Abstract
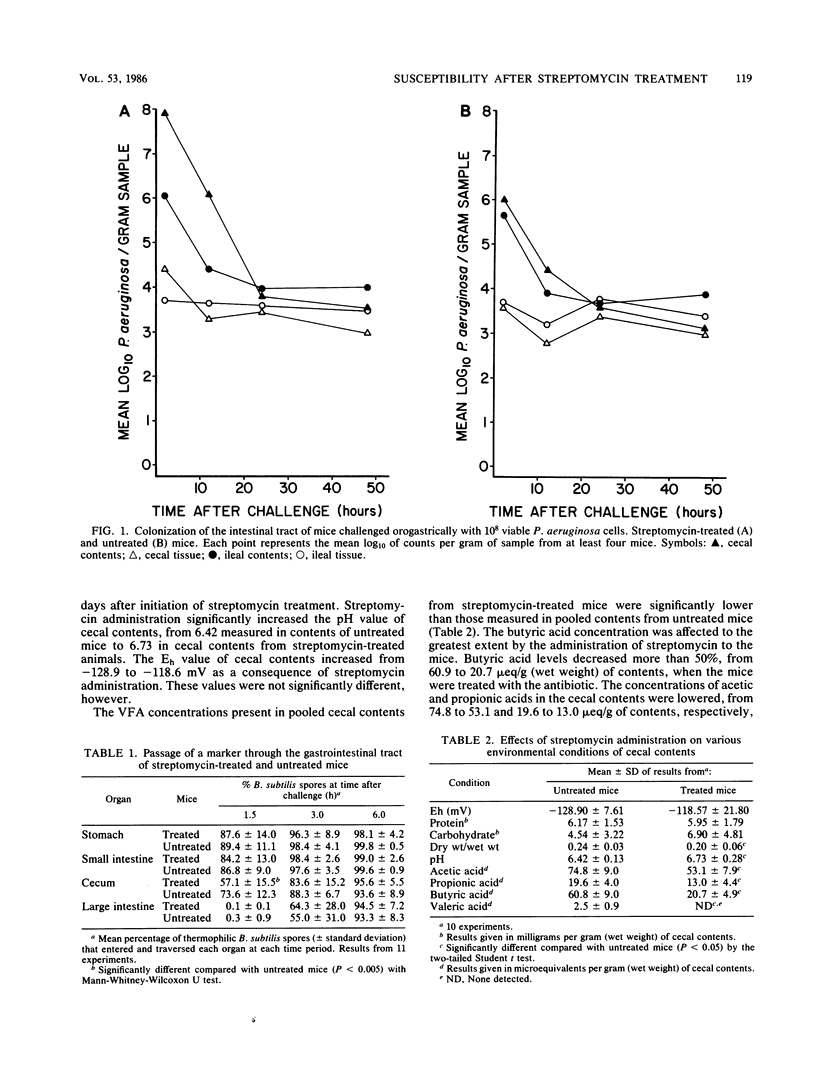
Streptomycin sulfate (5 mg/ml) was added to the drinking water of Swiss white mice. After treatment for 1 week, the mice were challenged orogastrically with 10(8) Pseudomonas aeruginosa cells. The organism failed to multiply in the intestinal tract of either treated or untreated animals, but could be recovered from contents and tissues after 48 h. In a previous study, Salmonella typhimurium was shown to multiply in the intestines of streptomycin-treated but not untreated mice when 10(3) organisms were used as inoculum. Streptomycin administration had little effect on Eh, protein or carbohydrate concentrations of cecal contents, or intestinal motility. However, it caused a statistically significant increase in water content and pH of contents and a decrease in the concentrations of acetic, propionic, butyric, and valeric acids. S. typhimurium multiplied in pooled cecal contents obtained from both streptomycin-treated and untreated animals, but its multiplication rate and total populations were significantly greater in contents from treated animals. P. aeruginosa did not multiply in contents from either treated or untreated mice. Similar results were obtained when the organisms were inoculated into nutrient broth adjusted to simulate the pH levels and volatile fatty acid (VFA) concentrations in cecal contents of treated and untreated mice. The addition of brain heart infusion broth to cecal contents from untreated animals, in concentrations that support multiplication of S. typhimurium and P. aeruginosa, did not reverse inhibition. The addition of VFA to cecal contents from treated animals to equal the concentration in cecal contents from untreated animals caused inhibition of a magnitude observed in cecal contents from untreated animals. The results indicate that VFA operating at the pH level of cecal contents of conventional mice inhibit the multiplication of both S. typhimurium and P. aeruginosa and restrict colonization of the intestine by these organisms. The decrease in VFA concentrations that occurs as a result of streptomycin administration adequately explains the increased susceptibility of treated mice to colonization with S. typhimurium. It does not explain the increased susceptibility of treated mice to P. aeruginosa colonization, however.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., DRAKE B. L., MILLER C. P. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954 May;86(1):132–137. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. I. FACTORS WHICH INTERFERE WITH THE INITIATION OF INFECTION BY ORAL INOCULATION. J Exp Med. 1964 Nov 1;120:805–816. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. II. FACTORS RESPONSIBLE FOR ITS LOSS FOLLOWING STREPTOMYCIN TREATMENT. J Exp Med. 1964 Nov 1;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel D. J., Aly R., Bayles C., Strauss W. G., Shinefield H. R., Maibach H. I. Competitive adherence as a mechanism of bacterial interference. Can J Microbiol. 1983 Jun;29(6):700–703. doi: 10.1139/m83-114. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- Davidson J. N., Hirsh D. C. Use of the K88 antigen for in vivo bacterial competition with porcine strains of enteropathogenic Escherichia coli. Infect Immun. 1975 Jul;12(1):134–136. doi: 10.1128/iai.12.1.134-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau R., Bellier M., Raibaud P. Transit Digestif de Divers Inoculums Bactériens Introduits "Per Os" Chez des Souris Axéniques et "Holoxéniques" (Conventionnelles): Effet Antagoniste de la Microflore du Tractus Gastro-Intestinal. Zentralbl Bakteriol Orig. 1970 May;213(4):533–548. [PubMed] [Google Scholar]
- FRETER R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J Exp Med. 1956 Sep 1;104(3):411–418. doi: 10.1084/jem.104.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R. The fatal enteric cholera infection in the guinea pig, achieved by inhibition of normal enteric flora. J Infect Dis. 1955 Jul-Aug;97(1):57–65. doi: 10.1093/infdis/97.1.57. [DOI] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J. Enteric pathogen--normal flora interactions. Am J Clin Nutr. 1970 Nov;23(11):1451–1456. doi: 10.1093/ajcn/23.11.1451. [DOI] [PubMed] [Google Scholar]
- Hentges D. J., Stein A. J., Casey S. W., Que J. U. Protective role of intestinal flora against infection with Pseudomonas aeruginosa in mice: influence of antibiotics on colonization resistance. Infect Immun. 1985 Jan;47(1):118–122. doi: 10.1128/iai.47.1.118-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikari N. S., Kenton D. M., Young V. M. Interaction in the germfree mouse intestine of colicinogenic and colicin-sensitive microorganisms. Proc Soc Exp Biol Med. 1969 Apr;130(4):1280–1284. doi: 10.3181/00379727-130-33773. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- Que J. U., Hentges D. J. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985 Apr;48(1):169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D. Role of volatile fatty acids in colonization resistance to Clostridium difficile. Infect Immun. 1984 Jul;45(1):185–191. doi: 10.1128/iai.45.1.185-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]


