Abstract
We describe the case of a patient with transcobalamin II deficiency, hypogammaglobulinemia, absent corpus callosum, and mental retardation who presented at an early age with colorectal cancer and multifocal anaplastic astrocytoma. He was found to have a possible germline mutation of the PMS2 gene, as evidenced by absent protein expression in both normal and tumor tissues. His parents were found to be carriers of a nonsense mutation of the PMS2 gene.
Keywords: Lynch syndrome, malignant glioma, PMS2
Hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, is usually autosomal dominant and accounts for about 2% – 5% of all colorectal cancers.1,2 The syndrome is characterized by polypoid ( < 100 polyps) or nonpolypoid colorectal adenomas or carcinomas, endometrial carcinoma, malignant gliomas (WHO grade III or IV), and tumors of the ovary, sebaceous glands, bone marrow, breast, larynx, and other sites.1 It is due to germline mutations of the mismatch repair (MMR) genes, including MLH1, MSH2, and MSH6.2 Rarely, mutations in the mutL homologue genes PMS1 and PMS2 can cause Lynch syndrome or childhood malignancies in an autosomal recessive fashion.3–6 The hallmark of defective or absent MMR proteins is microsatellite instability (MSI).2 Diagnosis of such patients is based on clinical criteria (Amsterdam or Bethesda criteria),2 molecular characterization of MSI and MMR defect, and demonstration of the absence of key MMR proteins by immunohistochemical (IHC) analysis of tumor tissue. Other congenital anomalies have not been commonly reported in association with Lynch syndrome. We now report the clinical characteristics, radiologic, pathologic, and molecular features of a patient with mental retardation, agenesis of the corpus callosum, and transcobalamin II (TCII) deficiency who developed colorectal cancer and multifocal anaplastic astrocytoma at an early age.
Case Report
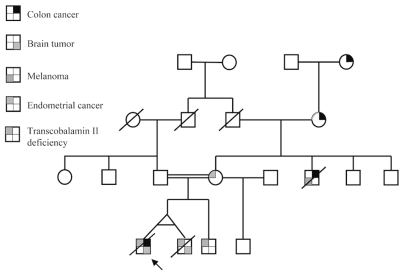
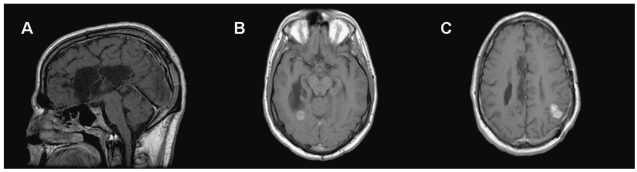
The patient was a 21-year-old white man who was one of identical twins born to consanguineous parents (Fig. 1). Both twins and an older male sibling were diagnosed with transcobalamin II deficiency and hypogammaglobulinemia as infants. The patient presented at age 3 years with developmental delay and seizures. An MRI scan of the brain revealed agenesis of the corpus callosum (Fig. 2). He was also noted to have multiple congenital compound nevi. At age 14 years, he presented with rectal bleeding and was found to have a colorectal mass on sigmoidoscopy. He underwent a pancolectomy. Pathologic examination showed a poorly differentiated adenocarcinoma. He was treated with 5-fluorouracil plus leucovorin for 6 months and underwent focal radiotherapy. Five years later, he presented with seizures, and an MRI scan of the brain revealed an enhancing lesion in the left parietal lobe. He underwent a subtotal resection of the mass. Pathologic examination found a grade III astrocytoma. He was given 63 Gy of focal radiotherapy to the tumor. Three months later, he was noted to have progressive disease and was started on therapy with oral temozolomide (Temodar; Schering-Plough, Kenilworth, NJ, USA). He had disease progression in the left parietal lobe, and a new tumor was now found in the right temporal lobe (Fig. 2) following one cycle of chemotherapy. He was then seen in consultation at Duke University Medical Center. He was given salvage chemotherapy with carmustine (BiCNU; Ben Venue Laboratories, Bedford, OH, USA) plus irinotecan (Camptosar; Pfizer, New York, NY, USA) and had a very good partial response to treatment. He completed chemotherapy about 18 months later with minimal enhancement in the sites of prior tumor and a fluorodeoxyglucose positron emission tomography (FDG-PET) of the brain that showed very minimal FDG uptake in the same areas. Four months following completion of chemotherapy, he developed progressive disease in the brain and died despite salvage therapy.
Fig. 1.
Family pedigree of patient with hereditary colon cancer, brain tumor, and transcobalamin II deficiency.
Fig. 2.
T1-weighted sagittal and axial gadolinium-enhanced images of the brain demonstrating absent corpus callosum (A) and enhancing lesions in the right temporal lobe (B) and left parietal lobe (C) consistent with malignant glioma.
Family history was positive for colorectal carcinoma in his maternal grandmother (at age 52 years), great-grandmother (at age 80 years), and maternal uncle (at age 60 years, and who was also treated for malignant melanoma at age 40 years) (Fig. 1) and satisfied the Amsterdam criteria for HNPCC. The patient’s twin brother died of anaplastic oligodendroglioma at age 11 years, but his neuroimaging studies did not show abnormalities of the corpus callosum. The patient’s mother was recently diagnosed at age 56 years with endometrial carcinoma. The patient’s paternal grandmother died of carcinoma of a paranasal sinus at age 62 years. The patient’s father has had several colonoscopies that have been reported as normal and has not been diagnosed with any cancer thus far. The patient’s older sibling (age 26 years) with transcobalamin II deficiency has had at least one colonoscopy (with normal results) and is currently alive and well without any history of malignancy; his neuroimaging studies did not reveal any abnormalities of the corpus callosum. Another half-brother (through his mother) who is now 36 years old, is currently alive without any history of cancer.
Methods
Signed informed consent was obtained from both parents to conduct IHC and other molecular studies on normal and tumor tissues obtained from the patient and parents. The mutation screening study was approved by the local Institutional Review Board of Ohio State University. The parents were informed of the genetic results obtained during the course of this study.
Using standard techniques, formalin-fixed, paraffin-embedded sections of tumor were processed and analyzed by light microscopy for IHC expression of MMR proteins as previously described.7 Adjacent normal tissue and surrounding tissue lymphocytes served as internal positive controls for each case. Nuclear staining of the tumor was scored as either present or absent compared with the corresponding internal control. The adenomatosis polyposis coli (APC) gene was sequenced from the genomic DNA obtained from the patient’s peripheral blood lymphocytes by sequence determination (forward and reverse directions) of 8,532 base pairs (bp) of 15 exons and 420 bp of the adjacent noncoding introns. Microsatellite instability analysis was performed using formalin-fixed, paraffin-embedded sections of tumor and corresponding normal tissue. DNA was extracted from microdissected areas of tumor and normal tissue from sections on glass slides. Following DNA amplification using fluorescent labeled primers, a panel of five microsatellites recommended by the NCI (BAT25, BAT26, D2S123, D5S346, and D173250) was analyzed for allelic shift using a multiplex fluorescence-based PCR assay. Tumors were classified as MSI-high (allelic shift with two or more markers), MSI-low (allelic shift with one marker), or MS-stable. Mutation screening within PMS2 was performed using a recently described method that avoided amplifying pseudogene sequences.8
Results
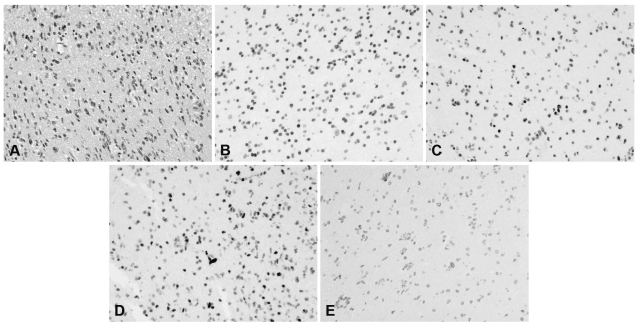
By IHC analysis, both the colon adenocarcinoma and malignant glioma retained positive nuclear expression for MLH1, MSH2, and MSH6 (Fig. 3, panels B, C, and D). However, unlike the usual finding in Lynch syndrome, the patient’s normal brain and colonic tissue, glioma, and colon adenocarcinoma were negative for PMS2 protein (Fig. 3, panel E). This was suggestive of a germline PMS2 mutation causing inactivation of both PMS2 alleles, although it is possible that other genetic mechanisms could mediate this gene suppression. Positive controls were analyzed for staining of all four proteins to ensure antibody reactivity and were found to be adequate. In addition, the positive expression of MLHI, MSH2, and MSH6 in the tumor tissues indicated that the lack of PMS2 staining was not due to fixation or other internal problems that could result in a false-negative reaction. However, MSI analysis of both tumors showed no allelic shift for any of the five microsatellites examined. Therefore, both the colon and brain tumors were considered MS-stable microsatellite stable (MSI assays were repeated twice for confirmation).
Fig. 3.
Photomicrographs of brain tumor. (A) Hematoxylin and eosin; (B – E) immunohistochemistry for MLH1 (B), MSH2 (C), MSH6 (D), and PMS2 (E). The tumor cell nuclei are strongly positive for MLH1, MSH2, and MSH6. However, tumor cell nuclei are negative for PMS2. All photomicrographs × 200.
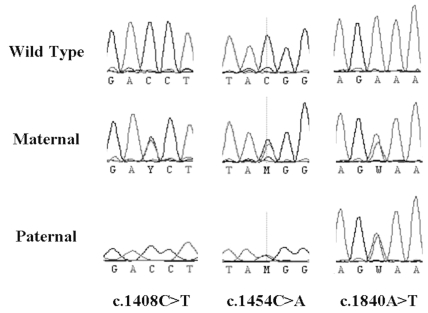
An aliquot of genomic DNA extracted from the patient’s peripheral blood lymphocytes was analyzed and found to be negative for known APC mutations. The remaining DNA sample was of poor quality and could not be analyzed further for MMR gene mutations. DNA analysis of the peripheral blood lymphocytes from the maternal sample revealed a single nucleotide polymorphism (SNP) that introduced a premature stop codon in exon 11 (c.1840A > T; p.Lys614Stp) (Fig. 4). Two additional SNPs were found in the maternal sample (c.1408C > T and c.1454C > A), both of which were also in exon 11 (Fig. 4). Single site analysis of the paternal sample showed the presence of the same premature stop codon in exon 11 as well as the c.1454C > A SNP (Fig. 4).
Fig. 4.
Sequencing chromatograms depicting the genomic variation identified within PMS2. Complete genomic sequencing of PMS2 from the maternal sample identified three variants, all of which lie within exon 11. c.1408C > T results in a missense change from proline to serine at residue 470; c.1454C > A results in a missense change from threonine to lysine at residue 485; and c.1840A > T results in the introduction of a stop codon at residue 614. Screening for these three variants in the paternal sample identified the same truncating mutation as well as the c.1454C > A SNP.
Discussion
The occurrence of polypoid colorectal carcinoma at a young age and a metachronous malignant glioma in the same patient along with a family history of colorectal carcinoma in three generations, endometrial carcinoma in the mother, a similar brain tumor in the identical twin, and absence of germline APC mutation suggests the presence of Lynch syndrome in our patient. The consanguinity of the parents and their carrier state is suggestive of an autosomal recessive pattern of inheritance. The patient also satisfied the modified Amsterdam criteria for Lynch syndrome.2
The PMS2 gene has been localized to chromosome 7p22 and is similar to the mutL MMR gene found in Escherichia coli.6 The mutL α heterodimer of PMS2 and MLH1 (another MutL homologue) functions as an MMR protein in humans.6 Germline PMS2 mutations are found in only about 2% of patients with Lynch syndrome and are mostly deleterious in nature. In general, PMS2 mutations are believed to have low penetrance in the heterozygous state but can cause tumors at an earlier age in those with homozygosity for the mutated gene, as seen in our patient. Although PMS2 knockout mice have mutational rates similar to MLH1 null counterparts, the incidence of cancer in PMS2-deficient individuals is extremely rare and has been reported in only about five families thus far.6 Mutation sites in the PMS2 gene have included exon 14 (R802X), junction of exon 10/11 (1145ins20), exon 11 and 14 (1221delG, 2361delCTTC), exon 5 and 13 (R134X, 2184delTC), and exon 12 (E705K).6 Our patient inherited a nonsense mutation of exon 11 (c.1840A > T) from both parents that resulted in the introduction of a stop codon at residue 614; this specific mutation, to the best of our knowledge, has not been reported previously.6,8
While MMR gene mutations are associated with MSI in > 95% of cases, occasional patients are apparently MS-stable as assessed by conventional methods in the face of impaired gene function. In a recent population-based analysis of MMR mutations that sought to identify Lynch syndrome in 543 women with endometrial carcinoma, Hampel et al.9 found that one patient, whose tumor was MS-stable, had absent MSH6 immunostaining and a corresponding deleterious MSH6 mutation. It is likely that our patient has a similar combination of MS stability (based on testing with the MSI markers as recommended by the NIH) in the face of a nonsense PMS2 mutation. Similarly, Kuismanen et al.10 have shown that in a group of patients with endometrial and colorectal cancers with identical predisposing mutations in MLH1 and MSH2, 5 (11%) of 44 colorectal tumors and 13 (23%) of 57 endometrial cancers were stable for all microsatellite markers studied, even though IHC analysis demonstrated the absence of the specific MMR protein. Possible reasons for the inability to demonstrate MS instability could be related to normal tissue contamination or intratumoral inhomogeneity and overrepresentation of a MS-stable clone.10 However, apparent MS stability in the tumor tissue of our patient might imply that the absence of PMS2 protein did not play a role in the development of the colorectal or brain tumors. Alterations in the PMS2 gene can also cause malignancies distinct from those found in Lynch syndrome. De Vos et al.6 have recently described an autosomal recessive syndrome in a family characterized by café-aulait spots and childhood malignancies (notably supratentorial primitive neuroectodermal tumors) associated with a homozygous mutation in exon 14 of the PMS2 gene. There was a notable absence of bowel cancers in this family, and the heterozygotes were clinically normal.
Additional features of interest in this patient are the cooccurrence of agenesis of the corpus callosum, mental retardation, and transcobalamin II deficiency, which have not been reported previously in patients with Lynch syndrome. Agenesis of the corpus callosum occurs in < 0.7% of children worldwide and can be associated with at least 17 syndromes of autosomal or X-chromosome-linked inheritance.11 Agenesis can be partial or complete. The neurologic manifestations of this condition are related partly to the agenesis and associated other CNS malformations. Patients usually present with mental retardation, seizures, and motor deficits.11 Transcobalamin II deficiency is a autosomal recessive disorder that leads to absence of transcobalamin II and symptoms of vitamin B-12 deficiency.12,13 Transcobalamin II (TCII) is a carrier protein for vitamin B-12 in the plasma and is responsible for delivery of the vitamin to the cell via the TCII receptor.14 The TCII gene is located on chromosome 22q12 – 13.15 Transcobalamin II deficiency usually presents following birth with megaloblastic anemia, irritability, failure to thrive, neurologic abnormalities, and immune deficiency usually in the form of hypogammaglobulinemia.13,14 The condition usually responds to weekly injections of high-dose vitamin B-12 (usually 1 mg) and can lead to impaired brain development due to delayed diagnosis and/or absence of specific treatment.13
There is an unclear relationship between these two specific genetic conditions and the germline PMS2 mutation in our patient. Although the parents of our patient are obligate carriers of a mutated TCII gene, the exact genetic mutation that resulted in agenesis of the corpus callosum in our patient is unknown. Of interest, agenesis of the corpus callosum has been associated with mutations in the region of 7p13 in the acrocallosal and Greig’s cephalopolysyndactyly syndromes.11 However, it is unclear whether the patient’s brain malformation was a familial trait, because none of his siblings with transcobalamin II deficiency have had abnormalities of the corpus callosum.
Footnotes
Presented in part at the Twelfth International Society of Pediatric Neuro-Oncology Meeting, held in Nara, Japan, June 6 – 9, 2006.
References
- 1.Merg A, Lynch HT, Lynch JF, et al. Hereditary colon cancer — part I. Curr Probl Surg. 2005;42:195 – 256. doi: 10.1067/j.cpsurg.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919 – 932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839 – 847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides NC, Papadopoulos NN, Liu BB, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75 – 80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 5.De Rosa M, Fasano C, Panariello L, et al. Evidence for a recessive inheritance of Turcot’s syndrome caused by compound heterozygous mutations within the PMS2 gene. Oncogene. 2000;19:1719 – 1723. doi: 10.1038/sj.onc.1203447. [DOI] [PubMed] [Google Scholar]
- 6.De Vos M, Hayward BE, Picton S, et al. Novel PMS2 pseudogenes can conceal recessive mutations causing a distinctive childhood cancer syndrome. Am J Hum Genet. 2004;74:954 – 964. doi: 10.1086/420796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa H, Lockman JC, Frankel WL, et al. Mismatch repair gene PMS2: disease-causing germline mutations are frequent in patients whose tumors stain negative for PMS2 protein, but paralogous genes obscure mutation detection and interpretation. Cancer Res. 2004;64:4721 – 4727. doi: 10.1158/0008-5472.CAN-03-2879. [DOI] [PubMed] [Google Scholar]
- 8.Clendenning M, Hampel H, LaJeunesse J, et al. Long-range PCR facilitates the identification of PMS2-specific mutations. Hum Mutat. 2006;27:490–495. doi: 10.1002/humu.20318. [DOI] [PubMed] [Google Scholar]
- 9.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810 – 7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 10.Kuismanen SA, Moisio AL, Schweizer P, et al. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Pathol. 2002;160:1953–1958. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamnasaran D. Agenesis of the corpus callosum: lessons from humans and mice. Clin Invest Med. 2005;28:267 – 282. [PubMed] [Google Scholar]
- 12.Hall CA. The neurologic aspects of transcobalamin II deficiency. Br J Haematol. 1992;80:117 – 120. doi: 10.1111/j.1365-2141.1992.tb06410.x. [DOI] [PubMed] [Google Scholar]
- 13.Monagle PT, Tauro GP. Long-term follow up of patients with transcobalamin II deficiency. Arch Dis Child. 1995;72:237 – 238. doi: 10.1136/adc.72.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamoun P. Transcobalamin II deficiency. [Accessed September 24, 2007];Orphanet Encyclopedia. 2003 :1–2. Available at http://www.orpha.net/data/patho/GB/uk-TCII.pdf.
- 15.Seetharam B, Li N. Transcobalamin II and its cell surface receptor. Vitam Horm. 2000;59:337 – 366. doi: 10.1016/s0083-6729(00)59012-8. [DOI] [PubMed] [Google Scholar]