Abstract
Glioblastoma multiforme (GBM) continues to be a difficult therapeutic challenge. Our study was conducted to determine whether improved survival and tumor control could be achieved with modern delivery of fast neutron radiation using three-dimensional treatment planning. Ten patients were enrolled. Eligibility criteria included pathologic diagnosis of GBM, age ≥ 18 years, and KPS ≥60. Patients underwent MRI and 18F-fluorodeoxyglucose PET (FDG PET) as part of initial three-dimensional treatment planning. Sequential targets were treated with noncoplanar fields to a total dose of 18 Gy in 16 fractions over 4 weeks. Median and 1-year overall survival were 55 weeks and 60%, respectively. One patient remains alive at last follow-up 255 weeks after diagnosis. Median progression-free survival was 16 weeks, and all patients had tumor progression by 39 weeks. Treatment was clinically well tolerated, but evidence of mild to moderate gliosis and microvascular sclerosis consistent with radiation injury was observed at autopsy in specimens taken from regions of contralateral brain that received approximately 6–10 Gy. Fast neutron radiation using modern imaging, treatment planning, and beam delivery was feasible to a total dose of 18 Gy, but tumor control probability was poor in comparison to that predicted from a dose-response model based on older studies. Steep dose-response curves for both tumor control and neurotoxicity continue to present a challenge to establishing a therapeutic window for fast neutron radiation in GBM, even with modern techniques.
Keywords: FDG PET, glioblastoma multiforme, neutron radiotherapy
Primary malignant brain tumors, in particular glioblastoma multiforme (GBM), continue to be a difficult therapeutic challenge, with media survival of approximately 10 months. Whereas malignancies originating outside the central nervous system kill their host primarily by metastasizing to distant organs, gliomas remain a local disease. Local failure of GBM treatment predominates even in the face of escalated radiation doses using photon radiation in the form of brachytherapy, with or without hyperthermia,1,2 stereotactic radiosurgery,3 and three-dimensional conformal external beam radiation.4 The University of Washington (UW) has reported results of a phase II trial in which patients were treated by conformal external beam photon radiation to 79.4 Gy, utilizing MRI for targeting the initial 59.4 Gy, and 18F-fluorodeoxyglucose PET (FDG PET) to target the final 20 Gy.5 Preliminary analysis of the first 27 patients on that trial revealed that FDG PET defined regions of interest that were different from those defined by MRI. Furthermore, the volume of abnormality on FDG PET was more predictive than MRI for survival and time to tumor progression after radiation.6 With early indications of the potential utility of FDG PET guidance combined with excellent tolerance of 79.4 Gy, it was decided to initiate a pilot study to investigate conformal fast neutron radiation using FDG PET guidance.
Fast neutron radiation for GBM was investigated in several clinical trials two to three decades ago.7–10 In these trials, fast neutrons were reported to sterilize GBM in a significant proportion of patients. Histological data (biopsy and autopsy) from those trials have been analyzed with respect to neutron dose.11 Differences in relative biological effectiveness between the beams used at participating neutron facilities were used to normalize doses to approximate equivalent doses for the current clinical UW fast neutron beam. That analysis revealed a tightly fit sigmoid dose-response curve (r2 = 0.997). This curve was steep, with calculated control rates of 10%, 50%, and 90% corresponding to 14 Gy, 16 Gy, and 18 Gy, respectively. Despite the unusual finding of GBM sterilization in these older neutron trials, no survival benefit was observed because patients suffered significant neurotoxicity as supported by histological findings in normal brain tissue.12
The treatment delivery of neutrons in previous GBM trials was primitive by today’s standards. Typically, whole brain, opposed lateral, and simple wedge pair fields were used, with limited ability to shield portions of the fields. Imaging was limited in the era of these studies to CT at best. Three-dimensional treatment planning was not yet available. Consequently, large volumes of normal tissue were treated, with no ability to optimize dose distributions around tumor volume or to assess and minimize dose heterogeneity in normal brain. The current UW fast neutron facility allows delivery of sophisticated dose distributions designed by three-dimensional computer planning. In fact, this neutron facility was used to treat a series of 20 patients with arteriovenous malformations (AVMs) of the brain with 9 Gy in a single dose (at isocenter) through 7–14 noncoplanar conformal fields.13 There were no cases of toxicity, including complete absence of new T2 signal abnormality on follow-up MRI scans. Dose to brain tissue at the periphery of the AVMs on that study averaged approximately 6 Gy.
Considering these observations with respect to neutron dosimetry, GBM control, brain toxicity, and imaging, a pilot study was designed to utilize MRI and FDG PET to guide noncoplanar three-dimensional conformal neutron radiation in the treatment of GBM using sequential target volumes.
Materials and Methods
Patient Eligibility
Patients were required to have histological diagnosis of GBM or gliosarcoma with the most recent operative procedure for the tumor performed no more than 21 days prior to registration on study. Minimum eligible age was 18 years. Patients having tumors with evidence of subependymal spread, meningeal spread, cerebral spinal fluid dissemination, or gadolinium enhancement within the brainstem were ineligible. KPS of 60 or higher, platelets greater than 50,000 per microliter, and neutrophils greater than 1,000 per microliter were required. Pregnant or nursing women were not eligible. All patients were informed of the investigational nature of this study, and gave written informed consent in accordance with institutional and federal guidelines.
Neutron Treatment Facility
The clinical UW fast neutron facility (Scanditronix, Uppsala, Sweden) uses a cyclotron to deliver 50.5 MV protons to a gantry-mounted beryllium target, producing a neutron beam with an average energy of 4 MV. This device administers treatment through an isocentric gantry with 360-degree rotation in combination with 180-degree couch rotation. Fields are shaped by an infinitely variable multileaf collimator, and wedges are available internally within the gantry head. An x-ray source is available within the gantry head for field verification. The neutron facility is interfaced with Prism, the UW computerized treatment planning system, providing the ability to deliver three-dimensional conformal neutron radiation comparable to that of conventional linear accelerators.
Imaging and Treatment
All patients underwent postoperative MRI scan with gadolinium. Within 10 days of study registration, patients were fitted with a head immobilization mask and underwent both CT and FDG PET scanning in the mask. Methods for interpretation of FDG PET, delineation of target volume, and coregistration with MRI and treatment planning CT scans have previously been described in detail.6
Three planning target volumes (PTV) were defined. Two of these PTVs were standard volumes based on postoperative MRI T2 signal abnormality with 2.5 cm margin (PTVT2) and T1 gadolinium enhancement plus resection cavity with 1.5 cm margin (PTVT1). The third PTV was based on the FDG PET region of abnormal uptake with 1 cm margin (PTVPET). The three PTVs were treated with sequential plans, each of which utilized three noncoplanar beams designed by three-dimensional computerized planning. Initially PTVPET received 4 Gy in two fractions of 2 Gy each. Then PTVT2 received 9 Gy in nine fractions of 1 Gy each. Finally, PTVT1 received 5 Gy in five fractions of 1 Gy each. The three sequential plans were designed to use unique beam angles in order to avoid repeatedly traversing nontargeted brain over the entire treatment course. All doses were prescribed to isocenter. The composite plan delivered a total of 18 Gy to PTVPET, giving one fraction daily, 4 days per week. In theory, if no abnormal region was identified on FDG PET, then PTVPET would have been eliminated from the treatment course, and the total dose would have been 14 Gy. In practice, all patients had abnormal FDG PET and were prescribed 18 Gy.
Patients were placed on dexamethasone at a dose of at least 2 mg p.o. twice per day at least 2 days prior to initiating fast neutron therapy. The dexamethasone dose was tapered during the course of therapy if tolerated by the patient, but a minimum dose of 2 mg per day was recommended, along with a histamine-2 blocker.
Follow-up and Study Endpoints
This study was intended to accrue 20 patients. Patients were assessed by physical exam at least weekly while receiving treatment. After completing treatment, follow-up with physical exam and MRI was planned at 3 weeks, then every 3 months for the first year, every 4 months for the second year, every 6 months for the third through fifth years, then every year thereafter. Endpoints were survival, time to tumor progression, site of progression relative to initial FDG PET volume of interest, and toxicity based on the National Cancer Institute Common Toxicity Criteria (version 2). Tumor progression was defined as greater than 25% increase in the product of the maximum perpendicular axial dimensions of contrast enhancement on MRI scan from the initial or nadir measurements after treatment. New sites of contrast enhancement distant to the original tumor bed were also considered to represent tumor progression. All recurrences based on MRI were confirmed with FDG PET, MR spectroscopy (MRS), and/or histopathology by reoperation or autopsy.
Results
Ten patients were enrolled in this study from April through October 1999. Initially, 20 patients were planned for enrollment, but the protocol was stopped early because of rapid tumor recurrence in these initial 10 patients (7 male, 3 female). Median age was 55 years (range 33–67 years) and median KPS was 90 (range 70–90). Nine patients underwent surgical resection and one had biopsy only. Eight patients were Radiation Therapy Oncology Group recursive partitioning analysis class 4, and two were class 3.14 All patients received the planned dose of 18 Gy.
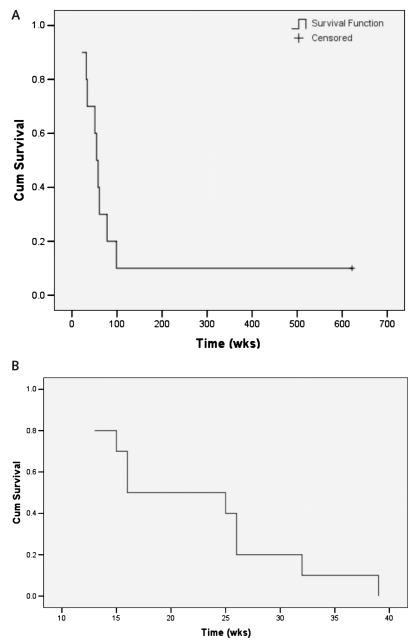
Overall survival and progression-free survival from date of diagnosis are shown in Fig. 1. Median survival was 55 weeks, with 1-year survival of 60%. One patient (10%) remains alive 255 weeks after diagnosis. That patient failed distantly with spinal leptomeningeal tumor that responded completely to intraommaya topotecan chemotherapy and focal radiotherapy (x-ray) without subsequent recurrence. Median time to progression was 16 weeks, and all 10 patients failed by 39 weeks. All but the one patient with spinal leptomeningeal tumor failed within the initial volume of abnormal FDG uptake on PET. Tumor progression as determined by MRI was confirmed by at least one other method in all cases. Seven patients had tumor progression confirmed by FDG PET. Among those seven patients with PET confirmation, four had histopathological confirmation at reoperation, and one had confirmation by MRS and histopathology at reoperation. Among the 3 patients who did not have a confirming FDG PET study after progression on MRI, one had confirmation by histopathology at reoperation, one had confirmation by MRS, and one had confirmation by MRS plus histopathology at autopsy.
Fig. 1.
Overall (A) and progression-free (B) survival after diagnosis.
The autopsied patient had developed a new right temporal enhancing satellite lesion on MRI, 2 cm from the initial primary enhancing mass, 3 weeks after completing radiation. The primary site also had increased size of enhancement. The satellite lesion was within the initial abnormal uptake volume on the pretreatment FDG PET, and received the full 18 Gy dose. MRS 1 week later suggested the primary gadolinium enhancing volume was necrotic tissue and the satellite lesion was tumor. The patient died 9 weeks after completion of neutron therapy. At autopsy both the primary site and the satellite contained confluent necrosis with perivascular islands of viable-appearing gemistocytic tumor cells. In the temporal cortex there were sheets of densely packed pleomorphic gemistocytes away from areas of necrosis. In all areas of the brain sampled ipsi- and contralateral to the GBM, there was mild to moderate reactive gliosis, worse in white matter than in gray matter except in the midbrain, where the red nucleus was the most gliotic structure. Microvascular sclerosis accompanied these changes. In ipsilateral white matter, there were areas of pallor of myelin staining, infiltrates of macrophages, and reduced density of axons. It is noteworthy that material from contralateral brain that had no signal abnormality on T2 MRI showed white matter gliosis and vascular changes consistent with mild radiation injury where the neutron dose was as low as 6–10 Gy.
Among the five patients who had reoperation, the amount of intralesional and perilesional necrosis was not remarkable compared to findings typical of patients undergoing reoperation for GBM after photon radiotherapy.
All patients had a drop in KPS below 70, with median time from diagnosis to establishing KPS below 70 of 33 weeks (range 21–54 weeks). In all cases, KPS dropped below 70 after documented tumor progression. The single living patient eventually recovered performance status, and currently has a KPS of 100.
Treatments were well tolerated, with no cases of grade 3 or higher acute toxicity. Two patients experienced grade 2 toxicity (headache in one, and fatigue in both), while the remainder of patients had toxicity limited to grade 1 headache, alopecia, dermatitis, and fatigue. No cases of late neurological decline were observed that could not be explained by progressive tumor except for one case of grade 1 tremor that developed prior to documented tumor progression.
Salvage therapies consisted of reoperation with implantation of carmustine wafers with or without systemic chemotherapy in four cases, reoperation with implantation of carmustine wafers and iodine-125 seeds on a separate protocol in one case, and systemic chemotherapy alone in three cases. One patient did not have salvage therapy; salvage treatment for the living patient was described above.
Discussion
Prior fast neutron trials in high-grade gliomas indicated an increased probability of tumor control compared to photon therapy.7–10 Dose on this trial was selected based on a dose-response relationship observed in older trials of fast neutrons.11 The isocentric clinical neutron facility at UW provided an opportunity to reinvestigate fast neutrons in GBM using modern three-dimensional treatment planning. Refinement of the fields based on FDG PET was undertaken to localize the highest neutron doses to the regions of highest risk for recurrence after establishing the ability to incorporate FDG PET in the treatment planning process for GBM.5 The planning process with fast neutrons differed from that on the photon trial using FDG PET guidance by obtaining the FDG PET scan for treatment planning prior to initiating radiation. Obtaining the FDG PET prior to the fast neutron radiation allowed the design of a composite radiation plan for all three target phases, allowing total plan optimization from the beginning. Because the overall treatment time was only 4 weeks, the additional time required in treatment planning with early incorporation of FDG PET information did not extend the overall interval from time of diagnosis to completion of treatment.
The prior clinical trials of fast neutrons in high-grade gliomas were plagued by neurotoxicity, precluding improvement in survival despite higher probability of tumor sterilization. Conversely, the present trial showed no improvement in tumor control in comparison to that commonly observed with photon therapy, and clinically apparent toxicity was remarkably low. Overall and progression-free survival on the two contemporary protocols using FDG PET guidance were similar (Table 1), with a trend toward better outcome among the patients treated with photons. However, one patient in the neutron trial, the youngest at age 33, continues to survive approximately 5 years after diagnosis after salvage chemotherapy and radiotherapy for spinal leptomeningeal recurrence. That patient’s clinical course is more indicative of unusual tumor behavior than efficacy of the local neutron therapy. The counterintuitive results of the current neutron trial may be explained by the conditions of treatment in older neutron trials and estimates that were used to derive the relationship between neutron dose and probability of tumor control based on those older trials. The dose-response relationship incorporated estimates of relative biological effectiveness of each beam in order to normalize the data.11 The resulting dose-response curve had a steep slope, giving the potential for significant discrepancies between actual and predicted tumor control probability at a given dose. The dose of 18 Gy selected for this trial was predicted from the dose-response relationship to provide a 90% probability of control, but that point exists on the threshold of the steep portion of the sigmoid curve. Furthermore, the older trials were not conducted with the modern noncoplanar beam delivery and three-dimensional planning used on the current trial. It is likely that the actual delivered dose on the older trials was substantially more heterogeneous, with regions of high dose in narrower areas of the head away from the central axis of treatment. Consequently, actual dose delivered on the older neutron trials may have been somewhat higher to some regions of tumor than the prescribed dose. Such heterogeneity was compensated by wedges and beam weighting in the present study, also explaining the lack of clinical toxicity observed on this trial.
Table 1.
Comparison of overall and progression-free survival times from diagnosis between the fast neutron and photon trials using FDG PET guidance
| Survival Category | Fast Neutron | Photon* |
|---|---|---|
| Median OS | 55 weeks | 70 weeks |
| 1-year OS | 60% | 70% |
| 2-year OS | 10% | 17% |
| Median PFS | 16 weeks | 24 weeks |
| 1-year PFS | 0% | 18% |
| 2-year PFS | 0% | 3% |
Abbreviations: OS, overall survival; PFS, progression-free survival.
Douglas et al.5
Based on these findings, it is tempting to explore a higher dose of fast neutrons with three-dimensional treatment planning. However, caution must be exercised as the steep dose-response curve for tumor control likely applies as well to that for toxicity. Histopathology from contralateral brain uninvolved by tumor at autopsy showed changes consistent with radiation damage. That region of brain received approximately 6–10 Gy based on the neutron isodose curves correlated to the anatomical position of the specimen. Other options could include utilization of a boron neutron capture agent with selective tumor uptake to take advantage of a small thermal neutron contaminant that is available within the UW fast neutron beam,15 or combining fast neutrons confined to the highest risk target volume with photons to lower risk regions.
Conclusions
Use of modern three-dimensional conformal treatment planning based on MRI and FDG PET targeting with an isocentric fast neutron delivery system was feasible for treatment of GBM. The total dose of 18 Gy resulted in tumor control that was worse than predicted based on past trials of fast neutrons for high-grade gliomas. Clinical tolerance was excellent. However, there was pathologic evidence of subclinical radiation brain injury at 6–10 Gy. The dose-response relationship is likely steep for both tumor control and neurotoxicity. Consequently, a therapeutic window for fast neutron radiation therapy of GBM remains elusive.
Acknowledgments
The authors wish to thank Evan Chapman, CMD, for his extensive work in designing the conformal fast neutron treatment plans used on this trial. This work was supported by NIH grant PO1 CA 42045.
References
- 1.Sneed PK, Gutin PH, Larson DA, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 2.Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost + hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- 3.Souhami L, Scott C, Brachman D, et al. Randomized prospective comparison of stereotactic radiosurgery (SRS) followed by conventional radiotherapy (RT) with BCNU to RT with BCNU alone for selected patients with supratentorial glioblastoma multiforme (GBM): report of RTOG 93-05 protocol [abstract] Int J Radiat Oncol Biol Phys. 2002;54:94–95. [Google Scholar]
- 4.Chan JL, Lee SW, Sandler HM, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 5.Douglas JG, Stelzer KJ, Tralins K, et al. [F-18]-fluorodeoxyglucose positron emission tomography targeting radiation to glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2006;64:886–891. doi: 10.1016/j.ijrobp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Tralins KS, Douglas JG, Stelzer KJ, et al. Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med. 2002;43:1667–1673. [PubMed] [Google Scholar]
- 7.Catterall M, Bloom HJ, Ash DV, et al. Fast neutrons compared with megavoltage x-rays in the treatment of patients with supratentorial glioblastoma: a controlled pilot study. Int J Radiat Oncol Biol Phys. 1980;6:261–266. doi: 10.1016/0360-3016(80)90131-5. [DOI] [PubMed] [Google Scholar]
- 8.Kurup PD, Pajak TF, Hendrickson FR, et al. Fast neutrons and misonidazole for malignant astrocytomas. Int J Radiat Oncol Biol Phys. 1985;11:679–686. doi: 10.1016/0360-3016(85)90298-6. [DOI] [PubMed] [Google Scholar]
- 9.Laramore GE, Diener-West M, Griffin TW, et al. Randomized neutron dose searching study for malignant gliomas of the brain: results of an RTOG study. Int J Radiat Oncol Biol Phys. 1988;14:1093–1102. doi: 10.1016/0360-3016(88)90384-7. [DOI] [PubMed] [Google Scholar]
- 10.Laramore GE, Griffin TW, Gerdes AJ, et al. Fast neutron and mixed (neutron/photon) beam teletherapy for grades III and IV astrocytomas. Cancer. 1978;42:96–103. doi: 10.1002/1097-0142(197807)42:1<96::aid-cncr2820420116>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Stelzer KJ, Lindsley KL, Cho PS, et al. Fast neutron radiotherapy: the University of Washington experience and potential use of concomitant boost with boron neutron capture. Radiat Protect Dosim. 1997;70:471–475. [Google Scholar]
- 12.Shaw CM, Sumi SM, Alvord EC, Jr, et al. Fast neutron irradiation of glioblastoma multiforme: neuropathological analysis. J Neurosurg. 1978;49:1–12. doi: 10.3171/jns.1978.49.1.0001. [DOI] [PubMed] [Google Scholar]
- 13.Stelzer K, Griffin B, Eskridge J, et al. Results of neutron radiosurgery for inoperable arteriovenous malformations of the brain. Med Dosim. 1991;16:137–141. doi: 10.1016/0958-3947(91)90123-j. [DOI] [PubMed] [Google Scholar]
- 14.Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz TA, Laramore GE, Stelzer KJ, et al. Boron neutron capture enhanced fast neutron radiotherapy for malignant glioma and other tumors. J Neurooncol. 1997;33:171–178. doi: 10.1023/a:1005798004420. [DOI] [PubMed] [Google Scholar]