Abstract
Reliable data on large cohorts of patients with glioblastoma are needed because such studies differ importantly from trials that have a strong bias toward the recruitment of younger patients with a higher performance status. We analyzed the outcome of 676 patients with histologically confirmed newly diagnosed glioblastoma who were treated consecutively at a single institution over a 7-year period (1997 – 2003) with follow-up to April 30, 2006. Survival probabilities were 57% at 1 year, 16% at 2 years, and 7% at 3 years. Progression-free survival was 15% at 1 year. Prolongation of survival was significantly associated with surgery in patients with a good performance status, whatever the patient’s age, with an adjusted hazard ratio of 0.55 (p < 0.001) or a 45% relative decrease in the risk of death. Radiotherapy and chemotherapy improved survival, with adjusted hazard ratios of 0.61 (p = 0.001) and 0.89 (p = 0.04), respectively, regardless of age, performance status, or residual tumor volume. Recurrence occurred in 99% of patients throughout the follow-up. Reoperation was performed in one-fourth of these patients but was not effective, whether performed within 9 months (hazard ratio, 0.86; p = 0.256) or after 9 months (hazard ratio, 0.98; p = 0.860) of initial surgery, whereas second-line chemotherapy with procarbazine, lomustine, and vincristine (PCV) or with temozolomide improved survival (hazard ratio, 0.77; p = 0.008). Surgery followed by radiotherapy and chemotherapy should be considered in all patients with glioblastoma, and these treatments should not be withheld because of increasing age alone. The benefit of second surgery at recurrence is uncertain, and new trials are needed to assess its effectiveness. Chemotherapy with PCV or temozolomide seems to be a reasonable option at tumor recurrence.
Keywords: chemotherapy, elderly, glioblastoma, radiotherapy, surgery, survival analysis
Glioblastoma multiforme is the most common primary malignant brain tumor, accounting for 50% – 60% of all intracranial gliomas and carrying one of the worst prognoses of all cancers. Primary (de novo) glioblastoma develops rapidly and without evidence of less malignant precursor lesions, typically in older patients. Secondary glioblastoma develops more slowly, by progression from low-grade or anaplastic astrocytoma, in middle-age patients.1
Current treatment of glioblastoma is usually surgical resection followed by radiotherapy and chemotherapy.2 Radiotherapy has proven to be effective in several randomized studies,3,4 whereas the effectiveness of surgical resection remains uncertain, although decompression is important in symptomatic patients.5–9 Nitrosourea-based chemotherapy in addition to postoperative radiotherapy improved median survival by 2 months (from 10 to 12 months) in a meta-analysis of individual data from 12 randomized studies.10 More recently, the European Organisation for Research and Treatment of Cancer (EORTC) 26981 study, which combined radiotherapy with oral temozolomide, reported a median survival benefit of 2.5 months (from 12.1 to 14.6 months).11
Most randomized studies of radiotherapy and chemotherapy have involved young patients or those with good performance status and less unfavorable prognosis. The benefit of radiotherapy has not been established in patients older than 70 years, and there is no conclusive evidence of survival benefit with chemotherapy in elderly patients or those with poor performance status.12,13 In the absence of experimental evidence from randomized studies, prospective cohort studies can produce useful information on the effectiveness and morbidity of surgical resection and adjuvant treatments in elderly and poor-prognosis patients, who form the majority of those who develop primary glioblastoma.14,15
The referral-based longitudinal Brain Cancer Register of the Fondazione I.R.C.C.S. Istituto Neurologico “Carlo Besta” in Milan, Italy, has been collecting comprehensive information for all patients presenting at the institute with a malignant or benign tumor of the nervous system since 1997. The information we collect is used in research into the causes of such tumors, in education and information programs, and in the planning of a strategy to deliver the best cancer care to patients. We report here the results of our experience in treating patients who present with primary glioblastoma, because the best management of such patients remains problematic. We sought to assess the role of patient characteristics, surgery, and adjuvant treatments in the prediction of overall survival and progression-free survival in these patients. Such findings will enable decision making on the basis of the risk of treatment compared with the benefit of improving survival at the first diagnosis of glioblastoma.
Materials and Methods
Patients
All consecutive patients older than 16 years with histologically confirmed primary glioblastoma (WHO grade IV astrocytoma),16 newly diagnosed between January 1997 and December 2003, were included and followed up to April 30, 2006. Secondary glioblastoma patients with previous histopathological or radiological diagnoses of low-grade or anaplastic astrocytoma (WHO grade II or III astrocytoma) were excluded, as were cases without histological verification. Pathological diagnosis was performed by two neuropathologists at our institute in agreement with WHO guidelines.16 All patients gave written consent to surgery or chemoradiotherapy. Each hospitalized patient admitted to our institute was asked for written consent for processing of his or her data for research purposes by health professionals subject to professional secrecy.
Outcomes
Clinical and radiological tumor progression was assessed at regular intervals at our institution, from the time of first surgery throughout the follow-up. Death certificates were collected at municipal offices yearly. Survival was defined as the time from first surgery to death or until April 30, 2006. Progression-free survival was defined as the time from first surgery to first evidence of tumor progression on CT or MRI or to death.17 Tumor progression was defined as the appearance of new lesions, an increase in tumor extension by 25% on CT or MRI, a worsening in the clinical/neurological condition, or an increased need for corticosteroids.18
Prognostic Variables
Patient characteristics at diagnosis included sex, age, preoperative KPS (assessed on the day before surgery), and tumor extension. The extent of surgical resection was determined by comparison of postoperative images obtained up to 48 h (CT) or 72 h (MRI) after surgery with the latest preoperative images. If CT or MRI was performed later than 48 or 72 h, respectively, debulking surgery was classified as of “undefined extent.”
Statistical Analysis
The completeness of follow-up was quantified according to the “completeness index.”19 Survival and progression- free survival were estimated by the Kaplan-Meier method, and pointwise confidence intervals (CI) were based on the Greenwood estimate of the SEM. The log-rank test was used to compare survival by sex, age at diagnosis (16 – 50, 51 – 65, > 65 years), preoperative KPS ( ≥ 70, > 70), tumor extension (single lobe or multiple lobes), and first surgery (surgical resection, biopsy only). Relevant clinical factors were entered into multivariable Cox proportional-hazards models to predict overall survival and progression-free survival; these factors were sex, age, KPS, tumor extension, surgery, radiotherapy, chemotherapy, and second surgery. A multivariable model to predict survival after tumor progression was generated that also incorporated tumor extension at progression and second-line chemotherapy in addition to the factors in the previous models.
Radiotherapy, chemotherapy, second surgery, and second-line chemotherapy were included in the Cox models as time-dependent covariates.20
Interaction terms (particularly age or KPS and surgery, radiotherapy, or chemotherapy interactions) in predicting survival were tested in the multivariable models. We regarded p values less than 0.05 as statistically significant.
Results
Patients
The study included 676 consecutive cases of primary glioblastoma. Most patients (623 cases, 92%) received histological diagnosis within 3 months of their first diagnostic CT or MRI, and the remaining 53 cases within 4 – 35 months. Survival status was verified for all patients, and the completeness index of follow-up was 100%. The reverse Kaplan-Meier median follow-up was 69.1 months (95% CI, 52.0 – 86.1). Nineteen patients were alive at the end of the study, and their follow-up time ranged from 28.0 to 103.2 months. The clinical characteristics of all patients are shown in Table 1. Median age was 58 years (range, 16 – 81 years), and 22% of the patients were older than 65 years. The male-to-female ratio was 1.6. In 389 (57.5%) cases, the tumor occurred in a single lobe (temporal in 24%, frontal in 19.5%, parietal in 11%, other in 2.5%); in the remaining cases, it extended across multiple lobes. Most patients (594, 88%) underwent surgical resection, and 82 (12%) underwent biopsy only; median age was similar in the two groups (58.0 years, range 16 – 81 years, vs. 55.5 years, range 21 – 78 years). Almost all patients (668, 99%) received perioperative corticosteroids. Precise evaluation of residual tumor volume by CT or MRI performed within 72 h after surgical intervention was obtained for 355 (60%) of the 594 patients who underwent resection. Information on radiotherapy was available in 635 (94%); of these, 546 (86%) received postoperative irradiation according to this protocol: focal external beam radiation therapy of 60 Gy (split in 1.8 – 2 Gy daily fractions) to the enhancing portion of the tumor and within a 2 – 3 cm margin. Information on whether or not chemotherapy was given was available in 648 cases (96%); of these, 505 (78%) received chemotherapy, and 472 (73%) were given concomitant chemotherapy and radiotherapy. The most widely used chemotherapy agents were carmustine, lomustine, and cisplatin administered soon after surgery.21 Delay between surgery and chemoradiotherapy ranged from 2 to 6 weeks. At progression, patients were considered for reoperation and/or second-line chemotherapy consisting of procarbazine, lomustine, and vincristine (PCV)22 or oral temozolomide.23
Table 1.
Clinical characteristics of the 676 patients at baseline
| Characteristic | No. of Patients (%) |
|---|---|
| Year of diagnosis | |
| 1997 | 79 (11.7) |
| 1998 | 84 (12.4) |
| 1999 | 82 (12.1) |
| 2000 | 105 (15.5) |
| 2001 | 117 (17.3) |
| 2002 | 99 (14.6) |
| 2003 | 110 (16.3) |
| Sex | |
| Male | 418 (61.8) |
| Female | 258 (38.2) |
| Age (years) | |
| ≥ 50 | 193 (28.6) |
| 51 – 65 | 336 (49.7) |
| > 65 | 147 (21.7) |
| KPSa | |
| ≥ 70 | 213 (31.5) |
| > 70 | 429 (63.5) |
| Tumor extensionb | |
| Frontal (C71.1) | 132 (19.5) |
| Temporal (C71.2) | 162 (24.0) |
| Parietal (C71.3) | 72 (10.7) |
| Occipital (C71.4) | 6 (0.9) |
| Other single site (C71.0, C71.5 – 71.7, C71.9) | 17 (2.5) |
| Multiple sites (C71.8) | 287 (42.5) |
| Surgeryc | |
| Biopsy only | 82 (12.1) |
| Surgical resection | 594 (87.9) |
| Gross total | 50 (7.4) |
| Partial | 120 (17.8) |
| Undefined extent | 424 (62.7) |
Missing information for 34 patients.
Assessed by first CT or MRI and coded according to the International Classification of Diseases for Oncology location code.
Determined by comparison of postoperative images obtained up to 48 h (CT) or 72 h (MRI) after surgery with the latest preoperative images.
Survival
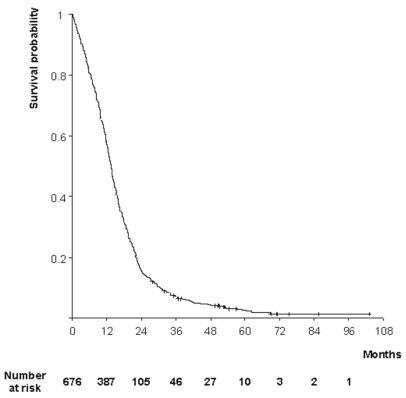
The estimate of overall survival is shown in Fig. 1. A total of 657 patients (97.2%) died during the follow-up. Median survival was 13.6 months (95% CI, 12.9 – 14.3), and survival probabilities were 57% (95% CI, 54 – 61%) at 1 year, 16% (95% CI, 13 – 18%) at 2 years, and 7% (95% CI, 5 – 9%) at 3 years.
Fig. 1.
Kaplan-Meier estimates of overall survival in patients with primary glioblastoma.
Table 2 shows the variables included in the survival analyses with the corresponding univariate log-rank tests estimated by the Kaplan-Meier method and hazard ratios for death estimated by a multivariable Cox proportional-hazards model. On multivariable analysis, the independent clinical prognostic factors of overall survival included patient age (p < 0.001), preoperative performance status (p < 0.001), and tumor extension (p = 0.023).
Table 2.
Factors affecting overall survival in patients with primary glioblastoma
| Kaplan-Meier Univariable Analysis (n = 676) | Multivariable Cox Proportional-Hazard Model (n = 598) | |||||
|---|---|---|---|---|---|---|
| Factor | No. of Events/ No. of Patients | Median Overall Survival, in Months (95% Confidence Interval) | p | No. of Events/ No. of Patientsa | Hazard Ratio (95% Confidence Interval) | p |
| Sex | ||||||
| Male | 407/418 | 13.6 (12.6 – 14.6) | 364/374 | 1 | ||
| Female | 250/258 | 13.6 (12.4 – 14.8) | 0.667 | 216/224 | 0.90 (0.76 – 1.08) | 0.261 |
| Age (years)b | ||||||
| ≥ 50 | 179/193 | 17.8 (15.9 – 19.7) | — | 1.03 (1.02 – 1.04) | < 0.001 | |
| 51 – 65 | 331/336 | 13.6 (12.9 – 14.3) | ||||
| > 65 | 147/147 | 9.6 (8.3 – 10.8) | < 0.001 | |||
| KPS | ||||||
| ≥ 70 | 211/213 | 9.9 (8.2 – 11.5) | 186/188 | 1 | ||
| > 70 | 413/429 | 14.7 (13.7 – 15.7) | < 0.001 | 394/410 | 0.66 (0.55 – 0.79) | < 0.001 |
| Tumor extension | ||||||
| Multiple lobes | 277/287 | 11.6 (10.5 – 12.7) | 242/251 | 1 | ||
| Single lobe | 380/389 | 15.1 (14.1 – 16.1) | < 0.001 | 338/347 | 0.82 (0.69 – 0.97) | 0.023 |
| Surgery | ||||||
| Biopsy only | 81/82 | 6.3 (4.8 – 7.9) | 70/71 | 1 | ||
| Surgical resection | 576/594 | 14.1 (13.2 – 15.0) | < 0.001 | 510/527 | 0.55 (0.42 – 0.72) | < 0.001 |
| Chemotherapyc | ||||||
| No | — | — | 130/130 | 1 | ||
| Yes | 450/468 | 0.89 (0.72 – 0.99) | 0.04 | |||
| Radiotherapyc | ||||||
| No | — | — | 73/73 | 1 | ||
| Yes | 507/525 | 0.61 (0.45 – 0.83) | 0.001 | |||
Patients with any missing value were excluded from multivariable analysis.
Age was used as a continuous variable in the Cox models.
Variables were included as time-dependent covariates.
The hazard ratio for death in patients who had undergone surgical resection versus those who had undergone biopsy only was 0.55 (95% CI, 0.42 – 0.72; p < 0.001), a 45% relative reduction in the risk of death, after adjustment for clinical factors and postoperative treatments. This effect is equivalent to an 8-month increase in median survival time; an absolute increase in 1-year survival of 29% (95% CI, 18 – 40), from 32% to 61%; and an absolute increase in 2-year survival of 12% (95% CI, 7 – 18), from 5% to 17%. When extent of resection was considered, the longest median survival (15.2 months; 95% CI, 13.4 – 18.0) was observed among patients who had undergone extensive resection, compared with 11.2 months (95% CI, 9.4 – 13.1) among those who had undergone partial resection. Kaplan-Meier estimates of survival in these two subgroups were significantly different (p = 0.006 by the log-rank test).
We found a significant interaction (p = 0.01) between the effect of surgery and preoperative performance status with respect to overall survival. Patients with KPS greater than 70 benefited from surgery (hazard ratio, 0.42; 95% CI, 0.26 – 0.66; p = 0.02); in contrast, those with a score less than 70 received no significant survival benefit (hazard ratio, 0.85; 95% CI, 0.58 – 1.39). The median survival was 15.7 months (95% CI, 14.3 – 17.6) among those with a score greater than 70 and 11.4 months (95% CI, 9.7 – 12.9) among those with a score less than 70, with 1-year survival rates of 66% and 43%, respectively.
There was no evidence of interaction between the effect of surgery and the patient’s age (p = 0.43).
Radiotherapy improved survival, with a one-third (hazard ratio, 0.61; 95% CI, 0.45 – 0.83; p = 0.001) relative reduction of the risk of dying. A significant but smaller effect was also observed for chemotherapy (hazard ratio, 0.89; 95% CI, 0.72 – 0.99; p = 0.04; Table 2). There were no interactions between the effect of radiotherapy or chemotherapy and age, performance status, or residual tumor volume.
Results were similar when the 53 patients who received their first diagnostic CT or MRI within 4 – 35 months before the histological diagnosis were excluded from the analysis.
Progression-Free Survival
Information on disease progression was available from 657 (97%) of the 676 individuals: 651 (99%) had tumor progression throughout the follow-up. Median progression-free survival was 6.0 months (95% CI, 5.5 – 6.5), and the probability was 15% (95% CI, 12 – 17%) at 1 year. On multivariable analysis, patient age, preoperative KPS, and tumor extension were independent prognostic factors for progression-free survival (Table 3).
Table 3.
Factors affecting progression-free survival in patients with primary glioblastoma
| Kaplan-Meier Univariable Analysis (n = 657) | Multivariable Cox Proportional-Hazard Model (n = 589) | |||||
|---|---|---|---|---|---|---|
| Factor | No. of Events/ No. of Patients | Median Progression- Free Survival, in Months (95% Confidence Interval) | p | No. of Events/ No. of Patientsa | Hazard Ratio (95% Confidence Interval) | p |
| Sex | ||||||
| Male | 403/407 | 5.9 (5.3 – 6.5) | 365/369 | 1 | ||
| Female | 248/250 | 6.0 (5.2 – 6.8) | 0.413 | 218/220 | 0.95 (0.80 – 1.13) | 0.552 |
| Age (years)b | ||||||
| ≥ 50 | 183/188 | 7.0 (6.4 – 7.7) | — | 1.02 (1.01 – 1.03) | < 0.001 | |
| 51 – 65 | 326/327 | 6.0 (5.3 – 6.7) | ||||
| > 65 | 142/142 | 3.9 (3.2 – 4.6) | < 0.001 | |||
| KPS | ||||||
| ≥ 70 | 203/203 | 4.0 (3.4 – 4.7) | 185/185 | 1 | ||
| > 70 | 414/420 | 6.8 (6.3 – 7.3) | < 0.001 | 398/404 | 0.63 (0.52 – 0.75) | < 0.001 |
| Tumor extension | ||||||
| Multiple lobes | 277/279 | 4.9 (4.2 – 5.6) | 245/247 | 1 | ||
| Single lobe | 374/378 | 6.5 (6.0 – 7.0) | 0.001 | 338/342 | 0.84 (0.71 – 1.00) | 0.054 |
| Surgery | ||||||
| Biopsy only | 77/77 | 3.2 (1.8 – 4.5) | 66/66 | 1 | ||
| Surgical resection | 574/580 | 6.2 (5.7 – 6.7) | < 0.001 | 517/523 | 0.63 (0.49 – 0.83) | 0.001 |
| Chemotherapyc | ||||||
| No | — | — | 127/127 | 1 | ||
| Yes | 456/462 | 0.89 (0.73 – 1.09) | 0.256 | |||
| Radiotherapyc | ||||||
| No | — | — | 72/72 | 1 | ||
| Yes | 511/517 | 0.85 (0.61 – 0.97) | 0.040 | |||
Patients with any missing value were excluded from multivariable analysis.
Age was used as a continuous variable in the Cox model.
Variables were included as time-dependent covariates.
The effect of surgery showed a pattern similar to that for survival. The hazard ratio of 0.63 (95% CI, 0.49 – 0.83; p = 0.001; Table 3) indicates a significant 37% reduction in the risk of progression or death. Median progression-free survival was increased by 3 months, from 3.2 months to 6.2 months. The effect of radiotherapy also showed a pattern similar to that for survival, with a hazard ratio of 0.85 (95% CI, 0.61 – 0.97; p = 0.04; Table 3) indicating a significant 15% reduction in the risk of progression or death and an absolute improvement in progression-free survival of 12% (95% CI, 9 – 15) at 1 year after histological diagnosis. The effect was less consistent for chemotherapy, with a hazard ratio of 0.89 (95% CI, 0.73 – 1.09; p = 0.256; Table 3).
Survival after Tumor Progression
Median survival time after progression was 6.1 months (95% CI, 5.6 – 6.6). The multivariable analysis showed no effect of reoperation on survival, whether performed within 9 months of the first surgery (hazard ratio, 0.86; 95% CI, 0.66 – 1.12; p = 0.256) or after 9 months (hazard ratio, 0.98; 95% CI, 0.77 – 1.25; p = 0.860; Table 4). Temozolomide or PCV chemotherapy in patients not initially treated with these drugs was administered as salvage or second-line treatment after disease progression to 275 (50%) of 554 patients. The hazard ratio of 0.77 (95% CI, 0.63 – 0.93; Table 4) indicated a significant (p = 0.008) reduction in the risk of death after progression for patients treated with chemotherapy compared with those who were not treated.
Table 4.
Factors affecting survival after tumor progression in patients with primary glioblastoma
| Multivariable Cox Proportional-Hazard Model (n = 544) | |||
|---|---|---|---|
| Factor | No. of Events/ No. of Patientsa | Hazard Ratio (95% Confidence Interval) | p |
| Sex | |||
| Male | 337/345 | 1 | |
| Female | 193/199 | 0.88 (0.74 – 1.06) | 0.183 |
| Ageb | — | 1.02 (1.01 – 1.03) | < 0.001 |
| Tumor extension at progression | |||
| Multiple lobes | 332/341 | 1 | |
| Single lobe | 198/203 | 0.78 (0.65 – 0.94) | 0.008 |
| First surgery | |||
| Biopsy only | 46/46 | 1 | |
| Surgical resection | 484/498 | 0.60 (0.43 – 0.82) | 0.002 |
| Chemotherapy | |||
| No | 156/159 | 1 | |
| Yes | 374/385 | 0.96 (0.79 – 1.17) | 0.667 |
| Radiotherapy | |||
| No | 69/71 | 1 | |
| Yes | 461/473 | 0.96 (0.74 – 1.25) | 0.776 |
| Second-line chemotherapyc | |||
| No | 276/279 | 1 | |
| Yes | 254/275 | 0.77 (0.63 – 0.93) | 0.008 |
| Second surgeryc | |||
| No | 357/362 | 1 | |
| Yes, within nine monthsd | 78/81 | 0.86 (0.66 – 1.12) | 0.256 |
| Yes, after nine monthsd | 95/101 | 0.98 (0.77 – 1.25) | 0.860 |
Patients with any missing value were excluded from multivariable analysis.
Age was used as a continuous variable.
Variables were included as time-dependent covariates.
Second surgery within or after nine months after the first operation.
Discussion
Surgical resection was an effective treatment for primary glioblastoma in adults with an adequate performance status regardless of patient age. The effect of surgery corresponded to a 45% reduction in the 1-year relative risk of dying and a 37% reduction in the risk of progression. Most important, surgery also showed an 8-month median prolongation of overall survival, after adjustment for clinical factors and tumor extension. We noted an interaction between the effect of surgery and the patient’s preoperative performance status in the multivariable analysis. The benefit of surgery appeared to be restricted to patients with good performance status, whatever the patient’s age, so surgery should also be considered for elderly patients. In contrast, there was no evidence that surgery was effective for either young or old patients with a poor performance status at diagnosis. These findings applied to the broad spectrum of young and old patients treated at a single institution.
Our finding that surgical resection was associated with a significant survival advantage for patients with glioblastoma is contrary to the conclusion of most recent reports, in which any survival advantage from surgery was not convincingly evident,6 unknown,7,8 or remains to be confirmed.9 A Cochrane review identified only one trial of biopsy versus resection for malignant glioma and showed a significant (p = 0.049) survival advantage for resection.6 However, this trial was small, with a total of 23 patients included in the analysis, and does not provide definitive evidence.
Furthermore, most of the previous studies concluded that patient’s age had the greatest effect on survival and that, in contrast with our findings, the benefits of surgery were confined to young patients only; our data provide strong evidence for the effectiveness of surgery even for elderly patients, provided they have an adequate performance status.
Whether the extent of resection is a factor significantly associated with the survival advantage is much debated, but this important question remains unanswered. Some reports found that more extensive resection was associated with longer survival,24–26 whereas others showed no relation.7,27–29 Interpretation of data relating resection to survival is complicated by the difficulty of defining the extent of resection.8 Our results also support a significant increase in survival associated with extensive surgical resection compared with partial resection, although precise evaluation of residual volume after surgery by postoperative imaging was available for 60% of the patients. Our data are insufficient for clear conclusions on the prognostic value of the extent of resection in this cohort of patients with primary glioblastoma.
Postoperative radiotherapy had an independent benefit on both overall and progression-free survival, with a one-third reduction in the relative risk of dying, regardless of patient age or performance status. Consistent with our results, one recent trial demonstrated that radiotherapy improved both survival and progression-free survival in patients older than 70 years compared with survival times obtained with best supportive care only.30 A prospective study, focused on 202 patients older than 70 years with glioblastoma treated between 1990 and 2000, also concluded that radiotherapy significantly improved survival in elderly patients.31
In this study, 70% of the patients received radiotherapy concomitantly with chemotherapy, which was found to correlate significantly with increased survival. Similar results have been reported in a high-quality review that demonstrated a significant prolongation of survival for patients who received nitrosourea-based chemotherapy plus radiotherapy compared with patients receiving radiotherapy alone (hazard ratio, 0.85; 95% CI, 0.78 – 0.91; p < 0.0001) and a 5% increase in 2-year survivors.10 In both our study and the review by Stewart,10 there was no evidence that the effect of chemotherapy differed in any group of patients defined by age, performance status, or extent of resection. More recently, the EORTC/National Cancer Institute of Canada (NCIC) trial has shown that radiotherapy plus concomitant and adjuvant temozolomide is an efficacious and well-tolerated treatment for glioblastoma.11 The EORTC/NCIC trial included selected patients (i.e., age < 70 years, WHO performance status ≥ 1, and surgery instead of biopsy), so the optimum choice of temozolomide regimens for elderly and poor-prognosis patients has not been established.
The optimum strategy for the treatment of tumor progression remains controversial.5,8,32,33 In our study, progression occurred in 99% of the patients throughout the follow-up, and we found no evidence of an independent benefit of reoperation on survival. Whereas some retrospective studies have reported a positive effect of second surgery for recurrent high-grade glioma, other studies that accounted for histology found evidence of a benefit from reoperation in patients with recurrent anaplastic astrocytoma but not in patients with recurrent glioblastoma.24,34,35 Moreover, we did not find any significant increase in survival with second surgery in patients with an interval between the first and second operations of more than 9 months compared with patients with an interval of 9 months or less. This result is in accord with those of other studies that used statistical modeling to account for prognostic factors.24,36 For the series of 55 patients reported by Ammirati and colleagues,24 there was no significant difference in survival after reoperation between patients whose tumor-free interval was 6 months or more and those whose interval was less than 6 months (p = 0.140). Young and colleagues36 reported that the disease-free interval was relevant to survival after reoperation by univariate analysis but not by multivariate analysis. On the contrary, other authors37,38 reported that the interval between the first and second operations was significantly related to survival after reoperation; however, most of these studies did not account for prognostic factors in the survival analysis, and selection bias may account for much of their results.
Our results showed benefits for the use of second-line chemotherapy (PCV or temozolomide) after progression. There was a 23% significant reduction in the hazard ratio for the survival outcome, with a narrow confidence interval. This result was consistent with the findings of previous studies involving patients with recurrent glioblastoma, in which nitrosoureas improved survival.33 Similar benefits have been documented for temozolomide, which has been found to be an active and useful option at the time of disease recurrence39,40 or to improve the quality of life after tumor progression41 and which has a better toxicity profile than other alkylating agents.
A few general comments must be added. Both randomized clinical trials and prospective cohort studies are needed to gain a fuller understanding of treatment effects and prognostic factors. Patients entered into randomized trials are not representative of patients at large, particularly elderly patients and those with adverse prognostic factors. However, nonrandomized studies may appear to overestimate the effect of treatments because of attrition, detection, or performance bias.14 In our study, many of these biases were avoided because there were no losses to follow up on, the outcome and prognostic variables were standardized, and the completeness and quality of the data were carefully checked. The result is that the median overall survival and progression-free survival of our patients lie in the range reported for patients with glioblastoma treated with temozolomide plus radiotherapy in one recent trial.11
The Glioma Outcomes Project reported data on a series of 565 patients with newly diagnosed glioma (WHO grade III or IV astrocytoma) treated in the United States between 1997 and 2000. Treatment at academic centers was associated with improved survival compared with treatment at community centers. The explanation given by the authors was that patients treated at academic institutions were younger and more likely to receive radiation and chemotherapy. Academic institutions were also more likely to treat a large volume of patients and use advanced technological resources to aid in tumor resection.42 A valuable aspect that our study adds to these results is related to the fact that more than 20% of our study population consisted of elderly patients treated at a single institution.
Confirming age, preoperative performance status, and tumor extension as independent prognostic factors for both overall survival and progression-free survival emphasizes the recommendation that in randomized trials these factors need to be clearly addressed during patient selection and appropriately balanced across treatment arms.
Our findings of the predominance of preoperative performance status over patient age in predicting survival after surgery may help to refine the clinician’s prediction and treatment decisions. Patients with primary glioblastoma should receive high-level surgery and appropriate radiotherapy and chemotherapy regimens, and these treatments should not be withheld because of increasing age alone. The benefit of second surgery at recurrence is uncertain, and new trials are needed to assess its effectiveness.
Acknowledgments
We are indebted to the patients and their families for agreeing to participate in this study and to the nurses for their collaboration.
Gr.F. designed the study. Gr.F., C.F., R.F., and M.F. acquired all of the data in the study and take responsibility for the integrity of the data included in the Cancer Register of the Fondazione Istituto Neurologico “Carlo Besta.” A.B., G.B., S.G., C.L.S., D.C., Gr.F., and L.F. provided and cared for study patients. M.S. and M.G.B. provided expert advice on CT and MRI. B.P. performed histological diagnoses. Gr.F. and C.F. developed the plan of analysis and C.F. performed the analysis. Gr.F. drafted the paper. M.S., M.G.V., and Ga.F. provided critical revision of the manuscript. All authors commented on drafts of the paper and approved the final manuscript. This work was supported by the Italian health ministry (RC 2004–2005).
Appendix
The following investigators at the Fondazione I.R.C.C.S. Istituto Neurologico “Carlo Besta” (Milan, Italy) provided and cared for study patients: S. Brock, F. Di Meco, I. Dones, A. Franzini, G. Lasio, and S. Lodrini, Department of Neurosurgery; M. Bricchi, C. Ferrazza, and B. Regi, Unit of Neuroanesthesia and Intensive Care; M. Eoli, E. Lamperti, A. Salmaggi, and A. Silvani, Unit of Neuro-oncology; A. Bizzi, L. Farina, and E. Maccagnano, Department of Neuroradiology; and I. Milanesi, Unit of Radiotherapy. Graziella Filippini is the guarantor for this article.
Footnotes
The contents of this article have not been copyrighted or published previously and are not now under consideration for publication elsewhere.
Participating investigators are listed in the Appendix.
References
- 1.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892 – 6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Pavlidis N, Jelic S for the ESMO Guidelines Task Force. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol. 2005;16(suppl 1):i64–i65. doi: 10.1093/annonc/mdi834. [DOI] [PubMed] [Google Scholar]
- 3.Laperriere N, Zuraw L, Cairncross G Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site Group. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol. 2002;64:259 – 273. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 4.Stuschke M, Thames HD. Hyperfractionated radiotherapy of human tumours: overview of the randomized clinical trials. Int J Radiat Oncol Biol Phys. 1997;37:259 – 267. doi: 10.1016/s0360-3016(96)00511-1. [DOI] [PubMed] [Google Scholar]
- 5.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323 – 331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 6.Grant R, Metcalfe SE. Biopsy versus resection for malignant glioma. Cochrane Database Syst Rev. 2001;3:CD002034. doi: 10.1002/14651858.CD002034. [DOI] [PubMed] [Google Scholar]
- 7.Hess KR. Extent of resection as a prognostic variable in the treatment of gliomas. J Neurooncol. 1999;42:227 – 231. doi: 10.1023/a:1006118018770. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol. 2005;4:413–422. doi: 10.1016/S1474-4422(05)70118-6. [DOI] [PubMed] [Google Scholar]
- 9.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol (Berl) 2005;109:93 – 108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 10.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomized trials. Lancet. 2002;359:1011 – 1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 11.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 12.Gupta T, Sarin R. Poor-prognosis high-grade gliomas: evolving an evidence-based standard of care. Lancet Oncol. 2002;3:557 – 564. doi: 10.1016/s1470-2045(02)00853-7. [DOI] [PubMed] [Google Scholar]
- 13.Shaw EG. Nothing ventured, nothing gained: treatment of glioblastoma multiforme in the elderly. J Clin Oncol. 2004;22:1540 – 1541. doi: 10.1200/JCO.2004.01.989. [DOI] [PubMed] [Google Scholar]
- 14.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 15.Simon R, Altman DG. Statistical aspects of prognostic factors studies in oncology. Br J Cancer. 1994;69:979 – 985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleihues P, Cavenee WK, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000. [Google Scholar]
- 17.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. [Accessed May 6, 2006];Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics. 2004 Available at http://www.fda.gov/cder/guidance/index.htm.
- 18.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277 – 1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 19.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309 – 1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables (with discussion) J Roy Stat Soc B. 1972;34:187 – 220. [Google Scholar]
- 21.Silvani A, Eoli M, Salmaggi A, E rbetta A, Fariselli L, Boiardi A. Intra-arterial ACNU and carboplatin versus intravenous chemotherapy with cisplatin and BCNU in newly diagnosed patients with glioblastoma. Neurol Sci. 2002;23:219 – 224. doi: 10.1007/s100720200044. [DOI] [PubMed] [Google Scholar]
- 22.Boiardi A. PCV chemotherapy for recurrent glioblastoma multiforme. Neurology. 2001;56:1782. doi: 10.1212/wnl.56.12.1782. [DOI] [PubMed] [Google Scholar]
- 23.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588 – 593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201 – 206. doi: 10.1227/00006123-198708000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190 – 198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 26.Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults: the prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487 – 493. doi: 10.3171/jns.1989.71.4.0487. [DOI] [PubMed] [Google Scholar]
- 27.Franklin CI. Does the extent of surgery make a difference in high grade malignant astrocytoma? Australas Radiol. 1992;36:44 – 47. doi: 10.1111/j.1440-1673.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 28.Gamburg ES, Regine WF, Patchell RA, Strottmann JM, Mohiuddin M, Young AB. The prognostic significance of midline shift at presentation on survival in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2000;48:1359 – 1362. doi: 10.1016/s0360-3016(00)01410-3. [DOI] [PubMed] [Google Scholar]
- 29.Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery. 1991;29:385 – 388. doi: 10.1097/00006123-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Keime-Guibert F, Chinot O, Taillandier F. Phase 3 study comparing radiotherapy with supportive care in older patients with newly diagnosed anaplastic astrocytomas or glioblastoma multiforme: an ANOCEF group trial [abstract] Neuro-Oncology. 2005;7:349. [Google Scholar]
- 31.Marijnen CA, van den Berg SM, van Duinen SG, Voormolen JH, Noordijk EM. Radiotherapy is effective in patients with glioblastoma multiforme with a limited prognosis and in patients above 70 years of age: a retrospective single institution analysis. Radiother Oncol. 2005;75:210 – 216. doi: 10.1016/j.radonc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Brandes A, Vastola A, Monfardini S. Reoperation in recurrent high-grade gliomas. Literature review of prognostic factors and outcome. Am J Clin Oncol. 1999;22:387 – 390. doi: 10.1097/00000421-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 1998;18:1303 – 1311. [PubMed] [Google Scholar]
- 34.Harsh GR, IV, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615 – 621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sipos L, Afra D. Re-operations of supratentorial anaplastic astrocytomas. Acta Neurochir (Wien) 1997;139:99 – 104. doi: 10.1007/BF02747187. [DOI] [PubMed] [Google Scholar]
- 36.Young B, Oldfield EH, Markesbery WR, et al. Reoperation for glioblastoma. Neurosurgery. 1981;55:917 – 921. doi: 10.3171/jns.1981.55.6.0917. [DOI] [PubMed] [Google Scholar]
- 37.Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36:271 – 275. [PubMed] [Google Scholar]
- 38.Kelly PJ, Rappaport ZH, Bhagwati SN, Ushio Y, Vapalahti M, de Tribolet N. Reoperation for recurrent malignant gliomas: what are your indications? Surg Neurol. 1997;47:39 – 42. doi: 10.1016/s0090-3019(96)00273-x. [DOI] [PubMed] [Google Scholar]
- 39.Brandes AA, Ermani M, Basso U, et al. Temozolomide in patients with glioblastoma at second relapse after first line nitrosourea-procarbazine failure: a phase II study. Oncology. 2002;63:38 – 41. doi: 10.1159/000065718. [DOI] [PubMed] [Google Scholar]
- 40.Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113 – 2115. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- 41.Osoba D, Brada M, Yung WK, Prados M. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000;18:1481 – 1491. doi: 10.1200/JCO.2000.18.7.1481. [DOI] [PubMed] [Google Scholar]
- 42.Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557 – 564. doi: 10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]