and mGluR5) are traditionally coupled to Gαq, leading to the stimulation of phospholipase Cβ. Group II mGluRs (mGluR2 and mGluR3) and Group III mGluRs (mGluR4, mGluR6, mGluR7, and mGluR8) are coupled to Gαi/o and inhibition of adenylate cyclase (Nakanishi, 1994). Due to the wide diversity, heterogeneous distribution, and diverse physiological roles of mGluR subtypes, the opportunity exists for developing therapeutic agents that selectively interact with mGluRs involved in only particular CNS functions. Large bodies of preclinical and recent clinical studies suggest that ligands for specific mGluR subtypes have potential for treatment of a range of CNS disorders, including depression (Palucha and Pilc, 2002), anxiety disorders (Chojnacka-Wojcik et al., 2001; Pilc, 2003), schizophrenia (Chavez-Noriega et al., 2002; Marino and Conn, 2002), chronic pain (Varney and Gereau, 2002), epilepsy (Doherty and Dingledine, 2002), Alzheimer’s disease (Wisniewski and Car, 2002), and Parkinson’s disease (Marino et al., 2002).
However, it has proven difficult to develop highly selective ligands to the glutamate-binding site (Conn and Pin, 1997), likely due to the high conservation of the orthosteric (glutamate) binding site across mGluR subtypes. Another approach that has been highly successful in a clinical setting is the use of selective positive allosteric modulators (PAMs) of specific receptor subtypes. The classic example of this is the use of benzodiazepines as PAMs of GABA-A receptors, which provide an effective and safe approach to the treatment of anxiety disorders without inducing the potentially lethal effects of direct-acting GABA receptor agonists (Mohler et al., 2002). We and others have expanded this concept to the mGluRs and developed highly selective PAMs of these receptors, including mGluR1 (Hemstapat et al., 2006; Johnson et al., 2003; Knoflach et al., 2001; Lindsley et al., 2004; Marino et al., 2003; Mathiesen et al., 2003; O'Brien et al., 2003). These small molecules do not activate the mGluRs directly but act at allosteric sites on the receptor to potentiate glutamate-induced receptor activation. These compounds provide an exciting advance in demonstrating the potential of this approach for developing novel therapeutic agents that increase activity of specific mGluR subtypes. However, little is known about the precise domains involved in the action of different classes of mGluR potentiators or the physiological impact of these compounds on mGluR signaling in native systems.
Orthosteric ligands have been previously demonstrated to differentially activate distinct signaling pathways of a single GPCR in a phenomenon most recently termed “ligand-induced differential signaling” (Berg et al., 1998; Brink et al., 2000; Gazi et al., 2003; Urban et al., 2007). Recently we found that allosteric potentiators of mGluR5 have differential effects on a variety of signaling pathways in cortical astrocytes (Zhang et al., 2005). These studies imply that allosteric potentiators can display features of ligand-induced differential signaling and imply that allosteric compounds could be developed which would potentially activate or inhibit specific signaling pathways. Differential effects of allosteric potentiators on coupling of mGluRs to diverse signaling pathways could have a tremendous impact on the in vivo actions and potential therapeutic utility of distinct classes of compounds. However, the effects of mGluR1 potentiators on coupling to different effector systems are not known. In the present study we have investigated the effects of three mGluR1 positive allosteric modulators on a number of mGluR1-induced signal transduction cascades in baby hamster kidney cells.
METHODS
Materials
R214127 was synthesized as described previously and [3H]R214127 (25 Ci/mmol) was custom labeled by American Radiolabeled Chemicals (St. Louis, MO) from the corresponding bromo analog (Mabire et al., 2005). Ro 67-4853, Ro 01-6128, and racemic Ro 67-7476 were synthesized as described previously (Knoflach et al., 2001). l-glutamate and LY341495 were obtained from Tocris Cookson Inc. (Ellisville, MO). Methotrexate was obtained from Calbiochem (San Diego, CA). Probenecid and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO). All tissue culture reagents were obtained from Invitrogen (Carlsbad, CA). Adenosine 3′,5′-cyclic monophosphate (cAMP), 3-Isobutyl-1-methylxanthine (IBMX), and Forskolin were obtained from Sigma-Aldrich (St. Louis, MO). [3H]-cAMP was obtained from Perkin-Elmer (Waltham, MA). The polyclonal rabbit anti-phospho-p42/p44 MAP Kinase (P-ERK1/2) antibody and the polyclonal rabbit anti-p42/p44 MAP Kinase (ERK1/2) antibody were obtained from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-conjugated goat anti-rabbit IgG were obtained from Vector Laboratories (Burlingame, CA). Odyssey Blocking Buffer was obtained from LI-COR Biosciences (Lincoln, NE). The anti-goat total ERK1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The donkey anti-rabbit IR800 antibody was obtained from Rockland (Gilbertsville, PA). The donkey anti-goat Alexafluor 680 antibody was obtained from Invitrogen (Carlsbad, CA).
Cell culture
Baby hamster kidney (BHK) cells stably expressing the rat mGlu1a receptor were generously provided by Dr. Betty Haldeman (Zymogenetics, Seattle, WA). Cells were grown in high glucose Dulbecco's modified Eagle's medium without l-glutamine (DMEM), supplemented with 5% heat-inactivated fetal bovine serum (FBS), 1 mM sodium pyruvate, 20 mM HEPES, 2 mM GlutaMAX I, antibiotic-antimycotic (100 units of penicillin, 100 µg of streptomycin, and 0.25 µg of amphotericin B), and 250 nM methotrexate. Cells were maintained at 37°C in an atmosphere of 95% air, 5% CO2.
Membrane Preparation and Radioligand Binding Studies
BHK-mGluR1a membranes were prepared as previously described (Hemstapat et al., 2006). Saturation binding studies with [3H]R214127 were performed as previously described (Lavreysen et al., 2003).
Calcium Fluorescence Measurement
BHK cells stably expressing rat mGluR1a were plated at 60,000 cells per well in standard growth media lacking methotrexate in clear bottom black wall plates 96 well plates (Corning) twenty-four hours prior to assay and were incubated overnight at 37°C in 5% CO2. On the day of the assay, media was removed from the cells and replaced with DMEM containing 5% heat-inactivated dialyzed fetal bovine serum (dFBS), 1 mM sodium pyruvate, and 20 mM HEPES. Cells were incubated in this media for 1 hour at 37°C in 5% CO2. Media was then removed and replaced with DMEM containing 20 mM HEPES, 2.5 mM Probenacid (Sigma), and 3 µM Fluo4-AM dye, pH 7.4 (Invitrogen). Cells were incubated for 45 minutes (37°C, 5% CO2) for dye loading. Fluo4-AM dye was removed and replaced with 160 µL Calcium Assay Buffer (Hanks Balanced Salt Solution (HBSS; Invitrogen), 20 mM HEPES, 2.5 mM Probenecid (Sigma), pH 7.4). For calcium fluorescence measurement of potentiator potency, vehicle or concentration response curves (CRCs) of potentiators made in Calcium Assay Buffer were added (40 µL, 5X) at the 20 second time point and either a glutamate CRC (for vehicle treated) or an EC20 of glutamate (for potentiator CRCs) (20 µL, 10X) was added at the 260 second time point via a Flexstation II (Molecular Devices). Fluorescence imaging continued for a total of 305 seconds acquisition time using an excitation of 488 nM, an emission of 525 nM, and a cutoff of 515 nM. Agonist, potentiator, and vehicle were added at a speed of 52 µL/s, and calcium flux was measured using the Flexstation II at 37°C. For fold shift assays, vehicle or fixed concentrations of potentiator were added manually (40 µL, 5X) so that the agonist CRC was added via a Flexstation II following a 10 minute incubation with potentiator. All of the peaks of the calcium response were normalized to the maximum response to a saturated dose of glutamate (ECMax). Kinetic data were transformed and fit with GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, CA) to a 4 parameter logistic equation to determine EC50 values.
ERK1/2 Phosphorylation Assay and Western Blotting
BHK cells stably expressing mGluR1a were plated in 6 well plates (Becton Dickinson and Company) at 600,000 cells/well in standard growth media lacking methotrexate twenty-four hours prior to assay and were incubated overnight at 37°C in 5% CO2. On the day of the assay, media was removed from the cells and replaced with 3 mL serum free DMEM containing 20 mM HEPES. Cells were incubated in serum free media for 3 hours at 37°C in 5% CO2. Media was removed and replaced with an additional 2 mL of serum free DMEM containing 20 mM HEPES, followed by an additional 30 minute incubation at 37°C in 5% CO2.
For studies involving the mGluR1 antagonists R214127 or LY341495, cells were first treated with either vehicle or antagonist (20 µL, 100X) for 10 minutes at 37°C in 5% CO2. For time course and concentration response studies, this step was omitted. Next, vehicle, agonist, or potentiators (20 µL, 100X) were added for the times indicated at room temperature. At the end of stimulation, medium containing the drug was aspirated, the cells were placed on ice, washed 2 times with ice-cold Tris-buffered saline (TBS) (50 mM Tris, pH 7.4, 150 mM NaCl), and incubated with 500 µL lysis buffer (50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 10 mM Na4P2O7, 1% v/v Triton X-100, 2 mM orthovanadate, 1X Complete™ EDTA-free protease inhibitor cocktail (Roche; Indianapolis, IN), 1X Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich), and 1X Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich), pH 7.4) for 15 minutes at 4°C. Following lysis, cells were harvested by scraping, transferred into microfuge tubes, and spun at 14,000 × g for 20 minutes at 4°C to clarify the lysates. Lysate protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard. Normalized protein lysates were prepared in 1x LDS sample buffer (Invitrogen). Samples were resolved on 12% SDS-polyacrylamide gels (Bio-Rad) and electroblotted onto nitrocellulose membranes (Bio-Rad).
For potentiator time course, R214127, and LY341495 ERK1/2 experiments, the membranes were blocked in 5% powdered milk and probed for phospho-ERK immunoreactivity using a 1:1000 dilution of polyclonal rabbit anti-phospho-p42/p44 MAP Kinase (P-ERK1/2) antibody (Cell Signaling Technology, Beverly, MA) and a 1:1500 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA). Total ERK immunoreactivity was probed for using a 1:1000 dilution of polyclonal rabbit anti-p42/p44 MAP Kinase (ERK1/2) antibody (Cell Signaling Technology, Beverly, MA). Immunoreactivity was revealed using LumiLight horseradish peroxidase substrate (Roche). Films were scanned and densitometric analysis was performed using Scion Image software (Scion Corporation, Frederick, MD). ERK1/2 phosphorylation was first normalized to total ERK1/2 and then expressed as percentage of maximal response or -fold above control. Student's t test was used to evaluate significance of differences between mean values for each study, and differences were considered significant for p < 0.05.
For potentiator concentration response experiments, membranes were first blocked in Odyssey Blocking Buffer (LI-COR Biosciences) and then probed for phospho-ERK1/2 and ERK1 total immunoreactivity using a mix of a 1:1000 dilution polyclonal rabbit anti-phosphop42/ p44 MAP Kinase (P-ERK1/2) antibody (Cell Signaling Technology, Beverly, MA) and a 1:1000 dilution of anti-goat total ERK1 (Santa Cruz Biotechnology) made up in Odyssey Blocking Buffer with 0.1% Tween 20. Blots were incubated in primary antibody overnight at 4°C. The following day, blots were washed 4 times for 5 minutes each in TBS with 0.1% Tween 20 (TBST). Primary antibodies were detected with a mix of a 1:10000 dilution of donkey antirabbit IR800 (Rockland) and a 1:10000 dilution of donkey anti-goat Alexafluor 680 (Invitrogen), made up in a 1:1 mix of Odyssey buffer and TBST for 30 min at room temperature. Membranes were scanned using Odyssey Imaging System (LI-COR, Lincoln, NE). ERK1/2 phosphorylation was first normalized to total ERK1 and then expressed as percentage of maximal response or - fold above control. Student's t test was used to evaluate significance of differences between mean values for each study, and differences were considered significant for p < 0.05.
Adenylate Cyclase Assays
Adenylate cyclase activation was measured using a protocol modified from Watts and Neve (1996) (Watts and Neve, 1996), which was originally adapted from Nordstedt and Fredholm (1990) (Nordstedt and Fredholm, 1990). BHK cells stably expressing rat mGluR1a were plated at 60,000 cells per well in standard growth media lacking methotrexate in clear bottom black wall plates 96 well plates (Corning) twenty-four hours prior to assay and were incubated overnight at 37°C in 5% CO2. On the day of the assay, media was removed from the cells and replaced with 200 µL of serum free DMEM containing 20 mM HEPES. Cells were incubated in serum free media for 2 hours at 37°C in 5% CO2. The media was removed and replaced with 200 µL of serum free DMEM containing 20 mM HEPES. Cells were incubated an additional 30 minutes at 37°C in 5% CO2, then washed with 80 µL 37°C stimulation buffer (DMEM, 15 mM HEPES, pH 7.4, 0.025% ascorbic acid) for 10 minutes at room temperature. Following this incubation, the media was removed and the cells were placed on ice. To assess mGluR1 receptor stimulation, 40 µL of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (2X, 500 µM final concentration), prepared in stimulation buffer, was first added to all wells on ice to prevent cAMP breakdown. Following IBMX addition, cells were treated with vehicle or varying concentrations of potentiator (20 µL, 4X) on ice. Finally, vehicle, 10 µM forskolin (as a positive control), or various concentrations of glutamate (20 µL, 4X) were added on ice followed by a 20 minute incubation at 37°C in a water bath. The reaction was terminated by aspiration and the addition of 35 µL ice-cold 3% trichloroacetic acid (TCA). Cell lysates were chilled for at least 2 hours at 4°C.
cAMP was quantified using a competitive binding assay adapted with minor modifications (Nordstedt and Fredholm, 1990). Briefly, TCA extracts (15 µL) were added to wells in a deep well 96 well plate (Axygen Scientific). Following TCA extract addition, [3H]-cAMP (Perkin-Elmer,) (1 nM final concentration), prepared in cAMP assay buffer (100 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA) was added to each tube, followed by cAMP-binding proteins (approximately 100 µg of crude extract from bovine adrenal cortex in 500 µL of cAMP assay buffer; prepared as described below). The reaction tubes were incubated on ice for 2 hours then harvested with a Brandel cell harvester onto Whatman GF/B filters. Filters were allowed to dry and radioactivity bound quantified by liquid scintillation counting. The concentration of cAMP in each sample was estimated from a standard curve ranging from 0.1 to 100 picomoles of cAMP.
To prepare cAMP-binding protein, five bovine adrenal cortices (Pel-Freez Biologicals; Rogers, AR) were partially thawed on ice, chopped into small pieces and homogenized in 125 mL of PKA purification buffer (100 mM Tris-HCl pH 7.4, 10 mM EDTA, 250 mM NaCl, 250 mM sucrose, and 0.1% 2-mercaptoethanol). The homogenate was filtered several times through cheesecloth to remove fatty tissue and stirred for 30 minutes in an ice-water bath. This suspension was spun three times at 30,000 × g at 4°C for 30 minutes, filtering the supernatant through cheesecloth between each spin. The final supernatant was brought up to 100 mL in PKA purification buffer, split into 1 mL aliquots and stored at −80°C for up to 2 years. Optimal dilution of the cAMP-binding protein was determined by testing several dilutions with standard curves of unlabeled cAMP.
RESULTS
Ro 67-4853, Ro 01-6128, and Ro 67-7476 are positive allosteric modulators of mGluR1 signaling as measured by Ca2+ mobilization
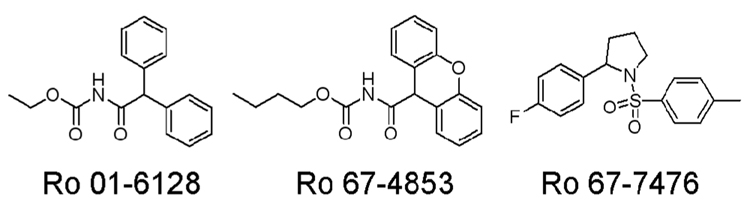
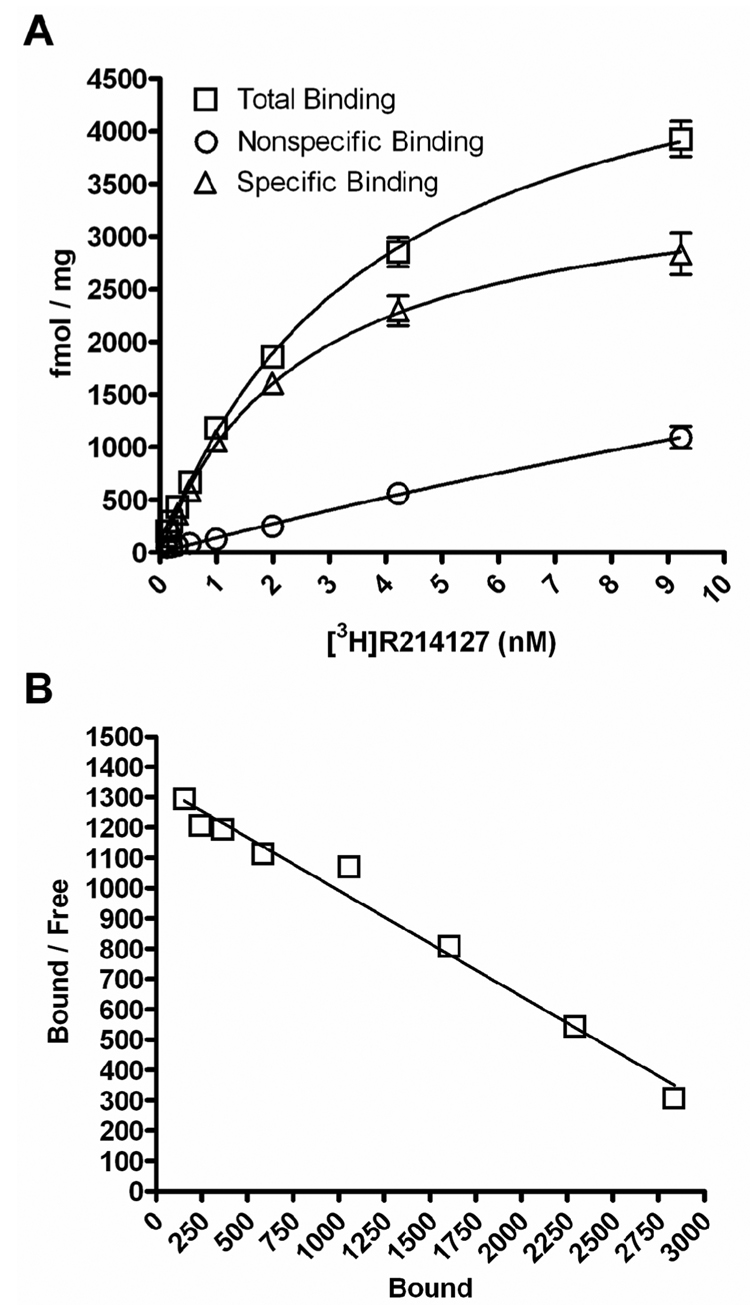
The first allosteric potentiators of mGluR1 discovered include (S)-2-(4-fluorophenyl)-1-(toluene-4-sulfonyl)pyrrolidine (Ro 67-7476), ethyl diphenylacetylcarbamate (Ro 01-6128), and butyl (9H-xanthene-9-carbonyl)carbamate (Ro 67-4853) (Knoflach et al., 2001; Vieira et al., 2005), whose chemical structures are shown in Figure 1. These compounds were identified by their ability to potentiate the signaling of an EC20 concentration of glutamate to Ca2+ mobilization and to have no effect on Ca2+ signaling in the absence of exogenously added glutamate (Knoflach et al., 2001). As we wanted to evaluate the effects of the mGluR1 PAMs in a variety of signal transduction pathways in the Baby Hamster Kidney (BHK) cells stably expressing mGluR1a, we first performed saturation binding studies to determine the expression level of mGluR1 in the BHK cells. Saturation binding studies were performed using a potent noncompetitive antagonist of mGluR1, [3H]R214127 (1-(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-2-phenyl-1-ethanone) (Lavreysen et al., 2003) (Figure 2). Nonlinear regression analysis of the saturation curve revealed an apparent Kd of 2.8 ± 0.3 nM and a Bmax of 3817 ± 226 fmol/mg of protein (Figure 2A). Scatchard analysis of these data indicated that [3H]R214127 bound to a single binding site (Figure 2B). These data are consistent with the high level of mGluR1 expression found in the rat cerebellum (4302 fmol/mg) (Lavreysen et al., 2003).
Figure 1. Chemical structures of mGluR1 positive allosteric modulators.
Figure 2. Saturation Binding of [3H]R214127 to BHK-mGluR1a membranes.
(A) Representative saturation binding curve for [3H]R214127 binding to membranes prepared from BHK-mGluR1 cells. Specific binding was determined by subtracting nonspecific binding measured in the presence of 10 µM R214127 from total binding. (B) Representative Scatchard plot of [3H]R214127 binding data. For each experiment, data points were determined in quadruplicate or greater. Experiments were replicated three independent times.
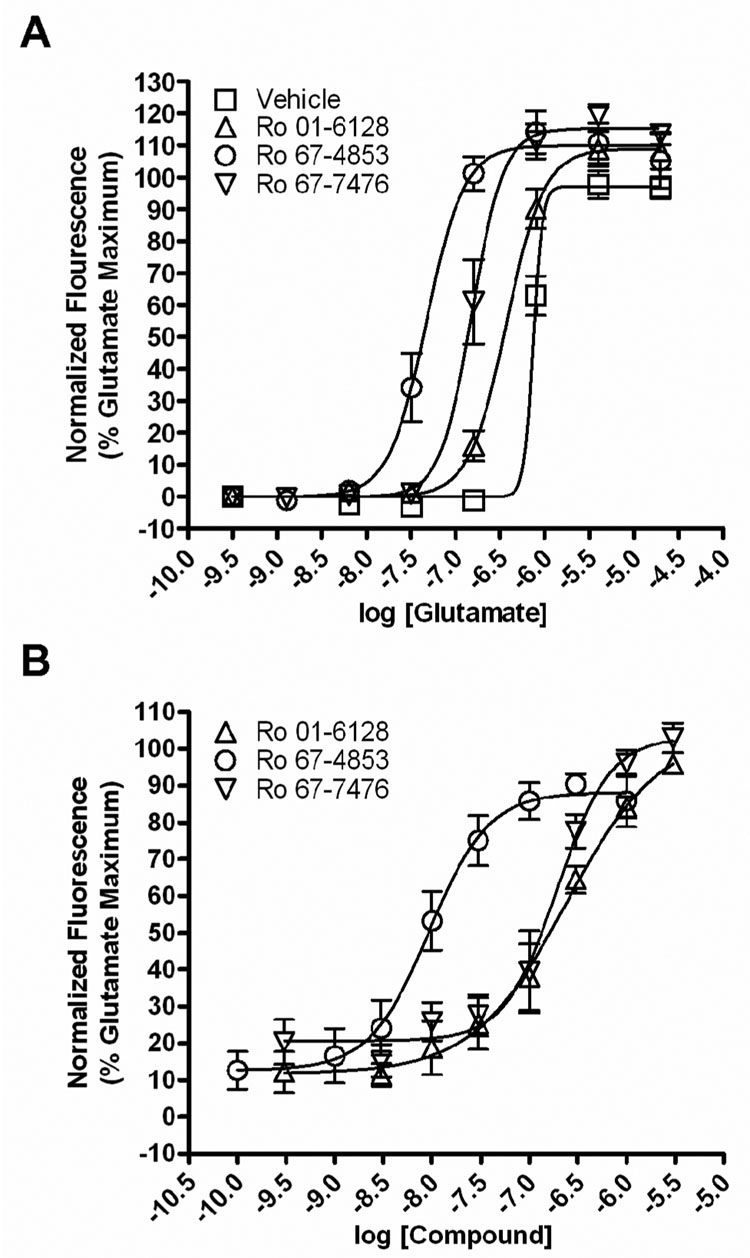
We next wanted to verify the behavior of these compounds in a calcium assay. As had been previously reported (Hemstapat et al., 2006; Knoflach et al., 2001), we found that fixed concentrations (1 µM) of Ro 01-6128, Ro 67-4853, or Ro 67-7476 shifted the concentration-response curve (CRC) of glutamate approximately 2-fold, 15-fold, and 4.5-fold to the left respectively (Figure 3A) in BHK cells stably expressing mGluR1a. These compounds also produced a concentration-dependent potentiation of the response of mGluR1a to an EC20 concentration of glutamate (250 – 450 nM) (Figure 3B). The EC50 values of potentiation were 223.8 ± 32.6 nM (Ro 01-6128), 10.0 ± 2.4 nM (Ro 67-4853), or 185.8 ± 45.6 nM (Ro 67-7476). No effect of the compounds alone was apparent, as was previously demonstrated (Knoflach et al., 2001). Together, these data support previous studies demonstrating the effect of these compounds on mGluR1 signaling, notably, a lack of an effect of these compounds on Ca2+ signaling in the absence of agonist accompanied by a potentiation of the response to mGluR1 agonists.
Figure 3. Ro 01-6128, Ro 67-4853, and Ro 67-7476 are positive allosteric modulators of mGluR1 as measured by calcium mobilization.
(A) BHK cells stably expressing mGluR1a were pre-incubated with a fixed concentration (1 µM) of each compound for 10 min before the addition of a range of concentrations of glutamate. These compounds have no agonist activity on mGluR1 when added alone but shift the concentration-response curve of glutamate approximately 2-fold (Ro 01-6128), 15-fold (Ro 67-4853), and 4.5-fold (Ro 67-7476) to the left. The fluorescence response was normalized as a percentage of the maximal response to 10 µM glutamate and is presented as the mean of four individual experiments performed in triplicate. Error bars are S.E.M. (B) Concentration-response curves of Ro 01-6128, Ro 67-4853, and Ro 67-7476 in the presence of an EC20 concentration of glutamate in BHK cells stably expressing mGluR1a. These compounds potentiated threshold responses to glutamate in the calcium mobilization assay, with EC50 values of 223.8 ± 32.6 nM (Ro 01-6128), 10.0 ± 2.4 nM (Ro 67-4853), or 185.8 ± 45.6 nM (Ro 67-7476). The fluorescence response was normalized as a percentage of the maximal response to 10 µM glutamate and is presented as the mean of three individual experiments performed in triplicate. Error bars are S.E.M.
Ro 01-6128, Ro 67-4853, and Ro 67-7476 are direct agonists of mGluR1 signaling as measured by the phosphorylation of ERK1/2
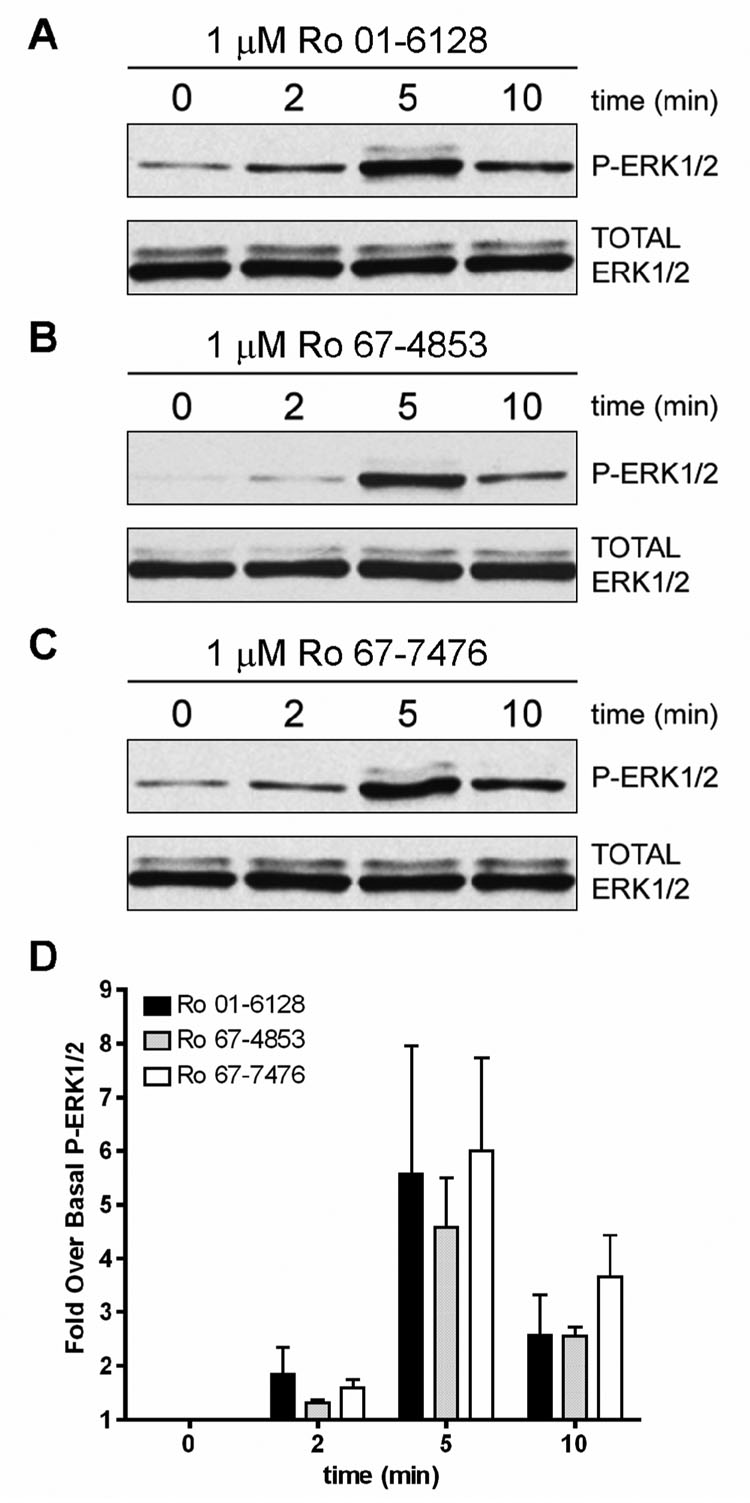
While agonist-induced Ca2+ mobilization is a commonly used measure of mGluR1 activation, mGluR1 can couple to a variety of signaling pathways, including activation of ERK1/2 phosphorylation and activation of adenylate cyclase (AC) (Aramori and Nakanishi, 1992; Conn and Pin, 1997; Ferraguti et al., 1999; Joly et al., 1995). If allosteric potentiators of mGluR1 have differential effects on these signaling pathways, this could dramatically impact their overall physiological effects or impact systems in which mGluR1 activators have been postulated to have potential therapeutic utility. We began by characterizing the effects of these mGluR1 PAMs on mGluR1-induced phosphorylation of ERK1/2. Initial studies (data not shown) demonstrated that 1 µM of the mGluR1 PAMs resulted in the phosphorylation of ERK1/2 in the absence of exogenously added agonist, with 1 µM of the PAMs demonstrating 119.5 ± 17.3% (Ro 01-6128), 114.4 ± 29.0% (Ro 67-4853), or 120.4 ± 1.9% (Ro 67-7476) of the response to a maximal concentration of glutamate. Thus, each of the PAMs acts as a full agonist of mGluR1, with a slightly higher efficacy than glutamate. Further studies, as shown in Figure 4, demonstrated that 1 µM of the PAMs activate P-ERK1/2 in the absence of agonist with a time course of activation peaking at 5 minutes.
Figure 4. Ro 01-6128, Ro 67-4853, and Ro 67-7476 are agonists of mGluR1 as measured by phosphorylation of ERK1/2.
(A–C) Shown are representative immunoblots from a single experiment repeated three times with equivalent results. BHK cells stably expressing mGluR1a were treated with 1 µM of either Ro 01-6128 (A), Ro 67-4853 (B), or Ro 67-7476 (C) for 0, 2, 5, or 10 minutes. The top immunoblot shows phosphorylated ERK1/2 levels and the bottom blot shows total ERK1/2 levels. (D) Quantification of the net pixel intensities of phosphorylated ERK1/2 normalized to total ERK1/2, expressed as the fold over basal ERK1/2 phosphorylation.
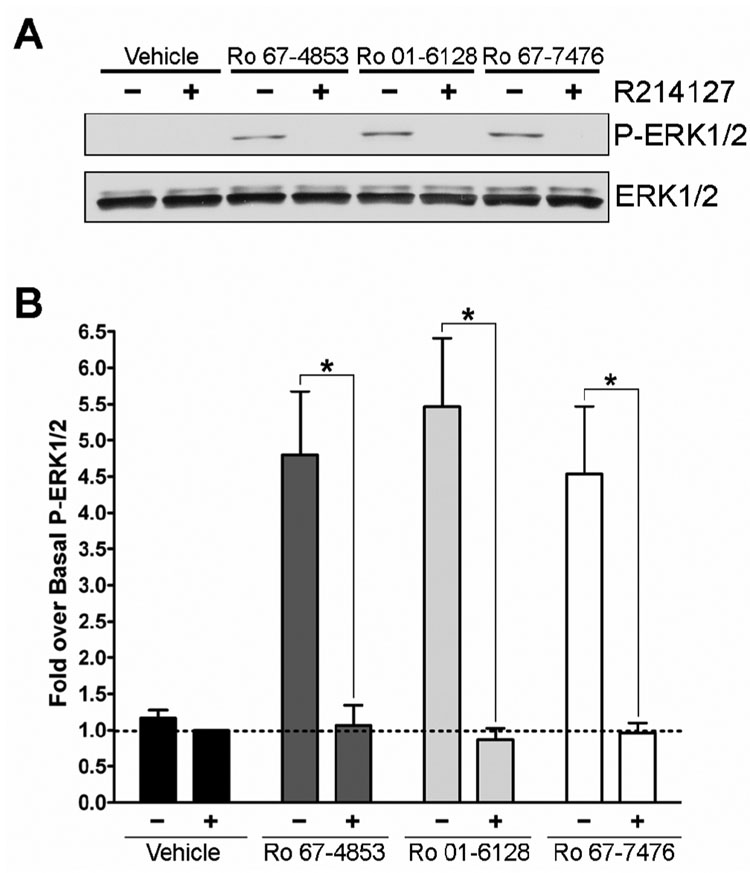
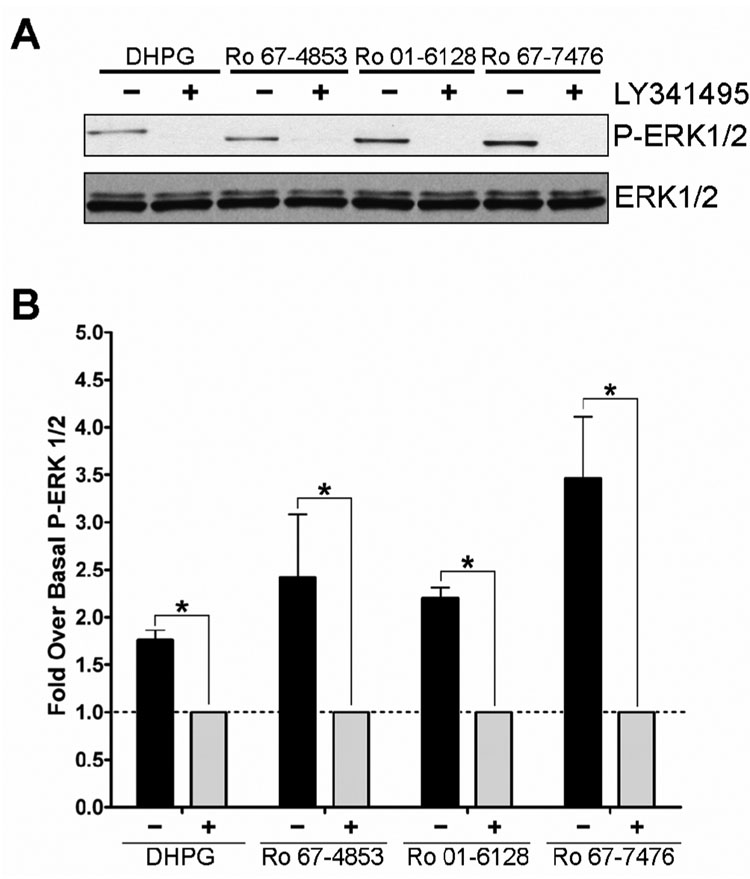
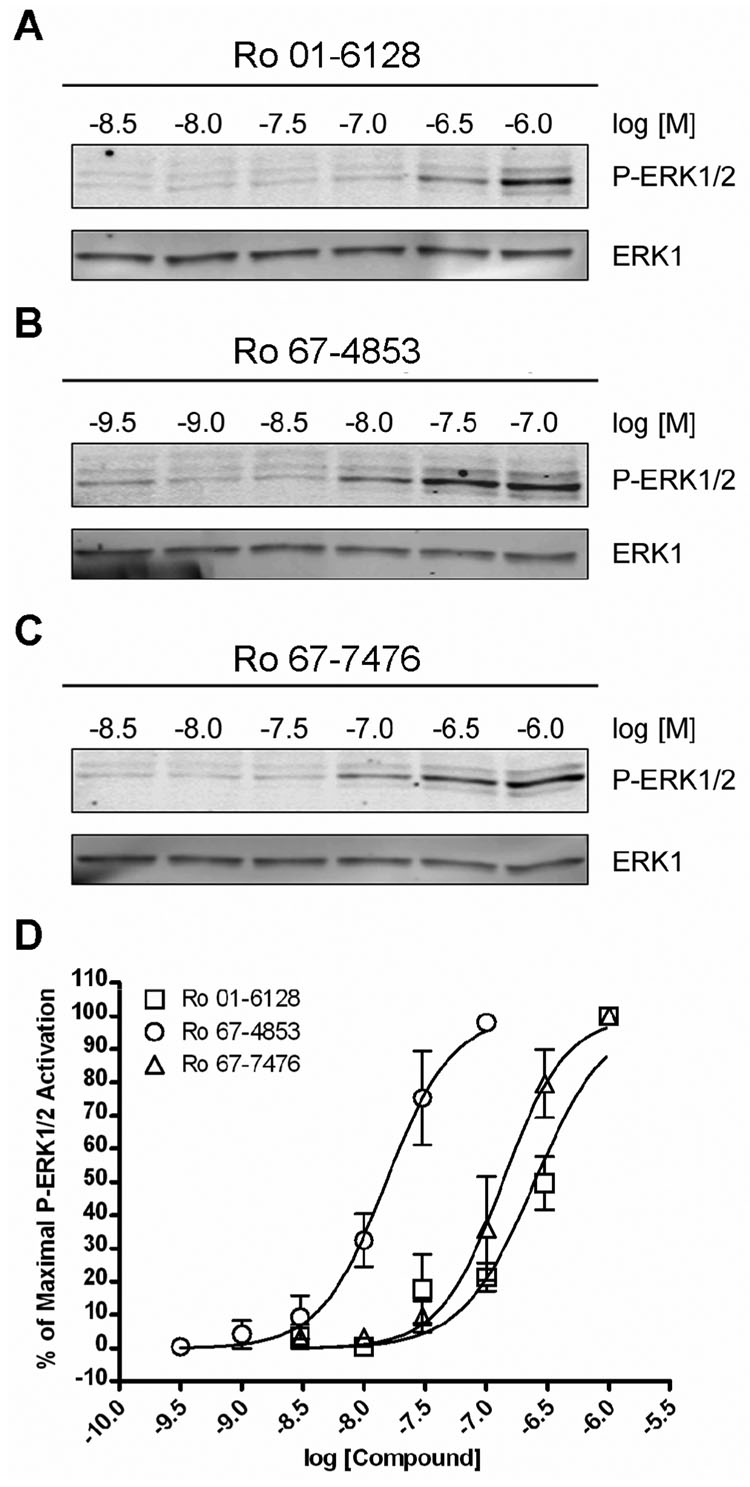
To verify that the activation of P-ERK1/2 was occurring via activation of mGluR1, studies were performed using the noncompetitive antagonist of mGluR1, R214127 (Lavreysen et al., 2003). Figure 5 shows the effect of 100 nM R214127 on 1 µM Ro 01-6128, Ro 67-4853, or Ro 67-7476 induced P-ERK1/2. R214127 completely inhibits the activity of these compounds but does not effect basal ERK1/2 phosphorylation, demonstrating that noncompetitive antagonists of mGluR1 can block the ability of the mGluR1 PAMs to cause P-ERK1/2 induction. To further verify that the PAM activation of ERK1/2 occurs via mGluR1, we evaluated a competitive orthosteric antagonist of mGluR1, LY341495 (Kingston et al., 1998), to determine if orthorsteric as well as allosteric antagonists of mGluR1 can block this PAM effect. Figure 6 demonstrates that 10 µM LY341495 also completely inhibits the ability of Ro 01-6128, Ro 67-4853, and Ro 67-7476 to activate P-ERK1/2. As we found for glutamate, the efficacy of PAM activation of ERK1/2 phosphorylation was greater than that of DHPG. In addition, as we found with R214127, LY341495 treatment did not lower the basal ERK1/2 phosphorylation (data not shown). Having demonstrated that the ability of these PAMs to activate P-ERK1/2 is not due to an off-target effect and is occurring via mGluR1, we then determined the concentrationdependence of P-ERK1/2 activation (Figure 7). The EC50 values P-ERK1/2 activation for the mGluR1 PAMs were all in the low to mid nanomolar range with a values of 247.9 ± 50.6 nM (Ro 01-6128), 9.2 ± 6.2 nM (Ro 67-4853), or 163.3 ± 44.8 nM (Ro 67-7476). Notably, the EC50s for full P-ERK1/2 activation are nearly identical to the EC50s for calcium mobilization potentiation. Together, these data demonstrate that Ro 01-6128, Ro 67-4853, and Ro 67-7476 activate mGluR1-induced ERK1/2 phosphorylation in the absence of exogenously added agonist, showing that these PAMs of mGluR1 have fundamentally different effects on mGluR1 coupling to calcium mobilization versus ERK1/2 phosphorylation.
Figure 5. Ro 67-4853, Ro 01-6128, and Ro 67-7476 activation of ERK1/2 phosphorylation is inhibited by the mGluR1 allosteric antagonist R214127.
(A) Shown are representative immunoblots from a single experiment repeated three times with equivalent results. BHK cells stably expressing mGluR1a were treated with vehicle (−) or 100 nM R214127 (+) for 10 minutes prior to potentiator addition. Following antagonist treatment, samples were treated with vehicle or 1 µM potentiator for 5 minutes. The top immunoblot shows phosphorylated ERK1/2 levels and the bottom blot shows total ERK1/2 levels. (B) Quantification of the net pixel intensities of phosphorylated ERK1/2 normalized to total ERK1/2, expressed as the fold over basal ERK1/2 phosphorylation. * = Statistically significant p<0.05
Figure 6. Ro 67-4853, Ro 01-6128, and Ro 67-7476 activation of ERK1/2 phosphorylation is inhibited by the mGluR1 orthosteric antagonist LY341495.
(A) Shown are representative immunoblots from a single experiment repeated three times with equivalent results. BHK cells stably expressing mGluR1a were treated with vehicle (−) or 10 µM LY341495 (+) for 10 minutes prior to agonist or potentiator addition. Following antagonist treatment, samples were treated with 10 M DHPG or 1 µM potentiator for 5 minutes. The top immunoblot shows phosphorylated ERK1/2 levels and the bottom blot shows total ERK1/2 levels. (B) Quantification of the net pixel intensities of phosphorylated ERK1/2 normalized to total ERK1/2, expressed as the fold over basal ERK1/2 phosphorylation. * = Statistically significant p<0.05
Figure 7. Ro 01-6128, Ro 67-4853, and Ro 67-7476 are potent agonists of P-ERK1/2.
(A–C) Shown are representative immunoblots from a single experiment repeated four to five times with equivalent results. BHK cells stably expressing mGluR1a were treated with a concentration response of either Ro 01-6128 (A), Ro 67-4853 (B), or Ro 67-7476 (C) for 5 minutes. The top immunoblot shows phosphorylated ERK1/2 levels and the bottom blot shows total ERK1 levels. (D) Quantification of the net pixel intensities of phosphorylated ERK1/2 normalized to total ERK1, expressed as the % of maximal P-ERK1/2 phosphorylation for each compound. The calculated EC50s from these data are 247.9 ± 50.6 nM (Ro 01-6128), 9.2 ± 6.2 nM (Ro 67-4853), or 163.3 ± 44.8 nM (Ro 67-7476). Error bars are S.E.M.
Ro 01-6128, Ro 67-4853, and Ro 67-7476 increase basal mGluR1 signaling and potentiate mGluR1 signaling as measured by the activation of adenylate cyclase
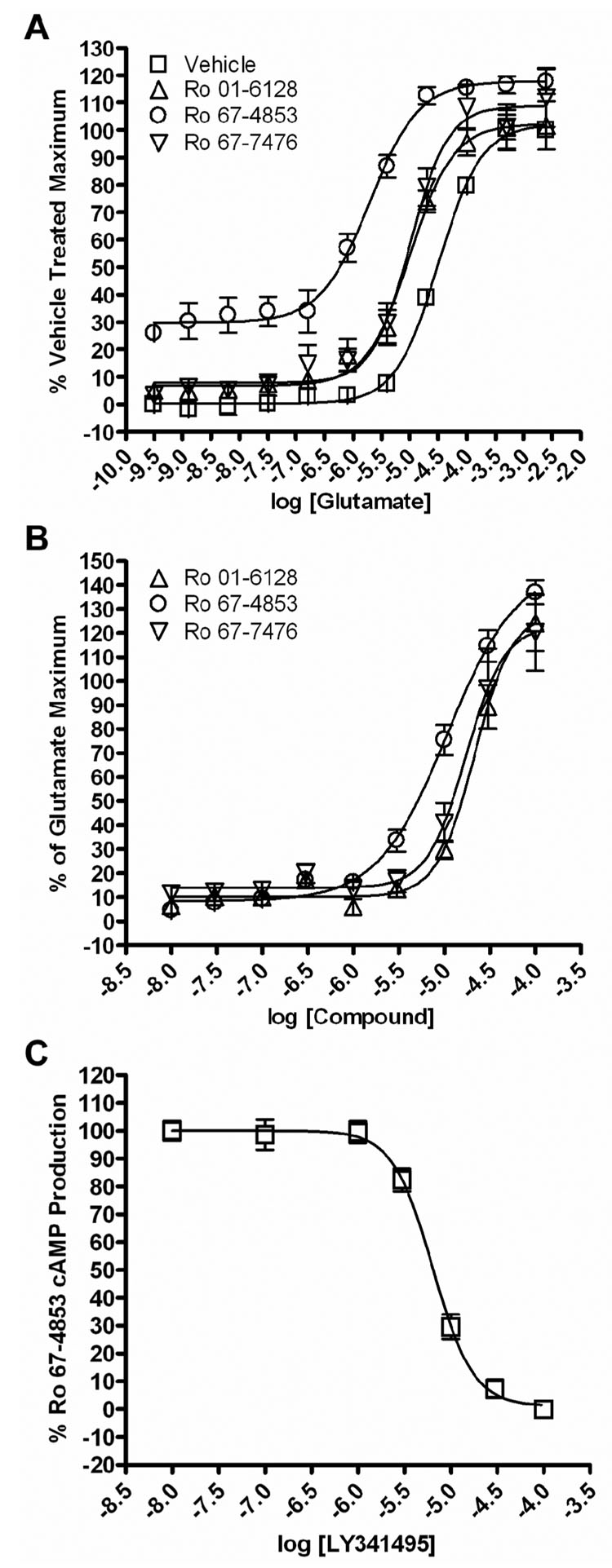
As mGluR1 can couple to Gαs and to the activation of AC in a number of systems (Aramori and Nakanishi, 1992; Francesconi and Duvoisin, 1998; Joly et al., 1995; Tateyama and Kubo, 2006), we also performed studies to investigate the effects of Ro 01-6128, Ro 67-4853, and Ro 67-7476 on mGluR1 signaling to the stimulation of cAMP production. Initial studies demonstrated that 500 nM Ro 01-6128, Ro 67-4853, or Ro 67-7476 potentiate glutamate-induced activation of mGluR1 as assessed by measures of cAMP production by 3-, 15-, and 3-fold respectively. Glutamate increased cAMP accumulation with an EC50 value of 32.08 ± 0.96 µM in the absence of an mGluR1 PAM (Vehicle). The EC50 values for glutamate in the presence of PAMs were 10.50 ± 2.29 µM (Ro 01-6128), 2.15 ± 0.43 µM (Ro 67-4853), or 10.93 ± 2.81 µM (Ro 67-7476) (Figure 8A). These studies also revealed that these PAMs increase basal cAMP production at the fixed concentration used (500 nM); this was especially evident for Ro 67-4853 which increased cAMP accumulation by 30% in the absence of glutamate. Effects of the other two mGluR PAMs on basal cAMP accumulation were minimal, with Ro 01-6128 potentiating basal cAMP by approximately 7%, and Ro 67-7476 by 8%. These studies suggest that these classes of mGluR1 allosteric potentiators, and especially Ro 67-4853, may have partial agonist activity at mGluR1 as measured by cAMP production. We also determined the effects of increasing concentrations of each mGluR1 PAM on responses of mGluR1a to an EC20 concentration of glutamate (10 µM) (Figure 8B). The EC50 values of the PAMs for potentiation of glutamate responses were 21.5 ± 1.8 µM (Ro 01-6128), 11.7 ± 2.4 µM (µM, Ro 67-4853), or 17.7 ± 0.2 (Ro 67-7476). Finally, we determined the effect of increasing concentrations of the orthosteric antagonist LY341495 on the cAMP production induced by 500 nM Ro 67-4853. LY341495 inhibited the cAMP production by 500 nM Ro 67-4853 with an IC50 of 5.95 ± 0.91 µM (Figure 8C). The allosteric mGluR1 antagonist (R214127) induced a similar inhibition of the effect of Ro 67-4853 on cAMP production (data not shown), These data demonstrate that Ro 01-6128, Ro 67-4853, and Ro 67-7476 increase basal mGluR1-induced cAMP accumulation and potentiate glutamate-induced cAMP accumulation but have lower potencies at modulating the cAMP response than at regulating ERK1/2 phosphorylation or calcium mobilization. In addition, these data provide further evidence that mGluR1 PAMs have either differential effects on the coupling of mGluR1 to different signaling pathways or system-dependent properties.
Figure 8. Ro 01-6128, Ro 67-4853, and Ro 67-7476 increase basal cAMP production and potentiate mGluR1 signaling to adenylate cyclase activation.
(A) BHK cells stably expressing mGluR1a were incubated with a fixed concentration (500 nM) of each compound for 1 min 25 before the addition of a range of concentrations of glutamate. These compounds shift the concentration-response curve of glutamate approximately 3-fold (Ro 01-6128), 16-fold (Ro 67-4853), and 3-fold (Ro 67-7476) to the left. The basal cAMP production at the fixed concentration used (500 nM) was increased, with Ro 01-6128 potentiating basal cAMP by approximately 7%, Ro 67-4853 by 30%, and Ro 67-7476 by 8%. The cAMP response was normalized as a percentage of the vehicle treated glutamate response and is presented as the mean of four individual experiments performed in triplicate. Error bars are S.E.M. (B) Concentration-response curves of Ro 01-6128, Ro 67-4853, and Ro 67-7476 in the presence of an EC20 concentration of glutamate in BHK cells stably expressing mGluR1a. These compounds potentiated threshold responses to glutamate in the cAMP accumulation assay, with EC50 values of 21.5 ± 1.8 µM (Ro 01-6128), 11.7 ± 2.4 µM (Ro 67-4853), or 17.7 ± 1.2 µM (Ro 67-7476). The cAMP response was normalized as a percentage of the maximum response to 3 mM glutamate response and is presented as the mean of four individual experiments performed in triplicate. Error bars are S.E.M. (C) BHK cells stably expressing mGluR1a were incubated with a fixed concentration (500 nM) of Ro 67-4853 for 1 min before the addition of a range of concentrations of LY341495. LY341495 inhibited the cAMP production induced by 500 nM Ro 67-4853 with an IC50 value of 5.95 ± 0.91 µM. The cAMP response was normalized as a percentage of the 500 nM Ro 67-4853 treated response and is presented as the mean of four individual experiments performed in triplicate. Error bars are S.E.M.
DISCUSSION
In recent years, our understanding of the basic mechanisms of GPCR function has been undergoing a radical change. It has become clear that a single GPCR can display a multitude of active states that can link to independent signaling pathways and that the regulation of GPCR signal transduction largely depends on the both the cellular milieu and the proteins which interact with the GPCR signaling complex. There are now numerous examples whereby the signal transduction and regulation of GPCRs are largely controlled by the particular proteins that interact with the receptor (Urban et al., 2007; Violin and Lefkowitz, 2007). Ligands stabilize different receptor states, altering particular protein interactions, thereby altering how a given receptor will respond to a drug. Independent signaling pathways for a particular receptor can also be differentially regulated by ligands, further complicating this picture. Numerous terms have been applied to this concept including “agonist-directed trafficking of receptor stimulus”, “biased agonism”, and “functional selectivity”, among others. Recently, it has been suggested that this concept be termed “ligand-induced differential signaling” (Urban et al., 2007). There are now a number of examples of ligand-induced differential signaling in the literature. These studies demonstrate differences in orthosteric ligand signal transduction and include studies with serotonin 2A and 2C receptors (Berg et al., 1998), β2 adrenergic receptors (Seifert et al., 1999), µ opioid receptors (Whistler et al., 1999), angiotensin II receptors (Hunyady et al., 1994), chemokine receptors (Blanpain et al., 2002), and complement factor 5a receptor, among many others. Although the aforementioned studies focus on traditional orthosteric ligands, it is equally likely that allosteric ligands would also display features of ligand-induced differential signaling. Indeed, recent studies suggest that distinct structural classes of allosteric potentiators of mGluR5 differ in their effects on coupling to multiple signaling pathways (Zhang et al., 2005).
The group I mGluR, mGluR1, is known to couple to multiple signaling pathways and its signal transduction to multiple second messenger systems can be differentially regulated (Aramori and Nakanishi, 1992; Conn and Pin, 1997; Ferraguti et al., 1999; Francesconi and Duvoisin, 2000; Joly et al., 1995). In addition, mGluR1 is expressed in a wide variety of neuronal populations and has physiological effects that vary depending on the neuronal population (Valenti et al., 2002). It is likely that varying physiological actions of mGluR1 in distinct neuronal populations depend on differential activation of signaling cascades. If allosteric potentiators of mGluR1 preferentially potentiate specific responses to receptor activation, this would have major implications when considering development of such compounds for therapeutic purposes. Thus, it will be critical to determine whether allosteric potentiators of mGluR1 have similar or different effects on the coupling of mGluR1 to multiple second messenger systems.
We now report the effects of Ro 01-6128, Ro 67-4853, and Ro 67-7476, three positive allosteric modulators of mGluR1, on mGluR1-induced calcium mobilization, ERK1/2 phosphorylation, and cAMP production in baby hamster kidney cells stably expressing mGluR1a. These three compounds behave similarly when measuring glutamate-induced calcium mobilization, inducing parallel leftward shifts of the agonist concentration-response relationships and displaying a rank order of potencies for potentiation of Ro 67-4853 > Ro 67-7476 ≥ Ro 01-6128. As has been previously reported, these modulators have no effect on basal calcium mobilization (Hemstapat et al., 2006; Knoflach et al., 2001).
Interestingly, all three allosteric potentiators acted as full agonists in the absence of exogenously added agonist in an ERK1/2 phosphorylation assay. The time course of ERK1/2 phosphorylation by the potentiators peaked at 5 minutes, a time point that is consistent with mGluR1 activation of ERK1/2 phosphorylation when activated by glutamate (Thandi et al., 2002). The maximal ERK1/2 phosphorylation induced by the PAMs was greater than that induced by either maximal concentrations of glutamate or DHPG, implying that these PAMs may couple to the ERK pathway better than orthosteric agonists, although further experimentation will be required to fully evaluate this possibility. We also found that the effects of the PAMs on ERK1/2 phosphorylation was fully inhibited by both orthosteric (LY341495) and allosteric (R214127) antagonists of mGluR1, demonstrating that the effect of these PAMs on ERK1/2 phosphorylation was not due to an off-target effect in BHK cells. In addition, the lack of an effect of orthosteric and allosteric antagonists on basal ERK1/2 phosphorylation demonstrates that it is unlikely that endogenous glutamate is contributing to the efficacy of the PAMs.
The rank order potencies for PAM activation of ERK1/2 phosphorylation were Ro 67-4853 > Ro 67-7476 ≥ Ro 01-6128. Notably, not only are the rank order potencies similar to that found for calcium mobilization potentiation, but the EC50 values for activation of ERK1/2 phosphorylation are nearly identical to the EC50 values for potentiation of calcium mobilization, implying that these differential effects on signal transduction cascades occur in nearly identical concentration ranges. Importantly, during the calcium mobilization assays, the compounds were incubated with the cells for 4 minutes prior to agonist addition without effect whereas there is already significant ERK1/2 phosphorylation occurring at the 2 minute time point as shown in Figure 4. If endogenous glutamate was contributing to the efficacy of these compounds, one would also expect to see agonist effects of the PAMs in a calcium assay, which we do not. In addition, neither orthosteric nor allosteric antagonists decreased basal ERK1/2 phosphorylation, consistent with a lack of endogenous glutamate. However, even given these controls, we cannot fully rule out the presence of endogenous glutamate in our assays. Regardless, the mGluR1 PAMs serve as robust agonists of mGluR1 coupling to ERK1/2 phosphorylation while acting as pure allosteric potentiators of coupling of the receptor to activation of calcium mobilization. These data are consistent with both ligand-induced differential signaling of the mGluR1 PAMs or the PAMs acting as agonists in a system-dependent manner.
Although a clear role of mGluR1 in the stimulation of cAMP has not been conclusively shown in vivo, in a number of in vitro systems, mGluR1 couples to Gαs, leading to the activation of AC and the production of cAMP (Aramori and Nakanishi, 1992; Francesconi and Duvoisin, 1998; Joly et al., 1995; Tateyama and Kubo, 2006). We found that mGluR1 activated cAMP production in BHK cells with an EC50 of 32 µM and that the PAMs induced parallel leftward shifts of the agonist concentration-responses, displaying a rank order potency for potentiation of Ro 67-4853 > Ro 67-7476 = Ro 01-6128. In addition, Ro 67-4853 increased basal cAMP production at the fixed concentration used and this increased basal was inhibited by both orthosteric and allosteric mGluR1 antagonists, implying that this PAM may have partial agonist activity. These PAMs display EC50s for potentiation of cAMP production in the low micromolar range, values nearly 1000-fold right shifted compared to the EC50s for calcium potentiation or the EC50s for ERK1/2 phosphorylation. Thus, the mGluR1 PAMs do not appear to potentiate mGluR1 coupling to Gαs as readily as they potentiate coupling to Gαq. Interestingly, we previously reported that mGluR5 PAMs have much higher potencies at potentiating responses of mGluR5 to glutamate than affinities at their allosteric site (Chen et al., 2007; de Paulis et al., 2006). This implies a high level of cooperativity for mGluR5 PAMs and orthosteric agonists in activating calcium mobilization. One possible explanation for the present results is that a similar cooperativity may exist for mGluR1 PAMs and orthosteric agonists in activating calcium mobilization but that a similar cooperativity does not exist in activating coupling of mGluR1 to Gαs. Unfortunately, radioligands are not available for measuring affinities of mGluR1 PAMs to their allosteric sites (Hemstapat et al., 2006). Until such ligands become available, the possibility of differential cooperativity between PAMs and orthosteric agonists at inducing coupling to Gαs and Gαq cannot be fully evaluated.
It is clear that mGluR1 can signal to a number of signal transduction pathways and that these pathways can be differentially regulated. The finding that Ro 01-6128, Ro 67-4853, and Ro 67-7476 have differential effects on calcium mobilization and ERK1/2 phosphorylation has important implications for the effects of these compounds on synaptic transmission and other functions in the CNS. For example, ERK1/2 phosphorylation by mGluR1 is thought to be involved in the induction of hippocampal long term depression (Volk et al., 2006), required for mGluR1-mediated effects on inflammatory pain (Karim et al., 2001), and dysregulated by mGluR1 in fragile X syndrome (Kim et al., 2008). The increasing number of examples of both orthosteric and allosteric ligand-induced differential signaling in the literature demonstrate the plausibility for development of compounds to regulate highly specific signal transduction responses, which could allow the fine tuning of specifically dysregulated signal transduction pathways in disease. Although further study will be required to determine if these effects are ligand-dependent or system-dependent for the mGluR1 PAMs, these possibilities highlight the critical importance of further analysis of mGluR1-induced allosteric ligand signal transduction effects in both heterologous and native systems.
ACKNOWLEDGEMENTS
This study was supported by grants from National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Alliance for Research on Schizophrenia and Depression, the Stanley Foundation (to P.J.C.), and a PhRMA foundation award (to D.J.S.). Vanderbilt is a site in the National Institutes of Health-supported Molecular Libraries Screening Center Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Blanpain C, Vanderwinden JM, Cihak J, Wittamer V, Le Poul E, Issafras H, Stangassinger M, Vassart G, Marullo S, Schlndorff D, Parmentier M, Mack M. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol Biol Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink CB, Wade SM, Neubig RR. Agonist-directed trafficking of porcine alpha(2A)-adrenergic receptor signaling in Chinese hamster ovary cells: 1-isoproterenol selectively activates G(s) J Pharmacol Exp Ther. 2000;294:539–547. [PubMed] [Google Scholar]
- Chavez-Noriega LE, Schaffhauser H, Campbell UC. Metabotropic glutamate receptors: potential drug targets for the treatment of schizophrenia. Curr Drug Targets CNS Neurol Disord. 2002;1:261–281. doi: 10.2174/1568007023339337. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol. 2007;71:1389–1398. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- Chojnacka-Wojcik E, Klodzinska A, Pilc A. Glutamate receptor ligands as anxiolytics. Curr Opin Investig Drugs. 2001;2:1112–1119. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–561. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- de Paulis T, Hemstapat K, Chen Y, Zhang Y, Saleh S, Alagille D, Baldwin RM, Tamagnan GD, Conn PJ. Substituent effects of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides on positive allosteric modulation of the metabotropic glutamate-5 receptor in rat cortical astrocytes. J Med Chem. 2006;49:3332–3344. doi: 10.1021/jm051252j. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doherty J, Dingledine R. The roles of metabotropic glutamate receptors in seizures and epilepsy. Curr Drug Targets CNS Neurol Disord. 2002;1:251–260. doi: 10.2174/1568007023339355. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C. Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;273:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci U S A. 2000;97:6185–6190. doi: 10.1073/pnas.97.11.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol. 2003;138:775–786. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ. A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators. Mol Pharmacol. 2006;70:616–626. doi: 10.1124/mol.105.021857. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Bor M, Balla T, Catt KJ. Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J Biol Chem. 1994;269:31378–31382. [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Jr, Britton TC, Large TH, Callagaro DO, Tizzano JP, Monn JA, Schoepp DD. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-37 methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci. 1995;15:3970–3981. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RWt. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Markham JA, Weiler IJ, Greenough WT. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc Natl Acad Sci U S A. 2008;105:4429–4434. doi: 10.1073/pnas.0800257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A. 2001;98:13402–13407. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Janssen C, Bischoff F, Langlois X, Leysen JE, Lesage AS. [3H]R214127: a novel high-affinity radioligand for the mGlu1 receptor reveals a common binding site shared by multiple allosteric antagonists. Mol Pharmacol. 2003;63:1082–1093. doi: 10.1124/mol.63.5.1082. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O'Brien JA, Lemaire W, Williams DL, Jr, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Mabire D, Coupa S, Adelinet C, Poncelet A, Simonnet Y, Venet M, Wouters R, Lesage AS, Van Beijsterveldt L, Bischoff F. Synthesis, structure-activity relationship, and receptor pharmacology of a new series of quinoline derivatives acting as selective, noncompetitive mGlu1 antagonists. J Med Chem. 2005;48:2134–2153. doi: 10.1021/jm049499o. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Awad-Granko H, Ciombor KJ, Conn PJ. Haloperidol-induced alteration in the physiological actions of group I mGlus in the subthalamic nucleus and the substantia nigra pars reticulata. Neuropharmacology. 2002;43:147–159. doi: 10.1016/s0028-3908(02)00097-7. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Chen TB, Chang RS, Jacobson MA, Ha SN, Lindsley CW, Schaffhauser HJ, Sur C, Pettibone DJ, Conn PJ, Williams DL., Jr A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol Pharmacol. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A. On the role of metabotropic glutamate receptors in the mechanisms of action of antidepressants. Pol J Pharmacol. 2002;54:581–586. [PubMed] [Google Scholar]
- Pilc A. LY-354740 (Eli Lilly) IDrugs. 2003;6:66–71. [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Seifert R, Gether U, Wenzel-Seifert K, Kobilka BK. Effects of guanine, inosine, and xanthine nucleotides on beta(2)-adrenergic receptor/G(s) interactions: evidence for multiple receptor conformations. Mol Pharmacol. 1999;56:348–358. doi: 10.1124/mol.56.2.348. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor 1alpha. Proc Natl Acad Sci U S A. 2006;103:1124–1128. doi: 10.1073/pnas.0505925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandi S, Blank JL, Challiss RA. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem. 2002;83:1139–1153. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- Varney MA, Gereau RWt. Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Targets CNS Neurol Disord. 2002;1:283–296. doi: 10.2174/1568007023339300. [DOI] [PubMed] [Google Scholar]
- Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J. 9H-Xanthene-9-carboxylic acid [1,2,4]oxadiazol-3-yl- and (2H-tetrazol-5-yl)-amides as potent, orally available mGlu1 receptor enhancers. Bioorg Med Chem Lett. 2005;15:4628–4631. doi: 10.1016/j.bmcl.2005.05.135. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically-induced hippocampal long-term depression. J Neurophysiol. 2006 doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol. 1996;50:966–976. [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Car H. (S)-3,5-DHPG: a review. CNS Drug Rev. 2002;8:101–116. doi: 10.1111/j.1527-3458.2002.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]