Abstract
The disseminated characteristics of human glioblastoma multiforme (GBM) make it a particularly difficult tumor to treat with long-term efficacy. Most preclinical models of GBM involve treatment of a single tumor mass. For therapeutic outcomes to translate from the preclinical to the clinical setting, induction of an antitumor response capable of eliminating multifocal disease is essential. We tested the hypothesis that expression of Flt3L (human soluble FMS-like tyrosine kinase 3 ligand) and TK (herpes simplex virus type 1–thymidine kinase) within brain gliomas would mediate regression of the primary, treated tumor mass and a secondary, untreated tumor growing at a distant site from the primary tumor and the site of therapeutic vector injection. In both the single-GBM and multifocal-GBM models used, all saline-treated control animals succumbed to tumors by day 22. Around 70% of the animals bearing a single GBM mass treated with an adenovirus expressing Flt3L (AdFlt3L) and an adenovirus expressing TK (AdTK + GCV) survived long term. Approximately 50% of animals bearing a large primary GBM that were implanted with a second GBM in the contralateral hemisphere at the same time the primary tumors were being treated with AdFlt3L and AdTK also survived long term. A second multifocal GBM model, in which bilateral GBMs were implanted simultaneously and only the right tumor mass was treated with AdFlt3L and AdTK, also demonstrated long-term survival. While no significant difference in survival was found between unifocal and multifocal GBM–bearing animals treated with AdFlt3L and AdTK, both treatments were statistically different from the saline-treated control group (p < 0.05). Our results demonstrate that combination therapy with AdFlt3L and AdTK can eradicate multifocal brain tumor disease in a syngeneic, intracranial GBM model.
Keywords: Flt3L, glioblastoma, HSV1-TK, immunotherapy, multiple tumor
Glioblastoma multiforme (GBM) is the most aggressive, rapidly progressing, and difficult to treat primary brain tumor. Classical GBMs do not have a single central tumor mass but disseminate throughout the brain.1–3 Even with the most aggressive treatments currently available, disease recurrence is nearly inevitable; only 29.1% of patients survive 1 year from diagnosis.4 During surgical resection of GBM, tumor cells cannot be removed completely due to the disseminated nature of this tumor, and this is believed to be the cause of tumor recurrence.5 Therefore, therapeutic approaches that are able to target and eliminate disseminated GBM foci would be anticipated to translate more efficaciously into the clinical setting. Gene therapy strategies utilizing oncolytic, replicating viral vectors have so far been the only treatment to show efficacy in models of multifocal GBM.6–10
Immunotherapy is another promising approach for treating disseminated GBM in human patients. Several immunotherapeutic approaches have used tumor vaccines to stimulate a highly specific and targeted immune response directed against tumor-specific antigens. These approaches require the recruitment and activation of antigen-presenting cells (APCs) within the tumor mass.11 Since dendritic cells (DCs) are the most efficient APCs,12,13 many preclinical and clinical trials employ DCs loaded with tumor lysates or specific tumor antigens14–18 to induce an immunotherapeutic antitumor effect.
Flt3L (human soluble FMS-like tyrosine kinase 3 ligand) stimulates maturation and proliferation of DCs19–21 and natural killer (NK) cells.22 Treatment of solid tumors with recombinant Flt3L protein slows disease progression in models of breast cancer and leukemia and decreases the number of metastases in models of colon adenocarcinoma, sarcoma, and melanoma.23–25 Also, in sarcoma models, the administration of recombinant Flt3L protein induces significant tumor regression and immunological memory.26–28 However, Flt3L recombinant protein has not been efficacious in the treatment of sarcoma and melanoma tumors when implanted in the brain.23 This has been attributed to the dose, short half-life, or distribution of the cytokine, as well as to tumor antigen availability.
We have recently demonstrated that injection of an adenoviral (Ad) vector expressing Flt3L (AdFlt3L) into the brain of rats induces a specific increase in the number of interferon alpha (IFNα)-secreting plasmacytoid dendritic cells (pDCs) within the parenchyma.19 In a syngeneic intracranial glioma model in rats, we found that AdFlt3L improves survival when tumors are treated within 3 days of tumor implantation.29 However, the efficacy of this treatment inversely correlates to tumor volume. Thus, we combined AdFlt3L with an Ad vector expressing herpes simplex virus type 1–thymidine kinase (AdTK), which prolonged survival in more than 70% of treated animals by inducing an antitumor immune response.30 In this experimental paradigm, TK in the presence of the prodrug ganciclovir (GCV) kills glioma cells,31,32 inducing the release of antigens that can be taken up by Flt3L-recruited DCs.
Although preclinical models of GBM share many pathological features with human GBM,33 they do not fully mimic the disseminated nature of the human disease. In this study, we aimed to determine whether the antitumor response generated by combined treatment with AdFlt3L and AdTK would be effective in a multifocal intracranial GBM model in rat. Thus, we implanted CNS-1 rat glioma cells bilaterally in both brain hemispheres and administered AdFlt3L combined with AdTK unilaterally, leaving the second tumor untreated. The results of this study demonstrates that the antitumor response generated by AdFlt3L and AdTK is effective at eliminating both the primary treated and the secondary, distant untreated tumor. Our results suggest that this gene therapeutic approach would be effective in eliminating glioma cells that disseminate from the main tumor mass throughout the normal brain parenchyma, and support further development of TK in combination with Flt3L for translation into a phase I clinical trial.
Materials and Methods
Adenoviral Vectors
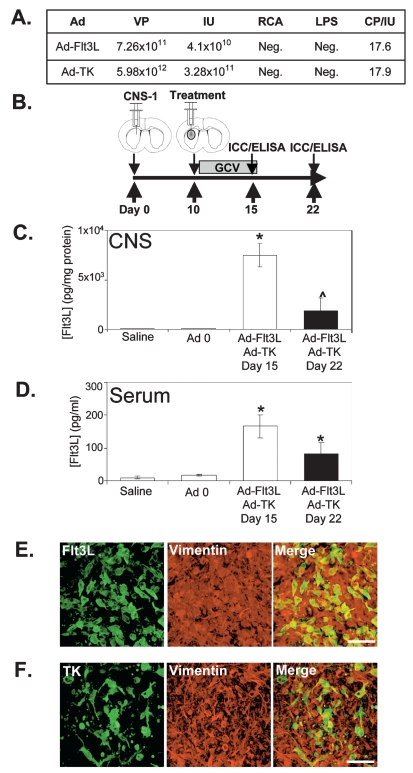
The first-generation replication-defective recombinant adenovirus type 5 vectors (Ad) expressed TK (AdTK) or soluble human Flt3L (AdFlt3L) under the transcriptional control of the human cytomegalovirus intermediate early promoter embedded within the E1 region.34 Ad 0 (containing no transgene) was used as a control virus. The construction of these vectors has been described in detail previously.19,29,30,35,36 The vectors were scaled up by infecting human embryonic kidney (HEK 293) cells with a multiplicity of infection of 3 infectious units (IU)/cell of vector seed stock. The cells were harvested 48 h later and lysed with 5% deoxycholate and DNase I, and Ad vectors were purified by ultracentrifugation over two cesium chloride step gradients.34,37 Vectors were titered in triplicate by end-point dilution, cytopathic effect (CPE) assay.34 The titers were 3.28 × 1011 IU/ml for AdTK29,30 and 4.10 × 1010 IU/ml for AdFlt3L.19,29,30 The vector preparations were screened for the presence of replication-competent adenovirus (RCA)34,38 and for lipopolysaccharide (LPS) contamination (Cambrex, East Rutherford, NJ, USA).34,39 Virus preparations used were free of RCA and LPS contamination (Fig. 1A).
Fig. 1.
Adenoviral (Ad) vector expressing human soluble FMS-like tyrosine kinase 3 ligand (AdFlt3L) and an Ad vector expressing herpes simplex virus type 1–thymidine kinase (AdTK) efficiently transduce intracranial CNS-1 rat glioma tumors. (A) Titers of the Ad viral vector preparations. Total viral particles (VP) were measured by optical density at 260 nm; infectious units (IU) were assessed by cytopathic effects (CPE) on human embryonic kidney (HEK 293) cells; lipopolysaccharide (LPS) contamination and replication- competent Ad (RCA) contamination by supernatant rescue on HeLa (ATCC Number: CCL-2) cells were evaluated for both of the vectors after cesium chloride purification. (B) Schematic representation of the experimental paradigm in which male Lewis rats were unilaterally implanted with 5,000 CNS-1 glioma cells and treated 10 days later by intratumoral injection of either saline, Ad 0, or AdFlt3L and AdTK. Twenty-four hours after Ad delivery, ganciclovir (GCV) was administered twice daily for 7 days (25 mg/kg, intraperitoneally). Animals were euthanized 15 days after CNS-1 cell implantation to determine levels of transgene expression. (C and D) Transgene expression was also detected in AdFlt3L/AdTK-treated survivors 22 days after tumor implantation. Flt3L ELISA was performed in homogenized brain extracts (C) and serum (D). Columns represent the mean ± SEM of Flt3L concentration; *p < 0.01 versus saline group; ^p < 0.05 versus AdFlt3L + AdTK day 15 (one-way analysis of variance followed by Bonferroni posttest; n = 4/group). (E and F) Transgene expression was also determined by immunocytochemistry in brain tumor sections 15 days after tumor implantation. Confocal images show colocalization of vimentin staining in CNS-1 cells (red) and Flt3L (E, green) or TK (F, green). Merged images show colocalization of vimentin and transgene (yellow). Scale bar = 50 μm.
Vector Characterization
Physical titration of Ad vectors by spectrophotometry was based on the absorption of pure adenovirus at 260 nm40 as previously described in detail.41 An optical density at 260 nm of 1.00 corresponds to a viral particle (VP) concentration of 1.1 × 1012 VP/ml based on a 36-kb size of the wild-type particle genome. Biological titrations of Ad vectors expressing TK and Flt3L were titrated by quantifying the IU per milliliter, using the end-point CPE method in 96-well plates.42,43
Syngeneic Intracranial Rat Tumor Models
Intracranial GBM model; single tumor mass
Male Lewis rats (220–250 g; Harlan, Indianapolis, IN, USA) were unilaterally injected into the striatum (+1 mm bregma, +3 mm lateral, and –4 mm from the dura) with 5,000 CNS-1 cells stably transfected with firefly luciferase. Ten days later, utilizing the same drill hole, saline, taAdflt3L and AdTK (5 × 107 IU each) were delivered within the tumor mass using the same coordinates as those used for implantation of CNS-1 cells. Twenty-four hours after delivery of viral vectors, animals that received AdTK began treatment with GCV (25 mg/kg, intraperitoneal [IP] injection), twice daily for 7 days. Animals were monitored daily and euthanized at the first signs of moribund behavior or at predetermined time points for neuropathological analysis and ELISA. Euthanasia was conducted under deep anesthesia by terminal perfusion. Animals were housed in a humidity- and temperature-controlled vivarium on a 12-h light/12-h dark cycle (lights on 07:00) with free access to food and water. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Multifocal tumor models
Rats were injected with 5,000 CNS-1 cells followed 10 days later by intratumoral injection of either saline or AdFlt3L and AdTK (5 × 107 IU each). Following saline or AdFlt3L and AdTK injection, 5,000 CNS-1 cells were injected into the contralateral striatum. Twenty-four hours later, animals were treated with GCV (25 mg/kg, IP) twice daily for 7 days. In the second multifocal GBM model used, animals were injected at the same time with 5,000 CNS-1 cells bilaterally, followed 4 days later by unilateral, intratumoral injection of saline or AdFlt3L and AdTK (5 × 107 IU each). Twenty-four hours later, all animals were treated with GCV (25 mg/kg, IP) twice daily for 7 days. Animals were monitored daily and euthanized at the first signs of moribund behavior or at day 4, 9, or 45 for neuropathological analysis. Results shown are representative of two independent experiments.
Human Soluble Flt3L ELISA
Human soluble Flt3L was assessed within homogenized tumor or striatal extracts taken day 15 or 22 after tumor cell implantation and from serum collected prior to perfusion (n = 4–10/group). Samples were measured utilizing a specific human soluble Flt3L ELISA system (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.19,29,31
Immunocytochemistry
Following perfusion, brains to be used for immunocytochemistry (ICC) were fixed in 4% paraformaldehyde (n = 4/group). Sixty-micrometer serial coronal sections were cut through the striatum. Free-floating ICC was performed to detect inflammatory and immune cell markers. Briefly, endogenous peroxidases were inactivated with 0.3% hydrogen peroxide, followed by blocking in 10% horse serum/phosphate-buffered saline. Sections were incubated overnight with primary antibody: anti-CD68 (clone ED1 to identify macrophages/activated microglia; 1:1,000), anti–major histocompatibility complex II (anti-MHC II; to identify immune cells; 1:1,000), and anti-CD8 (to identify cytotoxic T cells; 1:1,000), obtained from Serotec (Raleigh, NC, USA). Antivimentin (1:1,000; Sigma, St. Louis, MO, USA) was used to detect CNS-1 cells. To assess brain neuropathology, myelin basic protein (MBP; 1:1,000; Chemicon, Billerica, MA, USA) and tyrosine hydroxylase (TH; 1:1,000; Calbiochem, San Diego, CA, USA) immunoreactivity was evaluated. Binding of biotinylated secondary antibody against either mouse or rabbit immunoglobulins (1:1,000; Jackson Immunochemicals, West Grove, PA, USA) was detected using the Vectastain Elite ABC horseradish peroxidase method (Vector Laboratories, Burlingame, CA, USA). After developing with diaminobenzidine and glucose oxidase, sections were mounted on gelatinized glass slides and dehydrated through graded ethanol solutions. Tissues were analyzed and photographed using a Carl Zeiss Optical Axioplan microscope (Carl Zeiss, Chester, VA, USA).
Nissl staining was used to determine the histopathological features of the brains. Brain sections were mounted and incubated in cresyl violet (0.1%; Sigma). Sections were passed through destain solution (70% ethanol, 10% acetic acid) and dehydrated (100% ethanol and xylene). Sections were analyzed and photographed using a Zeiss Axioplan microscope.
Ad-expressed transgenes were detected utilizing custom-generated rabbit antibodies specific to TK35,44,45 or human soluble Flt3L19,30,31,44 (developed in rabbit; 1:1,000) combined with the mouse antivimentin antibody. Alexa 488 directly conjugated fluorescent secondary antibodies (1:1,000) were used to detect transgenes (Invitrogen, Carlsbad, CA, USA). Sections were mounted with ProLong Antifade mounting media (Invitrogen, Carlsbad, CA, USA).
Confocal Microscopy
Confocal micrographs were obtained using a Leica TCS SP2 confocal microscope with an acousto-optical beam splitter, equipped with a 405-nm violet diode ultraviolet laser, 488-nm argon laser, and 594- and 633-nm heliumneon lasers and using an HCX PL APO × 63 1.4 numerical-aperture oil objective (Leica Microsystems Heidelberg, Mannheim, Germany).
Statistical Analysis
Statistical analyses were performed using Graphpad Prism (version 3.03; Graphpad Software, San Diego, CA, USA). Flt3L ELISA measurements were compared by one-way analysis of variance tests followed by the Bonferroni multiple comparison test. Survival curves were compared using the Mantel-Haenszel log-rank test; p < 0.05 was considered significant. All experiments were performed at least twice.
Results
Quality Control of Ad Vectors
AdFlt3L and AdTK were generated as described previously.29,30,35 Recent FDA guidelines require the use of therapeutic Ad vectors with a ratio of total viral particles to therapeutic viral particles of no more than 30:1.46 The VP/IU ratio represents the proportion of the total particles that are infectious and can therefore express the encoded therapeutic transgenes. Moreover, because the viral particles themselves may mediate dose-dependent acute toxicity,47–51 high levels of noninfectious particles (reflected in a high VP/IU ratio) may increase toxicity and decrease benefit. Both viral preparations utilized for our experiments showed a VP/IU ratio of 18 and were LPS and RCA negative (Fig. 1A).
Flt3L and TK Expression within the Intracranial GBM Mass: Widespread Therapeutic Gene Expression within the Tumor and Levels of Flt3L in the Peripheral Circulation
Animals implanted with CNS-1 glioma cells succumb to tumors within 3 weeks if AdFlt3L and AdTK treatment does not eradicate the tumor.29,30 Flt3L and TK delivered by Ad vectors are hypothesized to induce high levels of tumor regression by infecting CNS-1 cells and inducing a systemic antitumor immune response.30 Male Lewis rats were unilaterally implanted with 5,000 syngeneic CNS-1 glioma cells into the striatum. Ten days later saline, Ad 0 (control virus expressing no transgene), or AdFlt3L and AdTK were intratumorally delivered, followed by the administration of GCV twice daily for 1 week. Animals either were euthanized at day 15 and day 22 postimplantation for analysis of transgene expression and tumor regression or were evaluated for long-term survival (Fig. 1B). At day 15 after tumor implantation, untreated animals began to succumb to tumors. At day 22, after the cessation of GCV, tumor regression, if successful, had been fully initiated.
Neither saline nor Ad 0 treatment induced detectable levels of human soluble Flt3L in CNS or serum (Fig. 1C,D). At both time points, animals treated with AdFlt3L and AdTK showed high levels of Flt3L, detectable both locally in the CNS (Fig. 1C) and in the peripheral circulation in serum (Fig. 1D). Brain levels of Flt3L expression diminished between day 15 and day 22 (Fig. 1C; p < 0.05), as the tumor regressed.
In order to detect intratumoral transgene expression, a group of animals did not receive GCV, to allow maximum detection of TK expression without GBM cell death. Expression of Flt3L (Fig. 1E) and TK (Fig. 1F) was widely distributed within the intracranial tumor mass 5 days after treatment in vimentin-positive CNS-1 glioma cells.
Combined GBM Treatment with AdFlt3L and AdTK: Tumor Regression, Neuropathology, and Immune Infiltrates
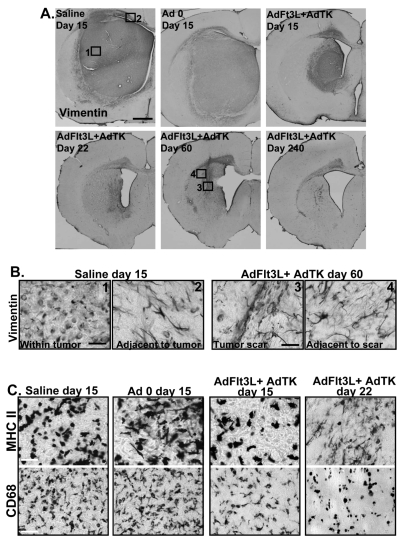
To assess tumor progression in control and AdFlt3L/AdTK-treated animals, neuropathological analysis was conducted at days 15 and 22 after CNS-1 cell implantation and in long-term survivors at days 60 and 240 postimplantation. Vimentin staining clearly delineated the tumor area encompassing most of the left striatum in saline- or Ad 0-treated tumors at day 15 (Fig. 2A,B). Conversely, vimentin staining at day 15 in AdFlt3L/AdTK-treated animals showed a smaller tumor mass that was absent by day 22 and was likewise not detectable 60 or 240 days after GBM implantation (Fig. 2A,B). Treated tumor-bearing animals euthanized at days 22, 60, and 240 after CNS-1 cell implantation showed vimentin staining consistent with reactive astrocytes, not CNS-1 tumor growth, both at the injection site and in adjacent areas (Fig. 2A). Positive vimentin staining for reactive astrocytes was limited to the ipsilateral hemisphere and corpus callosum, as shown in higher magnification images of treated animals euthanized 60 days after tumor implantation (Fig. 2B).
Fig. 2.
Combined gene therapy with adenoviral (Ad) vector expressing human soluble FMS-like tyrosine kinase 3 ligand (AdFlt3L) and an Ad vector expressing herpes simplex virus type 1–thymidine kinase (AdTK) induces regression of syngeneic glioblastoma multiforme in the rat brain. Lewis rats were unilaterally implanted with 5,000 CNS-1 glioma cells and treated 10 days later by intratumoral injection of either saline or AdFlt3L and AdTK. Twenty-four hours after viral delivery, ganciclovir (GCV) was administered twice daily for 7 days (25 mg/kg, IP). (A) Vimentin staining shows the appearance of the brains of animals after different treatments. Numbers in boxes indicate the approximate location of higher magnification images shown in B. Scale bar = 1,000 μ m. (B) High-magnification images of vimentin-stained brains from moribund saline-treated animals (1, 2) or AdFlt3L/AdTK-treated animals 60 days after tumor implantation (3, 4). For each condition, representative images show vimentin staining within the tumor or the scar area at the site of tumor regression. Areas adjacent to the immediate tumor mass or scar are shown. Scale bar = 50 μ m. (C) Representative images of brain sections from animals euthanized at the indicated time points after treatment that were evaluated for macrophage/activated microglia (CD68) and immune cell (major histocompatibility complex II [MHC II]) infiltration. Scale bar = 50 μ m.
In the presence of a lesion, astrocytes become activated, up-regulating synthesis of a number of structural proteins, such as glial fibrillary acidic protein and vimentin.52,53 Up-regulation of vimentin expression indicates the existence of astrocyte hypertrophy and gliosis, which is a typical response to a brain lesion.54 CNS-1 tumors, regardless of the treatment modality, are heavily infiltrated with immune cells (MHC II, Fig. 2C) absent from the normal brain parenchyma. Notably, CNS-1 tumors are highly infiltrated with macrophages/activated microglia (CD68, Fig. 2C). Both CD68- and MHC II-immunoreactive cells decreased by day 22 in AdFlt3L/AdTK-treated animals, indicating a gradual decrease in immune cell infiltration as tumor regression occurred (Fig. 2C).
Elimination of Multifocal Disease by Combined Flt3L and TK Expression
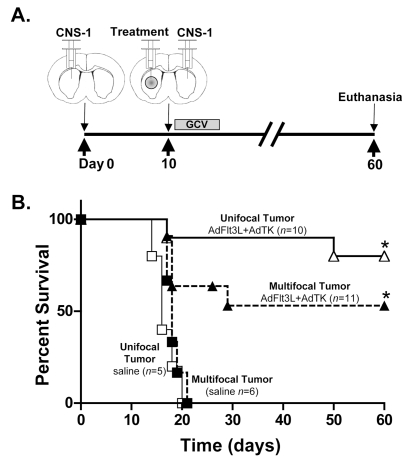
Considering that human glioblastoma is a disseminated disease, we aimed to test whether AdFlt3L and AdTK administration within a large single tumor mass would induce an effective antitumor response that would eliminate tumor cells located at a distant site in relation to the primary treated tumor. Thus, we determined whether AdFlt3L and AdTK would be effective in eliminating tumor cells implanted at the contralateral hemisphere from the primary treated lesion in a multifocal glioma model. CNS-1 cells were implanted into the striatum, followed 10 days later with injection of either saline or AdFlt3L and AdTK into the primary tumor mass. A second tumor was implanted in the contralateral hemisphere (Fig. 3A) on the same day the treatment was delivered to the primary tumor. As a control for treatment efficacy, single tumors were implanted and treated 10 days later with either saline or AdFlt3L and AdTK to evaluate long-term survival and therapeutic efficacy. All saline-treated animals implanted with either single or multifocal GBMs succumbed to tumors by day 22 (Fig. 3B) and showed tumors in one or both hemispheres, respectively. Approximately 50% of animals bearing multifocal GBM treated with AdFlt3L and AdTK survived to 60 days (Fig. 3B), and we observed > 70% long-term survival of the animals bearing a single GBM mass treated with AdFlt3L and AdTK (Fig. 3B). Both treatments were statistically different from the saline-treated control group (p < 0.05). No significant difference in survival was found between unifocal and multifocal GBM-bearing animals treated with AdFlt3L and AdTK.
Fig. 3.
Efficacy of human soluble FMS-like tyrosine kinase 3 ligand (Flt3L) and herpes simplex virus type 1–thymidine kinase (TK)-mediated gene therapy in a model of multifocal intracranial glioblastoma multiforme. (A) Diagram of the multifocal brain tumor model and treatment with adenoviral (Ad) vector expressing Flt3L (AdFlt3L) and Ad vector expressing TK (AdTK). Animals received 5,000 CNS-1 cells into the left striatum followed 10 days later by intratumoral injection of either saline or AdFlt3L and AdTK. Immediately after intratumoral Ad vector administration, a second distant GBM was implanted (5,000 CNS-1 rat glioma cells) in the contralateral striatum. No treatment was administered to the second tumor. Animals received ganciclovir (GCV) 24 h after Ad vector injection (25 mg/kg, twice daily for 7 days). Animals were monitored for survival and euthanized at the first signs of moribund behavior. AdFlt3L/AdTK-treated animals surviving to 60 days were euthanized for neuropathological analysis. (B) Survival curve for multifocal tumors treated with saline (n = 6, solid squares) or AdFlt3L and AdTK (n = 11, solid triangles) and unifocal tumors treated with saline (n = 5, open squares) or AdFlt3L and AdTK (n = 10, open triangles), which were used as positive controls of efficacious AdFlt3L and AdTK therapy; *p < 0.05 versus saline *p < 0.05 versus saline (Mantel-Haenszel log rank test).
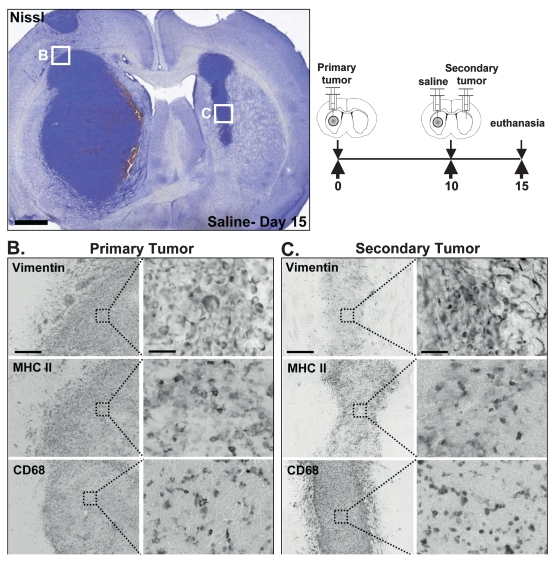
Untreated multifocal tumors were evaluated on the day of treatment (day 10) for the presence of primary and secondary tumor masses (Fig. 4A) and for tumor appearance and tumor-infiltrating immune cell markers (Fig. 4B,C). Both primary (Fig. 4B) and secondary tumors (Fig. 4C) were readily visible by Nissl and vimentin staining. They were profusely infiltrated with immune cells (Fig. 4B,C), as determined by MHC II staining, some of which were macrophages/activated microglia (CD68, Fig. 4B,C).
Fig. 4.
Characteristics of the syngeneic, intracranial, multifocal glioma model: histological features and immune infiltrates. (A) Representative Nissl staining of brains from animals bearing multifocal glioblastoma multiforme tumors treated with saline. Boxes represent approximate locations of higher magnification images. Scale bar = 1,000 μ m. The diagram represents the implantation of the primary and secondary tumor masses. The box represents approximate location of higher magnification images. (B) Immunocytochemistry of the primary tumor showing vimentin-expressing cells (CNS-1 rat glioma cells) and cells immunoreactive for major histocompatibility complex II (MHC II; immune cells) and CD68 (macrophages/activated microglia). Scale bar represents 500 μ m for lower magnification images and 50 μ m for higher magnification images. (C) Immunocytochemistry of secondary tumor showing vimentin-expressing cells (CNS-1 rat glioma cells and reactive astrocytes) and cells immunoreactive for MHC II (immune cells) and CD68 (macrophages/activated microglia). Scale bar represents 500 μ m for lower magnification images and 50 μ m for higher magnification images.
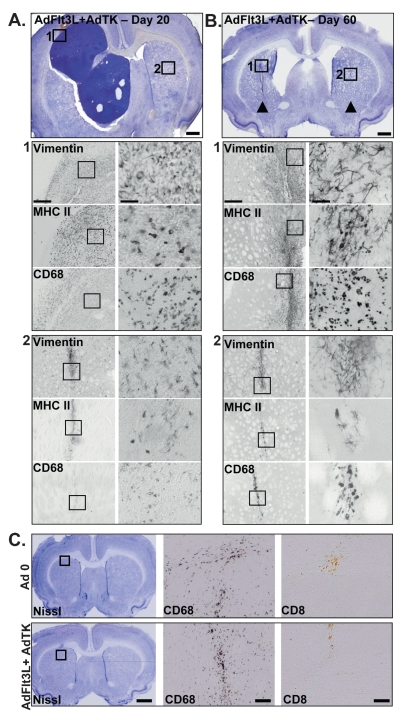
The AdFlt3L/AdTK-treated animals that did not survive to 60 days due to the primary tumor mass resolved the secondary contralateral tumor (Fig. 5A). The multi-focal tumor-bearing animals that had been treated with AdFlt3L and AdTK and survived to day 60 displayed injection scars at both the primary and secondary tumor implantation sites (Fig. 5B). The hemisphere where the primary tumor was implanted, treated, and resolved displayed an enlarged lateral ventricle consistent with striatal tissue loss due to the regression of a very large intracranial tumor mass. We determined whether the ventriculomegaly observed in the long-term survivors after successful gene therapy was due to tumor regression or Ad injection and GCV treatment. Naive animals were injected with Ad 0 or AdFlt3L and AdTK, and then all animals were treated with GCV and euthanized 60 days later. Nissl staining showed the site of injection without ventriculomegaly or any other noticeable neuropathological changes to the brain parenchyma (Fig. 5C). Staining for the inflammatory marker CD68 (macrophages/activated microglia) showed injectionsite–specific infiltration. However, no cytotoxic T cells (CD8) were detected in the brain parenchyma of the Ad-injected animals, indicating that low-level innate inflammation persisted 60 days after Ad injection only in the immediate area of injection in the area where a scar formed (Fig. 5C). No noticeable neuropathological changes were found in brains receiving AdFlt3L and AdTK, which induces death only of actively dividing cells upon GCV administration, indicating that this treatment alone does not induce damage to the normal brain parenchyma.
Fig. 5.
Human soluble FMS-like tyrosine kinase 3 ligand (Flt3L) and herpes simplex virus type 1–thymidine kinase (TK)-mediated gene therapy induces tumor regression in the multifocal, intracranial glioblastoma multiforme (GBM) model. (A and B) Representative Nissl stain of multifocal tumors, in which the primary tumor mass had been treated with AdFlt3L and AdTK followed by GCV administration. Animals received 5,000 CNS-1 rat glioma cells into the left striatum followed 10 days later by intratumoral injection of adenoviral (Ad) vector expressing Flt3L (AdFlt3L) and an Ad vector expressing TK (AdTK). Immediately after intratumoral viral vector administration, a second distant GBM was implanted (5,000 CNS-1 cells) in the contralateral striatum. No treatment was administered to the second tumor. Animals receiving AdTK began receiving ganciclovir (GCV) 24 h after Ad vector injection (25 mg/kg, twice daily for 7 days). Animals were euthanized as they became moribund or at 60 days postimplantation (long-term survivors) for neuropathological analysis. (A) Representative Nissl stain of an animal that required euthanasia at day 20 after implantation of the primary tumor. Boxes represent approximate location of the higher magnification images of the primary large tumor mass (1) and the site in the contralateral hemisphere where the second tumor was implanted (2). Scale bar = 1,000 μ m. Immunocytochemistry for cells immunoreactive for vimentin (CNS-1 cells and reactive astrocytes), major histocompatibility complex II (MHC II; immune cells), and CD68 (macrophages/activated microglia) within the primary (1) and secondary (2) tumors. Boxes represent approximate area shown in the higher magnification images to the right. Scale bars = 500 μ m and 50 μ m, respectively. (B) Representative image from the brain of a rat euthanized at the end of the experiment (day 60 after primary CNS-1 rat glioma cell implantation). Arrowheads indicate location of primary and secondary tumor injection sites. Boxes represent approximate location of the higher magnification images of site of primary tumor injection (1) and the site in the contralateral hemisphere were the second tumor was implanted (2). Immunocytochemistry for cells immunoreactive for vimentin (CNS-1 tumor cells and reactive astrocytes), MHC II (immune cells), and CD68 (macrophages/activated microglia) within the primary (1) and secondary (2) tumor sites are shown. Scale bars represent 500 μ m and 50 μ m, respectively. (C) Naive rats were unilaterally injected into the striatum with Ad 0 or AdFlt3L and AdTK. GCV administration began 24 h after viral vector injection in all animals. Animals were euthanized 60 days later and evaluated for neuropathology (Nissl) and immune infiltrates (CD68, CD8). Box in the Nissl stain indicates the approximate location of higher magnification images. Scale bars = 1,000 μ m and 50 μ m, respectively.
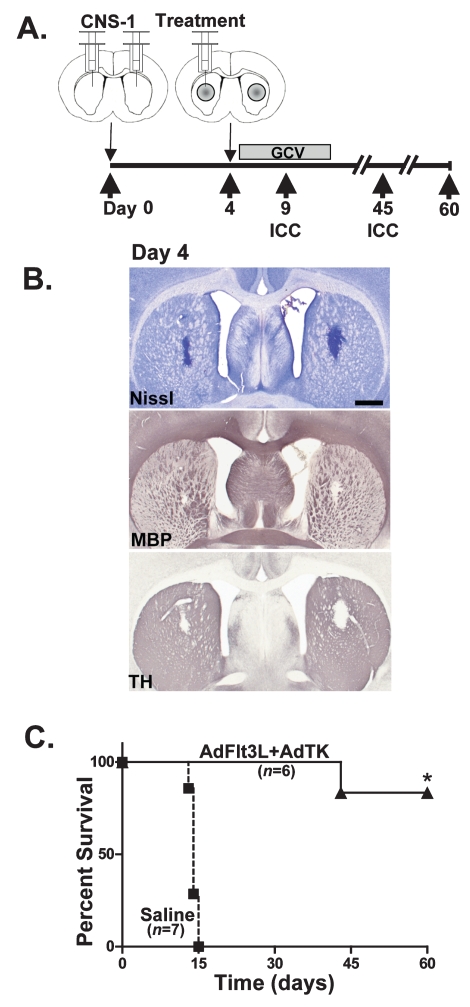
In order to assess whether the administration of AdFlt3L and AdTK to a tumor mass would elicit regression of a second established distant tumor, we developed a multifocal GBM model in which CNS-1 cells were simultaneously bilaterally implanted into the striatum of Lewis rats. Four days later, saline or AdFlt3L and AdTK were unilaterally injected into the right tumor mass (Fig. 6A). All animals were treated with GCV twice daily for 7 days beginning the day after viral vector administration. Groups of animals were euthanized at day 4 to examine tumor volume at the time of treatment, ensure growth of tumors in both hemispheres, and examine the structural integrity of the brain. Nissl staining was used to observe the appearance of the tumors, which were well developed in both hemispheres (Fig. 6B) in all experimental animals. MBP and TH immuno-reactivity was excluded from the growing tumor mass (Fig. 6B). Myelinated fibers were found throughout the corpus callosum and the fiber tracts of the striatum, as expected. No other gross alterations in MBP or TH distribution were apparent. Bilateral GBM tumors grew in all animals, and when they were treated with saline, they succumbed to their tumors by day 15 (n = 7; Fig. 6C). However, animals that received AdFlt3L and AdTK showed 80% survival to day 60 (n = 6; Fig. 6C), which was significantly different from the survival of untreated animals (p < 0.05).
Fig. 6.
Efficacy of adenovirus (Ad)-mediated gene therapy using Ad vector expressing human soluble FMS-like tyrosine kinase 3 ligand (AdFlt3L) in combination with an Ad vector expressing herpes simplex virus type 1–thymidine kinase (AdTK) plus ganciclovir (GCV) in a model of multifocal glioblastoma multiforme with two GBM tumors implanted simultaneously and treatment delivered into a single tumor mass. (A) Diagram of the experimental paradigm in which 5,000 CNS-1 rat glioma cells were simultaneously implanted into right and left striatum. Four days later, saline or AdFlt3L and AdTK were delivered into the right tumor mass. All animals were treated with GCV 24 h later, twice daily for 7 days (25 mg/kg). Animals were evaluated for survival or euthanized at different time points to evaluate tumor progression. (B) To ensure tumor growth and evaluate the neuropathology of tumors on the day of treatment, animals were euthanized on day 4 prior to treatment, and brains were evaluated by Nissl staining or by immunocytochemistry (ICC) for myelin basic protein (MBP) or tyrosine hydroxylase (TH). Scale bar = 1,000 μ m. (C) Survival curves of multifocal tumor-bearing rats treated with either saline (squares) or AdFlt3L and AdTK (triangles). Animals treated with saline (n = 7) all died by day 15. All animals treated with AdFlt3L and AdTK (n = 6) survived long term. *p < 0.05 versus saline (Mantel-Haenszel log rank test).
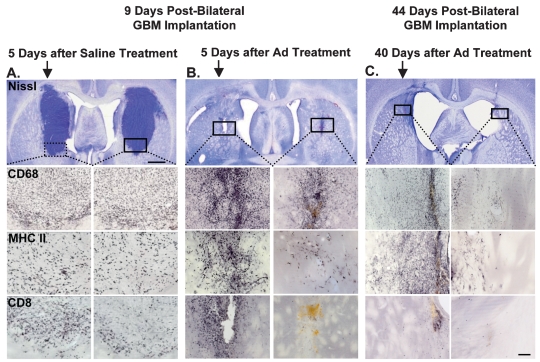
To evaluate tumor regression and immune cell infiltration into the tumor mass in control and AdFlt3L/AdTK-treated animals, groups of animals were euthanized 5 days after treatment, on day 9 after GBM implantation (Fig. 7A,B). In all saline-treated animals examined, large tumors were evident in both hemispheres (Fig. 7A). Although tumors were evident in AdFlt3L/AdTK-treated animals 5 days after treatment, they were much smaller than in the saline-treated animals (Fig. 7B). In all tumors, immune infiltration was detected by ICC using specific antibodies for macrophages/activated microglia (CD68), T cells (CD8), and APCs (MHC II) (Fig. 7A,B). Animals treated with AdFlt3L and AdTK and surviving to day 45 after GBM implantation were similarly evaluated for neuropathology and immune cell infiltration. Nissl staining revealed a scar at the injection site in both hemispheres and ventriculomegaly with no other gross alterations to the brain parenchyma (Fig. 7C). While CD68, MHC II, and CD8 were detected in both hemispheres as at earlier time points during the tumor regression process (Fig. 7B), immune infiltration was limited to the area immediate to the injection site 45 days after tumor implantation (Fig. 7C).
Fig. 7.
Human soluble FMS-like tyrosine kinase 3 ligand (Flt3L) and herpes simplex virus type 1–thymidine kinase (TK)-mediated gene therapy induces long-term regression of simultaneously implanted multifocal tumors. Five thousand CNS-1 rat glioma cells were simultaneously implanted into right and left striatum. Four days later saline (A) or adenoviral (Ad) vector expressing Flt3L (AdFlt3L) and an Ad vector expressing herpes simplex virus type 1–thymidine kinase (AdTK) (B and C) were delivered into the right tumor mass. Twenty-four hours later, all animals were treated with ganciclovir twice daily for 7 days (25 mg/kg). Animals were euthanized 5 days (A and B) and 40 days (C) after treatment to evaluate tumor regression and neuropathology. Representative photomicrographs depict tumor (Nissl staining) and immune infiltration (CD68, macrophages; major histocompatibility complex II [MHC II], antigen-presenting cells; CD8, T cells). Nissl staining in A reveals growth of tumor in both sides to appreciable sizes following saline treatment, whereas B shows very small tumor masses and C, absence of tumor, following Ad vector treatment in long-term survivors. Immunoreactivity to immune cells appears to be qualitatively similar in both sides following saline treatment, whereas there is intense immunoreactivity in the side injected with Ad vector in comparison to the contralateral side at day 9 after tumor cell implantation (5 days posttreatment). Immune cells could still be readily detected at 44 days after tumor implantation (40 days posttreatment). Scale bars = 1,000 μ m (top A, B, and C panels) and 250 μ m (photomicrographs in lower panels). Arrows indicate side of treatment.
Discussion
Attempts at brain tumor therapy through the induction of an antitumor immune response have been marred by their low translational efficacy in humans.55–58 Human tumors are spontaneous and slow growing, whereas preclinical models rely on implantation of syngeneic or allogeneic tumor cells into syngeneic or immunocompromised rodents, which results in death within weeks after tumor implantation. In order to increase the clinical translational efficacy of preclinical trials, testing of novel therapeutics should be performed in models that mimic characteristics of human GBM. To model the disseminated nature of human GBM, we examined the efficacy of AdFlt3L and AdTK in eradicating intracranial, syngeneic, CNS-1 tumors implanted in two sites, when the treatment is administered only to the primary tumor mass.
We previously demonstrated that alterations to the local brain tumor microenvironment by TK-mediated tumor cell killing and Flt3L-induced recruitment of antitumor macrophages and CD4 cells30 are critical to mounting an effective antitumor response. In the CNS-1 syngeneic intracranial tumor model of glioma, untreated or saline-treated animals begin to succumb to tumor as early as 15 days after tumor implantation.29,30,35 Animals succumb to the disease because of compression of the brainstem caused by the growing tumor. When the combination of AdFlt3L and AdTK is used to treat intracranial syngeneic GBM in Lewis rats, the efficacy of the treatment decreases with increased tumor burden. If a single large tumor is treated 6 days postimplantation, 100% of the animals survive up to 2 months; if treatment is delivered 10 days postimplantation, the treatment rescues around 70% of the tumor-bearing rats.30 By implanting a second tumor on day 10 postimplantation and delivering the therapeutic vectors only into the original tumor, we have increased tumor burden at the time of treatment, which could reduce treatment efficacy. Also, the host immune system plays a critical role in eliminating the tumor, establishing a battle between activating an effective antitumor immune response and tumor progression.30
Our treatment is effective in approximately 80% of the treated, multifocal GBM-bearing animals in which both tumors were implanted at the same time and only one tumor mass was treated 4 days after tumor implantation. In the multifocal tumor model in which the secondary tumor was implanted 10 days after implantation of the primary tumor and at the time of treatment, the efficacy of the combined therapy is approximately 50%. The delayed death of some of the tumor-bearing, treated animals attests to the effects of the combined therapy in inducing a delay in tumor growth. This is a result of individual variability in the therapeutic responses, as was observed by us and other groups implementing anti-GBM therapies in vivo.29,30,59–64
Elimination of secondary untreated tumors located in a different area of the brain could have relevance for a wider application of AdFlt3L and AdTK in brain cancer treatment. Our results could have implications for leptomeningeal tumors and tumors metastasizing to the brain from primary lung, breast, and melanoma cancers,65–67 as well as tumors that often disseminate through the cerebrospinal fluid (CSF), such as those seen in pediatric medulloblastoma.68 Novel strategies for these cancers have utilized replication-deficient adenovirus,69,70 replication-competent reovirus,68,71 herpes simplex virus,6–10,72 and immunotoxin73 approaches to slow but not eliminate tumor growth at therapeutically efficacious levels. Only the use of a replication-competent herpes simplex virus vector resulted in long-term survival in a 9L model of disseminated glioma.8,9 Similar effects have been observed in multifocal tumor models outside of the CNS, including models of melanoma, colorectal carcinoma, prostate cancer, and gallbladder carcinoma, where treatment with replication-competent herpes simplex virus resulted in an antitumor immune response to eliminate tumors never directly transduced by virus.74–77
Multifocal brain tumor models are preclinically useful in at least two contexts: to develop novel treatments that address the disseminated nature of gliomas,6–9,78 and to treat metastatic brain tumors.10 The majority of studies to date have utilized oncolytic replication-competent herpes simplex virus vectors to target multiple tumor foci in the brain6,7,10 or the brain and CSF.8,9,78 In models of disseminated disease, treatment using intraarterial delivery of oncolytic viruses is more effective in short-term immunosuppressive environments where innate immune responses to virus have been disabled.6,7,10 We have demonstrated that the antitumor effects mediated by expression of Flt3L in combination with TK plus GCV30 within the intracranial brain tumor is able to eliminate a distant tumor mass and prolong the survival of the tumor-bearing immune-competent rodents.
Regardless of tumor treatment, CNS-1 tumors such as human GBMs are highly infiltrated with macrophages. AdFlt3L/AdTK-mediated tumor regression requires both macrophages and CD4 cells.30 Whether the macrophages critical for tumor elimination are new infiltrating cells or are resident cells that shift phenotypically depending on the microenvironment of the tumor remains to be determined. The high levels of survival with AdFlt3L and AdTK suggest a synergy between altered microenvironment and systemic immune activation that eliminates the tumor. While AdTK alone has been shown to trigger immune activation preclinically,79–83 in clinical trials the strength of AdTK alone was insufficient to provide more than modest survival improvements.55,57 AdFlt3L alone has been shown to induce influx of IFNα-secreting plasmacytoid DCs into the brain19 and improves survival in small brain tumor models.29 The combination therapy of AdFlt3L with AdTK is effective against large, intracranial syngeneic GBMs,30 providing very long-term survival. Importantly, long-term survivors do not exhibit any noticeable neuropathological changes beyond ventriculomegaly. We conclude that this ventriculomegaly would be a consequence of tumor regression in response to the treatment rather than induced by the combined Ad delivery and therapeutic transgene expression, since non-tumor-bearing animals receiving AdFlt3L and AdTK and treated with GCV did not exhibit any structural or neuropathological changes. This indicates that this treatment, which upon GCV administration induces death only of actively dividing cells, does not induce damage to the normal brain parenchyma.
In summary, we have demonstrated that treatment with AdFlt3L and AdTK/GCV can induce tumor regression of the primary, treated tumor mass and also of tumor foci growing at distant sites in the contralateral hemisphere without any long-term neuropathological adverse side effects. These results suggest that this gene therapeutic approach would be effective in eliminating cells that disseminate from the main tumor mass throughout the normal brain parenchyma, and provide a strong rationale for further developing these therapeutic targets in order to implement this approach in a phase I clinical trial for GBM.
Acknowledgments
We thank Dr. Shlomo Melmed for his academic leadership and Mr. Richard Katzman and Dr. David Meyer for their support. Work described herein was funded by the National Institute of Neurological Disorders and Stroke (NINDS), grants RO1 NS4456.01, RO3 TW006273-01, and R21 NS054143-01 to M.G.C. and grants RO1 NS42893, U54 4 NS04-5309, and R21 NS47298 to P.R.L.; the Linda Tallen & David Paul Kane Annual Fellowship (PRL); the Bram and Elaine Goldsmith Endowed Chair to P.R.L.; the Medallions Group Endowed Chair to M.G.C.; and the Board of Governors at Cedars-Sinai Medical Center. G.D.K. is supported by NINDS F32 NS0503034-01.
References
- 1.Barnard RO, Geddes JF. The incidence of multifocal cerebral gliomas: a histologic study of large hemisphere sections. Cancer. 1987;60:1519–1531. doi: 10.1002/1097-0142(19871001)60:7<1519::aid-cncr2820600719>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Silbergeld DL, Rostomily RC, Alvord EC., Jr The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10:179–185. doi: 10.1007/BF00146880. [DOI] [PubMed] [Google Scholar]
- 3.Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 4.CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2004. [Google Scholar]
- 5.Giese A, Bjerkvig R, Berens ME, et al. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramm CM, Rainov NG, Sena-Esteves M, et al. Long-term survival in a rodent model of disseminated brain tumors by combined intrathecal delivery of herpes vectors and ganciclovir treatment. Hum Gene Ther. 1996;7:1989–1994. doi: 10.1089/hum.1996.7.16-1989. [DOI] [PubMed] [Google Scholar]
- 9.Kramm CM, Rainov NG, Sena-Esteves M, et al. Herpes vector- mediated delivery of marker genes to disseminated central nervous system tumors. Hum Gene Ther. 1996;7:291–300. doi: 10.1089/hum.1996.7.3-291. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Martuza RL, Rabkin SD. Intracarotid delivery of oncolytic HSV vector G47Delta to metastatic breast cancer in the brain. Gene Ther. 2005;12:647–654. doi: 10.1038/sj.gt.3302445. [DOI] [PubMed] [Google Scholar]
- 11.Furumoto K, Soares L, Engleman EG, et al. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774–783. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 13.Lowenstein PR. Dendritic cells and immune responses in the central nervous system. Trends Immunol. 2002;23:70. doi: 10.1016/s1471-4906(01)02151-2. [DOI] [PubMed] [Google Scholar]
- 14.Broder H, Anderson A, Kremen TJ, et al. MART-1 adenovirus-transduced dendritic cell immunization in a murine model of metastatic central nervous system tumor. J Neurooncol. 2003;64:21–30. doi: 10.1007/BF02700017. [DOI] [PubMed] [Google Scholar]
- 15.Liau LM, Black KL, Prins RM, et al. Treatment of intracranial gliomas with bone marrow–derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90:1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 16.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka R, Yajima N, Abe T, et al. Dendritic cell–based glioma immunotherapy (review) Int J Oncol. 2003;23:5–15. [PubMed] [Google Scholar]
- 18.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate–pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 19.Curtin JF, King GD, Barcia C, et al. FMS-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand–treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shurin MR, Pandharipande PP, Zorina TD, et al. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 22.Shaw SG, Maung AA, Steptoe RJ, et al. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161:2817–2824. [PubMed] [Google Scholar]
- 23.Averbook BJ, Schuh JL, Papay R, et al. Antitumor effects of Flt3 ligand in transplanted murine tumor models. J Immunother. 2002;25:27–35. doi: 10.1097/00002371-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Chen K, Braun S, Lyman S, et al. Antitumor activity and immunotherapeutic properties of Flt3-ligand in a murine breast cancer model. Cancer Res. 1997;57:3511–3516. [PubMed] [Google Scholar]
- 25.Wang A, Braun SE, Sonpavde G, et al. Antileukemic activity of Flt3 ligand in murine leukemia. Cancer Res. 2000;60:1895–1900. [PubMed] [Google Scholar]
- 26.Borges L, Miller RE, Jones J, et al. Synergistic action of FMS-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol. 1999;163:1289–1297. [PubMed] [Google Scholar]
- 27.Lynch DH, Andreasen A, Maraskovsky E, et al. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 28.Peron JM, Esche C, Subbotin VM, et al. FLT3-ligand administration inhibits liver metastases: role of NK cells. J Immunol. 1998;161:6164–6170. [PubMed] [Google Scholar]
- 29.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble FMS-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Candolfi M, Curtin JF, Xiong WD, et al. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol Ther. 2006;14:371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maleniak TC, Darling JL, Lowenstein PR, et al. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Ther. 2001;8:589–598. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- 33.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–48. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southgate T, Kingston P, Castro MG. Gene transfer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. New York: John Wiley; 2000. pp. 4.23.1–4.23.40. [Google Scholar]
- 35.Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 36.Thomas CE, Schiedner G, Kochanek S, et al. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 39.Cotten M, Baker A, Saltik M, et al. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–246. [PubMed] [Google Scholar]
- 40.Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. E vidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 41.Puntel M, Curtin JF, Zirger JM, et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther. 2006;17:531–544. doi: 10.1089/hum.2006.17.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowenstein PR, Thomas CE, Umana P, et al. High-capacity, helper-dependent, “gutless” adenoviral vectors for gene transfer into brain. Methods Enzymol. 2002;346:292–311. doi: 10.1016/s0076-6879(02)46062-4. [DOI] [PubMed] [Google Scholar]
- 43.Umana P, Gerdes CA, Stone D, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol. 2001;19:582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- 44.Candolfi M, Kroeger KM, Pluhar GE, et al. Adenoviral-mediated gene transfer into the canine brain in vivo. Neurosurgery. 2007;60:167–178. doi: 10.1227/01.NEU.0000249210.89096.6C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Candolfi M, Pluhar GE, Kroeger KM, et al. Optimization of adenoviral vector mediated expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro-oncology. 2007;9:245–58. doi: 10.1215/15228517-2007-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simek S, Byrnes A, Bauer S. FDA perspective on the use of the adenovirus reference material. Bioprocessing. 2002;31:40–42. [Google Scholar]
- 47.Brunetti-Pierri N, Palmer DJ, Beaudet AL, et al. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 48.Muruve DA, Barnes MJ, Stillman IE, et al. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 49.Muruve DA, Cotter MJ, Zaiss AK, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnell MA, Zhang Y, Tazelaar J, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Chirmule N, Gao GP, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, Cai W. Effect of vimentin on reactive gliosis: in vitro and in vivo analysis. J Neurotrauma. 2004;21:1671–1682. doi: 10.1089/neu.2004.21.1671. [DOI] [PubMed] [Google Scholar]
- 53.Pekny M, Johansson CB, Eliasson C, et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelmsson U, Li L, Pekna M, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Immonen A, Vapalahti M, Tyynela K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Kikuchi T, Akasaki Y, Irie M, et al. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 58.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 59.El Andaloussi A, Sonabend AM, Han Y, et al. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54:526–535. doi: 10.1002/glia.20401. [DOI] [PubMed] [Google Scholar]
- 60.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 mono-clonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 61.Heimberger AB, Crotty LE, Archer GE, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103:16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 62.Jiang H, Gomez-Manzano C, Alemany R, et al. Comparative effect of oncolytic adenoviruses with E1A-55 kDa or E1B-55 kDa deletions in malignant gliomas. Neoplasia. 2005;7:48–56. doi: 10.1593/neo.04391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 64.Kuwashima N, Nishimura F, Eguchi J, et al. Delivery of dendritic cells engineered to secrete IFN-alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol. 2005;175:2730–2740. doi: 10.4049/jimmunol.175.4.2730. [DOI] [PubMed] [Google Scholar]
- 65.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 66.Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 67.Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 68.Yang WQ, Senger D, Muzik H, et al. Reovirus prolongs survival and reduces the frequency of spinal and leptomeningeal metastases from medulloblastoma. Cancer Res. 2003;63:3162–3172. [PubMed] [Google Scholar]
- 69.Colak A, Goodman JC, Chen SH, et al. Adenovirus-mediated gene therapy in an experimental model of breast cancer metastatic to the brain. Hum Gene Ther. 1995;6:1317–1322. doi: 10.1089/hum.1995.6.10-1317. [DOI] [PubMed] [Google Scholar]
- 70.Vincent AJ, Esandi MD, van Someren G, et al. Treatment of leptomeningeal metastases in a rat model using a recombinant adenovirus containing the HSV-tk gene. J Neurosurg. 1996;85:648–654. doi: 10.3171/jns.1996.85.4.0648. [DOI] [PubMed] [Google Scholar]
- 71.Yang WQ, Senger DL, Lun XQ, et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- 72.Toda M, Rabkin SD, Martuza RL. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 73.Urch CE, George AJ, Stevenson GT, et al. Intra-thecal treatment of leptomeningeal lymphoma with immunotoxin. Int J Cancer. 1991;47:909–915. doi: 10.1002/ijc.2910470621. [DOI] [PubMed] [Google Scholar]
- 74.Endo T, Toda M, Watanabe M, et al. In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer. Cancer Gene Ther. 2002;9:142–148. doi: 10.1038/sj.cgt.7700407. [DOI] [PubMed] [Google Scholar]
- 75.Nakano K, Todo T, Chijiiwa K, et al. Therapeutic efficacy of G207, a conditionally replicating herpes simplex virus type 1 mutant, for gallbladder carcinoma in immunocompetent hamsters. Mol Ther. 2001;3:431–437. doi: 10.1006/mthe.2001.0303. [DOI] [PubMed] [Google Scholar]
- 76.Toda M, Martuza RL, Rabkin SD. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol Ther. 2000;2:324–329. doi: 10.1006/mthe.2000.0130. [DOI] [PubMed] [Google Scholar]
- 77.Toda M, Rabkin SD, Kojima H, et al. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 78.Rewers AB, Redgate ES, Deutsch M, et al. A new rat brain tumor model: glioma disseminated via the cerebral spinal fluid pathways. J Neurooncol. 1990;8:213–219. doi: 10.1007/BF00177354. [DOI] [PubMed] [Google Scholar]
- 79.Bi W, Kim YG, Feliciano ES, et al. An HSVtk-mediated local and distant antitumor bystander effect in tumors of head and neck origin in athymic mice. Cancer Gene Ther. 1997;4:246–252. [PubMed] [Google Scholar]
- 80.Dilber MS, Abedi MR, Bjorkstrand B, et al. Suicide gene therapy for plasma cell tumors. Blood. 1996;88:2192–2200. [PubMed] [Google Scholar]
- 81.Perez-Cruet MJ, Trask TW, Chen SH, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39:506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- 82.Vile RG, Castleden S, Marshall J, et al. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intra-tumoural cytokine expression. Int J Cancer. 1997;71:267–274. doi: 10.1002/(sici)1097-0215(19970410)71:2<267::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 83.Wilson KM, Stambrook PJ, Bi WL, et al. HSV-tk gene therapy in head and neck squamous cell carcinoma. Enhancement by the local and distant bystander effect. Arch Otolaryngol Head Neck Surg. 1996;122:746–749. doi: 10.1001/archotol.1996.01890190042011. [DOI] [PubMed] [Google Scholar]