Abstract
Cytotoxic chemotherapy that induces lymphopenia is predicted to ablate the benefits of active antitumor immunization. Temozolomide is an effective chemo-therapeutic agent for patients with glioblastoma multiforme, but it induces significant lymphopenia. Although there is monthly fluctuation of the white blood cell count, specifically the CD4 and CD8 counts, there was no cumulative decline in the patient described in this case report. Depriving patients of this agent, in order to treat with immunotherapy, is controversial. Despite conventional dogma, we demonstrated that chemotherapy and immunotherapy can be delivered concurrently without negating the effects of immunotherapy. In fact, the temozolomide-induced lymphopenia may prove to be synergistic with a peptide vaccine secondary to inhibition of regulatory T cells or their delayed recovery.
Keywords: active immunotherapy, antibody, antigen, CNS neoplasms, cytotoxic T lymphocyte, epidermal growth factor receptor, glioma
Despite aggressive surgical resection, high-dose focused radiation therapy, and chemotherapy, patients diagnosed with glioblastoma multiforme (GBM) have a median survival time of 14 months after diagnosis.1 Failure of therapy can be attributed, at least in part, to its lack of specificity for neoplastic tissue, which results in dose-limiting systemic or neurological toxicity. The use of immunotherapy has held promise for the potential treatment of these tumors, but until recently, few attempts have demonstrated clinical efficacy. Several clinical trials with selected glioma patients, involving vaccinating them with dendritic cells and either acid-eluted peptides2 or an antigen-specific peptide,3 have demonstrated promise by increasing median survival time up to 31 months. Temozolomide (TMZ), a methylating chemotherapeutic agent, has recently been shown to prolong survival in patients with GBM and has become part of the standard regimen used to treat them,1 but TMZ also often induces a profound and long-lasting lymphopenia4 that may limit such promising and specific immunotherapeutic approaches.
To test the hypothesis that chemotherapy and immunotherapy can be administered sequentially over time, we treated a patient with GBM using TMZ both concurrently with radiation and in postradiation cycles every 28 days, while also administering a tumor-specific peptide vaccine targeting the epidermal growth factor receptor variant III (EGFRvIII).5,6 The target antigen is the most frequent tumor-specific genetic alteration associated with GBM. The amplification of the EGFR gene, which results in overexpression of EGFR, a transmembrane tyrosine kinase receptor,7 is associated with the mutant EGFR gene EGFRvIII.8 Previous work has shown that EGFR amplification is evident in all GBMs expressing EGFRvIII9 and that GBMs lacking the amplified EGFR are not positive for EGFRvIII protein.10
Multiple preclinical model systems have demonstrated that the depletion of immune cell subsets can abrogate the efficacy of several types of immunotherapeutic approaches,11 indicating that chemotherapy administered during the effector stages of immunotherapy may be deleterious. However, this does not preclude using these agents together when appropriately timed to minimize the aforementioned effects. Furthermore, the depletion of certain suppressive lymphocyte subsets, such as regulatory T cells (Tregs),12,13 may be a highly desirable outcome of chemotherapy, including TMZ,4 yielding greater immunotherapeutic efficacy or possibly promoting a desirable cytokine profile14 for adequate tumor control.
Case Material and Results
In May 2005, a 51-year-old Caucasian male was evaluated following complaints of a 3-week history of persistent morning headaches without associated nausea. An MR image revealed a multilobular, irregularly enhancing lesion measuring 6.6 × 5.3 × 4.3 cm in the anterior aspect of the right temporal lobe. The Sylvian fissure was bowed upward, and there was a midline shift of 6 mm (Fig. 1). The patient underwent a gross total resection of the tumor, which was histologically determined to be a biphasic glioblastoma and malignant sarcoma. Immunohistochemical staining with EGFR-528 and EGFRvIII antibodies was positive,9 with the EGFRvIII staining demonstrating strong diffuse reactivity (Fig. 2) within the glioma component, whereas the EGFR-528 staining was more focal. Staining for PTEN protein was strongly positive, and p53 reactivity was present in more than 30% of tumor nuclei. The methylguanine-DNA methyl-transferase DNA-repair gene was methylated.15 Postoperatively, the patient underwent conventional external beam radiotherapy of 6,000 cGy in 30 fractions. TMZ (75 mg/m2) was administered concurrently daily during radiotherapy.1 An MR image taken at the completion of radiotherapy was unchanged and demonstrated no evidence of progression (Fig. 1).
Fig. 1.
Contrast-enhanced axial (top) and coronal (bottom) MR images in a patient with glioblastoma multiforme showing extent of the tumor prior to resection, after resection, and 1 year after treatment with temozolomide and immunotherapy.
Fig. 2.
Photomicrograph of immunohistochemically stained operative specimen demonstrating the features indicative of glioblastoma multiforme and with positive staining for epidermal growth factor receptor variant III (original magnification, ×200).
We then treated the patient with three intradermal injections of an EGFRvIII-specific peptide, PEPvIII (LEEKKGNYVVTDHC), conjugated to keyhole limpet hemocyanin (PEPvIII-KLH) to stimulate antibody responses at a 1:1 ratio (wt/wt; 500 μg/immunization), along with granulocyte-macrophage colony-stimulating factor (140 μg/immunization) every 2 weeks over an interval of 6 weeks. Prior to vaccination, the patient underwent leukapheresis to obtain sufficient cells for monitoring immunological responses to the vaccine. A second leukapheresis was also obtained after the third vaccination for the same purpose. After the third vaccination, maintenance cycles of TMZ were started at a dose of 150 mg/m2 on days 1–5 of each 28-day cycle. Beginning on day 19 of each cycle, complete blood counts were monitored every other day until there was evidence of recovery of the white blood cell count nadir, at which point the patient received the vaccine intradermally, usually on day 23 (range = 19–25) of his 28-day cycle (Fig. 3).
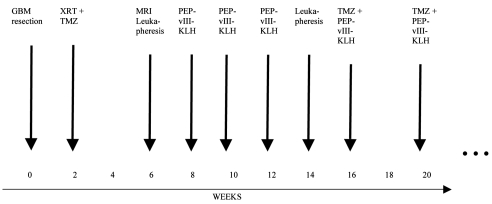
Fig. 3.
Study schema in a patient with glioblastoma multiforme (GBM) receiving temozolomide (TMZ) and the PEPvIII keyhole limpet hemocyanin (PEPvIII-KLH) vaccine. Abbreviation: XRT, radiation therapy.
Delayed-type hypersensitivity (DTH) testing to common recall antigens and the components of the vaccine was evaluated prior to the start of vaccine administration, after the third vaccination, and monthly during his maintenance cycle, on day 26. Prior to the initiation of vaccine administration and after the completion of radiation therapy with concurrent TMZ administration, the patient was reactive only to Candida and had no DTH reaction to the components of the vaccine, PEPvIII or KLH. After the 10th vaccination, and while concurrently receiving TMZ, he developed a DTH response to the PEPvIII component of the vaccine. A third leukapheresis was performed at this point to evaluate induced immune responses. The patient has continued to show marked DTH induration (16 × 15 mm) at the PEPvIII injection site. This indicates that the TMZ did not negatively influence the development of DTH responses, at least in this particular patient.
To determine if PEPvIII-specific humoral responses were induced, serum was obtained from the patient monthly and analyzed in a PEPvIII-Dynabead assay (Invitrogen, Carlsbad, CA, USA) in which PEPvIII or the extracellular domain of EGFRvIII (EGFRvIII-ECD) was covalently linked to magnetic microspheres that were used to capture specific antibodies from the patient’s serum. To determine specificity, an additional sample set was preincubated for 15 min with 500 ng of the PEPvIII peptide to block any anti-PEPvIII that would be captured by the PEPvIII-conjugated Dynabeads. Standards of human-mouse chimeric anti-PEPvIII antibody (81–0.11 ng/ml) were run with each assay along with a positive patient sample and negative (normal donor serum) controls. Prior to the administration of the vaccine, no humoral responses to the EGFRvIII were detected by either PEPvIII-labeled or EGFRvIII-ECD-labeled beads. After vaccination, there was a significant increase in the immunoglobulin G (IgG) response to EGFRvIII. The magnitude of the response was equivalent against both PEPvIII and EGFRvIII-ECD. These humoral responses have been maintained despite the continued administration of TMZ (Fig. 4). In preclinical models systems, in vivo depletion assays and serum transfers demonstrated that antibody-dependent cellular cytotoxicity contributed to the efficacy of the PEPvIII-KLH vaccine.11
Fig. 4.
Flow cytometric analysis of PEPvIII-specific humoral responses induced in the peripheral blood of a patient with glioblastoma multiforme. Before the patient received any vaccine (pre), there was no detectable humoral response to PEPvIII, but after the third vaccination, there was a marked induction of PEPvIII IgG-specific responses (post). These induced humoral responses did not appear to be diminished during the sequential administration of temozolomide and the PEPvIII-keyhole limpet hemocyanin vaccine. The numbers on the x-axis denote the vaccination number; mean fluorescence intensity (MFI) is indicated on the y-axis.
To determine if there was a cumulative decline of the patient’s overall white blood count, absolute CD4 or absolute CD8 counts were monitored at least every 2 weeks from the time of diagnosis. As anticipated, there was monthly variability in response to the administration of TMZ, but there was no cumulative decline. To further clarify whether TMZ would affect the induced CD8+ cytotoxic responses to PEPvIII, the patient’s peripheral blood mononuclear cells (PBMCs) from each leukapheresis and monthly PBMC were stimulated with PEP-1 (HDTVYCVKGNKELE; 10 μg/ml) as a negative control or with the PEPvIII (10 μg/ml) vaccine component. Mouse antihuman CD28 and CD49d antibodies (eBioscience, San Diego, CA, USA) at final concentrations of 2 μg/ml provided T-cell costimulation.16 The negative control consisted of unstimulated cells. The cells were stained for surface markers (CD3, CD4, CD8) by incubation with the appropriate fluorescein isothiocyanate (FITC)–labeled and allophycocyanin (APC)-labeled primary antibody or isotype control (BD Biosciences Pharmingen, San Diego, CA, USA). Peptide-specific intracellular cytokine analysis was performed as previously described17 and included corresponding isotype controls. After staining, cells were washed, and a minimum of 1 × 105 live, gated events were assessed by flow cytometry on a FACSCalibur flow cytometer using Cellquest software (BD Immunocytometry Systems, San Jose, CA, USA). Prior to receiving the vaccine, the patient had minimal response with the unstimulated control (0.11% CD3+CD8+ gamma-interferon [γ-IFN]–producing T cells) and with the PEP-1–negative control (0.08% CD3+CD8+ γ-IFN–producing T cells). Prior to receiving the vaccine, there were only 0.06% CD3+CD8+ γ-IFN–producing PEPvIII-specific T cells. After receiving the first three vaccinations, there was an increase in PEPvIII-specific γ-IFN–producing T cells to 0.81%. Despite the sequential administration of TMZ and the peptide vaccine, the percentage of responding CD3+CD8+ γ-IFN–producing PEPvIII-specific T cells persisted at 0.57% (Fig. 5). Furthermore, CD3+CD8+ alpha-tumor necrosis factor (α-TNF)–producing and CD3+CD4+ α-TNF–producing PEPvIII-specific T cells were induced (Fig. 5),17 indicating the induction of proinflammatory and antitumor immune activity.
Fig. 5.
Flow cytometric analysis of PEPvIII-specific CD4 and CD8 T-cell responses induced in the peripheral blood of a patient with glioblastoma multiforme. To further clarify whether the temozolomide would affect the induced CD8+ cytotoxic responses to PEPvIII, the patient’s peripheral blood mononuclear cells (PBMCs) from each leukapheresis and monthly PBMCs were stimulated with the PEP-1 protein (HDTVYCVKGNKELE; 10 μg/ml) as a negative control or PEPvIII (10 μg/ml), a vaccine component. The induced immune responses were specific to the components of the vaccine and were sustained despite the sequential administration of temozolomide. As anticipated, gamma-interferon (γ-IFN) responses were initially detected, but later the patient developed PEPvIII-specific alpha-tumor necrosis factor (TNFα) responses. Abbreviations: FITC, fluorescein isothiocyanate–labeled; APC, allophycocyanin-labeled.
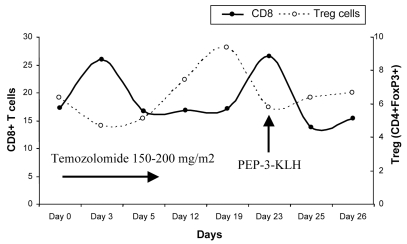
To characterize the response of the various T-cell populations during a cycle of TMZ (5/28 schedule) and concurrently administered vaccine (day 23 in this example), we obtained peripheral blood on days 0, 3, 5, 12, 19, 23, 25, and 26. Using flow cytometry, we investigated the percentage of the CD8+ T-cell and CD4+FoxP3+ Treg subsets during this cycle. All fluorescently labeled mono-clonal antibodies (mAbs; PerCP[Cy5.5]-CD3, FITC-CD8, APC-CD4) were purchased from BD Biosciences, except for the FITC-labeled mAb of FoxP3, which was made by eBioscience. The surface and intracellular staining of peripheral blood cells was performed according to the standard procedures provided by the manufacturer. In contrast to the decline of the CD8+ T-cell subset, the Treg population started to increase after the administration of TMZ for 3 days and reached its peak (9.38% of total CD4+ T cells) on day 19. The Tregs then began to drop until day 23, while the CD8+ T-cell numbers started to recover. At the end of the course, both the CD8+ T-cell and Treg populations recovered to pretreatment levels (Fig. 6). The vaccination resulted in a boost in numbers of CD8+ cytotoxic T cells at a time when Tregs were relatively diminished.
Fig. 6.
Flow cytometric determination of the absolute percentage of CD8+ T cells (gated on total lymphocytes) and regulatory T cells (Treg) (CD4+CD25+Foxp3+; gated on CD4+ T cells) in the peripheral blood of a glioblastoma multiforme patient during the course of treatment with temozolomide and immunotherapy. The vaccine was administered on day 23 during this particular cycle. Abbreviation: KLH, keyhole limpet hemocyanin.
Over 30 months, the patient underwent a complete physical examination and brain MR imaging at 2-month intervals. His exam remained stable, and he worked full time as a physician without impairment with a KPS of 100% and a Mini Mental State Exam score of 30/30. At 30 months he progressed and is receiving adjuvant therapy.
Discussion
First and foremost, this report demonstrates that concurrent antitumor active immunization is not contraindicated during chemotherapy with TMZ in patients with GBM. We present several findings that indicate that the coadministration of TMZ has not affected the efficacy of the PEPvIII-KLH vaccine. First, the patient developed DTH responses to the PEPvIII component of the vaccine, even while receiving TMZ, and the area of PEPvIII DTH reactivity has continued to increase with subsequent vaccinations despite continued treatment with TMZ. Similarly, PEPvIII-specific CD3+CD8+ γ-IFN–producing T cells induced by vaccination do not appear to be diminished during cycles of concurrently administered TMZ, which was monitored during the third leukapheresis. In addition, PEPvIII-specific IgG responses were induced after the third vaccination and have been maintained while the concurrent TMZ was administered. Finally, we have followed the CD8+ T-cell and Treg populations during a single treatment cycle and found that there appears to be a window of T-effector (CD8+ T cell) responsiveness with a relative diminution of Tregs. Thus, the concurrent administration of TMZ during active immunization, in the manner we described, does not appear to diminish the induced immune responses.
The use of lymphodepletion to augment immunological responses has been described both in murine model systems18,19 and in human cancer patients.20,21 Multiple mechanisms have been proposed to be responsible for these enhanced antitumor responses. Lymphodepletion may remove competition at the surface of antigen-presenting cells;22 enhance the availability of cytokines such as interleukin-7 and interleukin-15, which augment T-cell activity;14 and deplete the immune inhibitory Tregs.23 Chemotherapy could also potentially augment immunological responsiveness by enhancing immune priming and presentation,24 antigen expression,25 and targets for immune eradication.26 In a dose-intensive schedule of TMZ of 75 mg/m2/day in cycles of 6 weeks followed by a 2-week break, lymphopenia was induced in 60% of patients and was sustained in most patients for at least 2 months after the drug was discontinued.4 Although this schedule may result in sustained suppressed Tregs, it would theoretically inhibit desirable effector T-cell responses as well. Therefore, we elected to administer a short course of TMZ to allow for the clonotypic expansion of responding PEPvIII-specific T cells. We hypothesized that when a vaccination is administered during the nadir of TMZ, there may be an enhanced effector response. Such effector responses may be secondary to a lag in the recovery of Tregs, thus allowing a greater clonotypic expansion than otherwise would have been seen without TMZ. This was certainly observed during monitored chemotherapy/immunotherapy cycles in this particular patient. The lag of recovery of Tregs relative to effector T cells is not surprising given the normal physiological roles of immune cell responses. In order to mount an immune response, T effectors would need to become activated, proliferate, and mediate their response quickly. However, if this heightened response remained unchecked by hemostatic mechanisms such as Tregs, then the T-cell proliferation would escalate unabated. Therefore, the delay of Treg response would allow for efficacious immune responses but eventual down-modulation/ regulation of this response.
Conclusion
In conclusion, this case report suggests that sequential administration of chemotherapy and immunotherapy may not be deleterious; however, studies with additional patients are needed for confirmation of our findings.
Acknowledgments
We thank David M. Wildrick, Ph.D., for editorial assistance with the manuscript. This work was supported by the Commonwealth Cancer Foundation, the Adam Sliger Foundation, the Rose Foundation, and the National Brain Tumor Foundation (A.B.H.), by NIH grants SPORE 1P50 CA108786 (D.D.B./J.H.S.) and R01 CA09722 (J.H.S.), and by grants from the Brain Tumor Society (J.H.S.) and Accelerate Brain Cancer Cure Foundation (J.H.S./D.D.B.).
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 3.Archer G, Bigner D, Friedman A, et al. Dendritic cell vaccine for intracranial tumors I (DC Victori Trial). Paper presented at: Society for Neuro- Oncology, Education Day and Ninth Annual Scientific Meeting; 2004; Toronto, Ontario, Canada. [Google Scholar]
- 4.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Heimberger AB, Archer GE, Crotty LE, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50:158–164. doi: 10.1097/00006123-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48:2231–2238. [PubMed] [Google Scholar]
- 7.Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 8.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 9.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 10.Aldape K, Ballman K, Furth A, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- 11.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–4254. [PubMed] [Google Scholar]
- 12.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 13.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Dunn HS, Suni MA, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 17.Karanikas V, Lodding J, Maino VC, McKenzie IF. Flow cytometric measurement of intracellular cytokines detects immune responses in MUC1 immunotherapy. Clin Cancer Res. 2000;6:829–837. [PubMed] [Google Scholar]
- 18.Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125:711–714. [PubMed] [Google Scholar]
- 19.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber J, Salgaller M, Samid D, et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2’-deoxycytidine. Cancer Res. 1994;54:1766–1771. [PubMed] [Google Scholar]
- 25.Aquino A, Prete SP, Greiner JW, et al. Effect of the combined treatment with 5-fluorouracil, gamma-interferon or folinic acid on carcino-embryonic antigen expression in colon cancer cells. Clin Cancer Res. 1998;4:2473–2481. [PubMed] [Google Scholar]
- 26.Ciusani E, Perego P, Carenini N, et al. Fas/CD95-mediated apoptosis in human glioblastoma cells: a target for sensitisation to topoisomerase I inhibitors. Biochem Pharmacol. 2002;63:881–887. doi: 10.1016/s0006-2952(01)00837-1. [DOI] [PubMed] [Google Scholar]