Abstract
Analytical instruments that can measure small amounts of chemicals in complicated biological samples are often useful as diagnostic tools. However, it can be challenging to optimize these sensors using actual clinical samples, given the heterogeneous background and composition of the test materials. Here we use gas chromatography differential mobility spectrometry (GC/DMS) to analyze the chemical content of human exhaled breath condensate (EBC). Ultimately, this system can used for non-invasive disease diagnostics. Many parameters can be adjusted within this instrument system, and we implemented a factorial design-of-experiments to systematically test several combinations of parameter settings while concurrently analyzing effects and interactions.
We examined four parameters that affect sensitivity and detection for our instrument, requiring a 24 factorial design. We optimized sensor function using EBC samples spiked with acetone, a known clinical biomarker in breath. Two outputs were recorded for each experiment combination: number of chemicals detected, and the amplitude of acetone signal. Our goal is to find the best parameter combination that yields the highest acetone peak while also preserving the largest number of other chemical peaks in the spectra. By optimizing the system, we can conduct further clinical experiments with our sensor more efficiently and accurately.
Keywords: design-of-experiments, breath analysis, differential mobility spectrometer (DMS), biosensor, detection
INTRODUCTION
More than 1000 trace volatile organic compounds (VOC’s) have been detected in human breath [1–6]. Because of this, breath analysis provides a wide range of opportunities for diagnosis of diseases and other clinical concerns, and this method for testing is favorable due to the fact that it is both inexpensive and non-invasive [1, 7, 8]. In the case of assessing chronic obstructive pulmonary disease (COPD), breath analysis is useful for its suitability in longitudinal studies as well as its possible ability to monitor responses to inflammatory therapy [7, 8]. Combined with glucose analysis, investigating acetone content in breath has proved useful in differentiating between non-diabetic and diabetic patients [9]. Analysis of exhaled breath condensate has also been used to detect markers of airway inflammation in patients with primary ciliary dyskinesia (PCD) [10]. In a different disease system, Mazzone, et al. found a pattern of VOC’s in exhaled breath of patients with lung cancer to be unique from control non-diseased patients. They were able to predict the presence of lung cancer with a sensitivity of 73.3% and a specificity of 72.4% [11].
Analysis of breath condensate can be carried out through the use of a gas chromatograph / mass spectrometer (GC/MS) or other sensitive chemical analysis instruments. A smaller and less expensive, yet highly sensitive, alternative is the miniature differential mobility spectrometer (DMS). It has been demonstrated that this device has the potential to detect both potential biological and chemical warfare agents [12, 13]. Detection of explosive residues in air at low parts-per-billion (ppb) levels has also been demonstrated [14]. Focusing on bacteria classification, we have been able to not only successfully find markers that identify bacterial species based on their volatile signatures [15, 16], but have also been able to distinguish between species of spore-forming bacteria which may be found in environmental samples [17]. DMS has also been employed in the classification of fuel, with correct classification rates of 95 ± 0.3% [18]. We have recently begun using this instrument for breath analysis applications [19].
A key challenge in using a detection system like the GC/DMS, resides in optimizing its parameters to best suit the sample of interest. When so many variables are involved, testing each parameter individually can be both time consuming and expensive research. A 2n factorial design-of-experiments (DOE’s) provides investigators with a systematic statistical approach to examining all possible effects and interactions of many (n) variables simultaneously, while also observing complex non-linear interaction effects between variables at two different values.
Previously, factorial design-of-experiments has been used to optimize many types of instrumentation systems, such as selection of biodiesel production parameters [20], optimization of bulk manufacturing methods [21, 22], characterization of catalyst systems [23], design of protonic exchange membrane (PEM) fuel cells [24], biochemistry process characterization [25–31], and the drug discovery process [32]. Another critical application of this statistical approach is optimizing gas chromatography and/or mass spectrometry parameter settings [33–36].
To date, no group has demonstrated using a factorial DOE approach to optimization of breath analysis instrumentation, despite the complexity of these systems. In the case of exhaled breath condensate analysis, a factorial design-of-experiments approach may allow for tailor optimized protocols and instrumentation design that in turn will allow for precision detection for better clinical diagnostics.
MATERIALS AND METHODS
Collection/preparation of exhaled breath condensate samples
Exhaled breath condensate (EBC) samples were collected from 6 individuals using established protocols [19] and commercially available R-tubes (Respiratory Research, Inc; Charlottesville, VA). Briefly, study participants exhaled through the R-tube surrounded by a pre-chilled aluminum sleeve with a starting temperature of −20 °C. EBC was collected for 5 minutes, and then transferred to storage vials. Finally, 1 mL of EBC from each person was mixed together to form a heterogeneous mixture to prevent the method from being tailored for a single individual sample type. Acetone (Sigma-Aldrich; St. Louis, MO) was used to spike the samples, resulting in a concentration of 833 parts-per-billion (ppb). Replicates of test samples were dispensed into 100 µL aliquots and stored in 2 mL borosilicate vials containing polytetrafluoroethylene (PTFE) silicone septa (Supelco; Bellefonte, PA) and were stored in a −10 °C freezer. (Restek; Bellefonte, PA) before testing.
Design of experimental instrumentation platform
Headspace sampling was performed on each sample using either 85 µm polyacrylate or 65 µm polydimethylsiloxane-divinylbenzene (PDMS-DVB) coated solid phase microextraction (SPME) fibers (Supelco; Bellefonte, PA) for 30 minutes at 37°C under agitation. The concentrated EBC samples were then analyzed using a 4000 Varian gas chromatograph GC System (Varian, Inc.; Palo Alto, CA) which contains a 30 m long and 0.25 mm wide, FactorFour Capillary column. After desorption into the GC, the analytes are carried through the column using 1.0 mL min−1 helium as a carrier gas. During the cryogenic cooling cycle the column oven maintains temperature at 0 °C for 10 minutes. It is then heated according to the following protocol: 50 °C at 10 °C min−1, 75°C at 10°C min−1 with a hold time of 5 minutes, 100 °C at 10 °C min−1 with a hold time of 5 minutes, 125°C at 10 °C min−1 with a hold time of 10 minutes, 150°C at 10 °C min−1 with a hold time of 10 minutes, and a final temperature of 200 °C at 10 °C min−1 with a hold time of 10 minutes. During non-cryo-cooling cycles the GC starts at 50 °C but then follows the same GC profile as outlined above.
Upon exiting the GC, sample ions are then carried through the miniature differential mobility spectrometer (DMS) prototype (Sionex Corporation; Bedford, MA) with nitrogen, where they are first ionized by a 63Ni source before passing through the sensor drift tube region. The flow of nitrogen is controlled by a mass flow controller and is set at either 250 mL min−1 or 500 mL min−1, depending on the experiment. RF voltage is set at either 1200 V or 1400 V, depending on the experiment, while the compensation voltage is scanned between −43 V and +12 V, at 6 msec intervals.
Design-of-experiments approach to data analysis
A “design-of-experiments” approach is an efficient alternative to optimizing system performance and is frequently used in engineering and industry to optimize complex electromechanical systems. We use an nk factorial design to test all different configurations of a chosen set of instrument parameters, each at 2 different settings (p<0.05 significance). Three replicate samples are measured for each instrumentation configuration. For our optimization process, we are testing four specific parameters in our system: RF voltage (1200 V or 1400 V), nitrogen carrier gas flow rate (250 mL/min or 500 mL/min), SPME fiber type (Polyacrylate or PDMS-DVB coated), and GC cryogenic cooling (on or off). Outcome for our experiments is measured in two ways: total number of peaks which represent putative distinct chemicals in the samples, and the amplitude of the spiked acetone signal. Two criteria were used for peak selection: the intensity of a selected peak should be higher than neighboring pixels, and the intensity of a selected peak should be higher than the mean intensity plus 2X the standard deviation of the amplitude of the entire spectra. These two criteria can be expressed below:
where, i and j denote retention time index and compensation voltage index, respectively, and p and q represent the neighborhood of the pixel (i, j). In this study they are set to be 10 and 3, respectively, as the retention time has a higher resolution than the compensation voltage. The multiplier factor k is set to be 3.
RESULTS
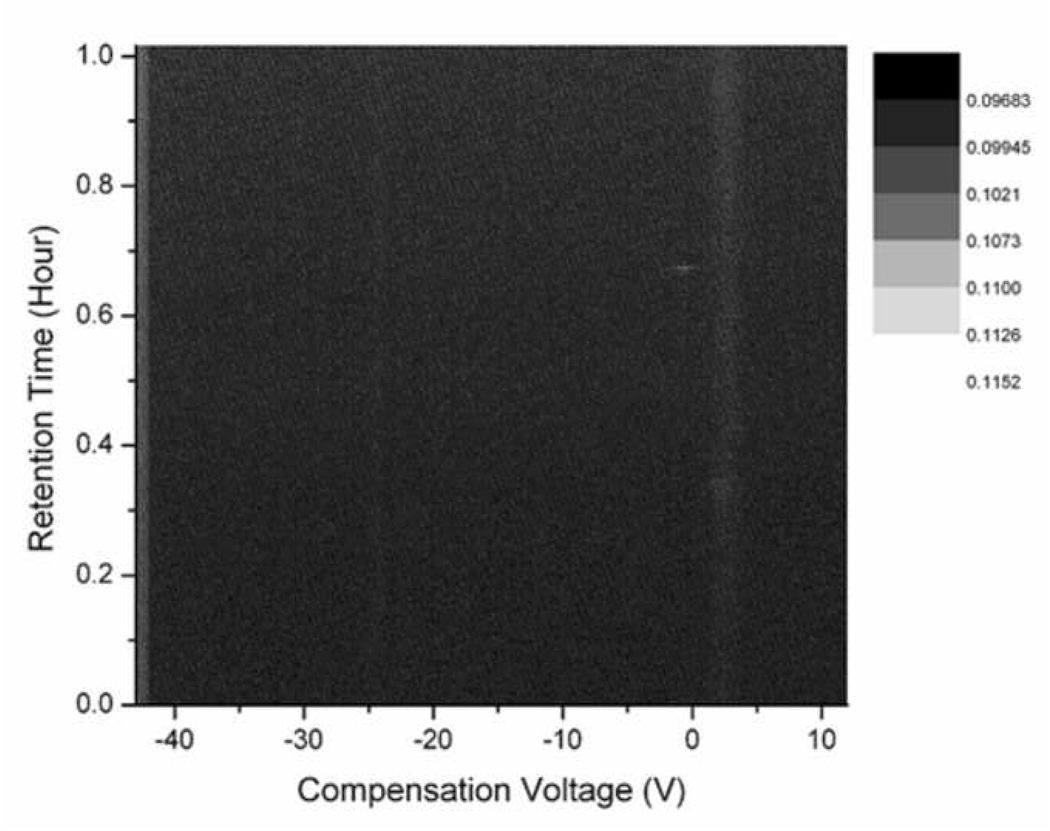
Figure 2 shows a representative spectra trace obtained from the GC/DMS system, and we record both positive ion spectra (top panel) and negative ion spectra (bottom panel) from distinct electrodes. As can be seen, many high amplitude peaks are recorded in the positive spectra, as represented by light colored bands scattered through the plot. These peaks correspond to specific chemicals which are exiting the GC at various retention times. While major large amplitude peaks are clearly visible without magnification, there are also many smaller amplitude peaks that can be measured above the baseline noise. The negative spectra showed fewer high amplitude peaks, but had comparable numbers of smaller amplitude peaks as the positive spectra. These positive and negative ion spectra were acquired three times for each of the instrumentation configurations noted in the DOE approach.
Figure 2. Exhaled breath condensate (EBC) samples spiked with acetone as measured by GC/DMS.
Both positive (top panel) and negative (bottom panel) ion spectra are shown as representative data plots. Color intensity of the points in the spectra indicate chemical abundance, and the chemicals are resolved along both the time and compensation voltage axes by both the gas chromatograph and the differential mobility spectrometer, respectively.
Table 1 through Table 3 portray the results of the factorial design-of-experiments conducted. For each system configuration, we calculated the average number of peaks and the average amplitude of the acetone peak. Table 1 shows that the use of configuration 6 yields the maximum average number of peaks. The maximum of 388 peaks was acquired using an RF voltage of 1400 V, carrier gas flow rate 250 mL min−1, polyacrylate coated SPME, and a non-cryogenic GC profile. The maximum average acetone amplitude was acquired using configuration 9, where RF voltage was set at 1200 V, carrier gas flow rate at 500 mL/min, SPME was polyacrylate coated, and a cryogenic cooling GC profile was used.
Table 1. Design-of-experiments variation of parameters.
Several user-defined parameters were selected factorial experiment optimization: (A) the RF voltage of the DMS sensor (1200 V or 1400V); (B) nitrogen carrier gas flow rate through the DMS (250 or 500 mL min−1); (C) solid phase microextraction (SPME) filter type (Polyacrylate or PDMS-DVB); (D) GC cooling profile (cryogenic and non-cryogenic). A total of 16 different combinations of instrument settings were tested with n=3 replicates for each configuration. Plus signs denote where the larger values of each parameter are used, whereas minus signs require the use of lower parameter value. Signs of the interactions are determined by the resulting sign of the multiple of interacting variables. Two outputs are measured: total number of chemical peaks (Y), and the amplitude of acetone peak (Z).
| Factor | Interaction of factors | Outputs | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Config. | A | B | C | D | AB | AC | AD | BC | BD | CD | ABC | ABD | BCD | ABCD | Y (avg) | Y (std) | Z (avg) | Z (std) |
| 1 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 332 | 6.9 | .1200 | 0.0005 |
| 2 | + | + | + | − | + | + | − | + | − | − | + | − | − | − | 361 | 35.1 | .1193 | 0.0005 |
| 3 | + | + | − | + | + | − | + | − | + | − | − | + | − | − | 313 | 28.3 | .1196 | 0.0003 |
| 4 | + | + | − | − | + | − | − | − | − | + | − | − | + | + | 335 | 14.1 | .1192 | 0.0005 |
| 5 | + | − | + | + | − | + | + | − | − | + | − | − | − | − | 245 | 104.2 | .1186 | 0.0001 |
| 6 | + | − | + | − | − | + | − | − | + | − | − | + | + | + | 388 | 70.8 | .1188 | 0.0002 |
| 7 | + | − | − | + | − | − | + | + | − | − | + | − | + | + | 264 | 61.8 | .1191 | 0.0002 |
| 8 | + | − | − | − | − | − | − | + | + | + | + | + | − | − | 370 | 58.8 | .1186 | 0.0004 |
| 9 | − | + | + | + | − | − | − | + | + | + | − | − | + | − | 137 | 5.0 | .1210 | 0.0002 |
| 10 | − | + | + | − | − | − | + | + | − | − | − | + | − | + | 126 | 3.2 | .1195 | 0.0004 |
| 11 | − | + | − | + | − | + | − | − | + | − | + | − | − | + | 133 | 9.0 | .1196 | 0.0001 |
| 12 | − | + | − | − | − | + | + | − | − | + | + | + | + | − | 137 | 3.2 | .1191 | 0.0003 |
| 13 | − | − | + | + | + | − | − | − | − | + | + | + | − | + | 258 | 18.9 | .1192 | 0.0002 |
| 14 | − | − | + | − | + | − | + | − | + | − | + | − | + | − | 202 | 75.6 | .1194 | 0.0002 |
| 15 | − | − | − | + | + | + | − | + | − | − | − | + | + | − | 224 | 22.2 | .1188 | 0.0002 |
| 16 | − | − | − | − | + | + | + | + | + | + | − | − | − | + | 233 | 90.0 | .1188 | 0.0004 |
Table 3. Effects and interactions of all tested parameters including signal-to-noise t-ratios for each case, based on number of peaks in the output spectra.
(A) Effects for each parameter were calculated using the average number of peaks found for each of the 16 configurations. Signal-to-noise-ratios were then found by dividing each effect by the calculated standard deviation. (B) Similarly, interactions and their respective t-ratios were also calculated by using the average number of peaks in accordance with the standard deviation.
| A. Main effects | ||||
|---|---|---|---|---|
| Factors of Interest | ||||
| A | B | C | D | |
| Main Effects | 144.6 | −38.79 | 5.042 | −30.63 |
| signal-to-noise t-ratio | 9.993 | −2.680 | 0.348 | −2.116 |
| significance (|te|>t95) | YES | YES | NO | YES |
| B. Interaction effects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interactions Between Factors | ||||||||||
| AB | AC | AD | BC | BD | CD | ABC | ABD | BCD | ABCD | |
| Interaction Effects | 57.04 | 6.208 | −44.29 | 4.292 | 19.46 | 4.458 | 6.958 | 29.96 | −2.458 | 57.04 |
| signal-to-noise t-ratio | 3.941 | 0.429 | −3.060 | 0.297 | 1.345 | 0.308 | 0.481 | 2.070 | −0.170 | 0.705 |
| significance (|te|>t95) | YES | NO | YES | NO | NO | NO | NO | YES | NO | NO |
We calculated the signal-to-noise t-ratios for each effect and interaction and compared these values to the critical t-value of 2.038, based on average acetone peak amplitude analysis. We found statistically significant effects for all of our main variables tested in this system (95% confidence level). However, significance of the interactions was found only in half of all possible combinations, including RF voltage with SPME type, carrier gas flow rate with SPME type, carrier gas flow rate with GC cooling profile, RF voltage with both carrier gas flow rate and GC cooling profile, and carrier gas flow rate with both SPME type and GC cooling profile.
When we performed similar analyses based on the average number of peaks recorded in the spectra, we found statistical significance in the effects of RF voltage, carrier gas flow rate, and GC cooling profile (95% confidence level). Significance was also found in the interactions of RF voltage with carrier gas flow rate, RF voltage with GC cooling profile, and RF voltage with both carrier gas flow rate and GC cooling profile. Interpretation of these results follows in the discussion section.
DISCUSSION AND CONCLUSIONS
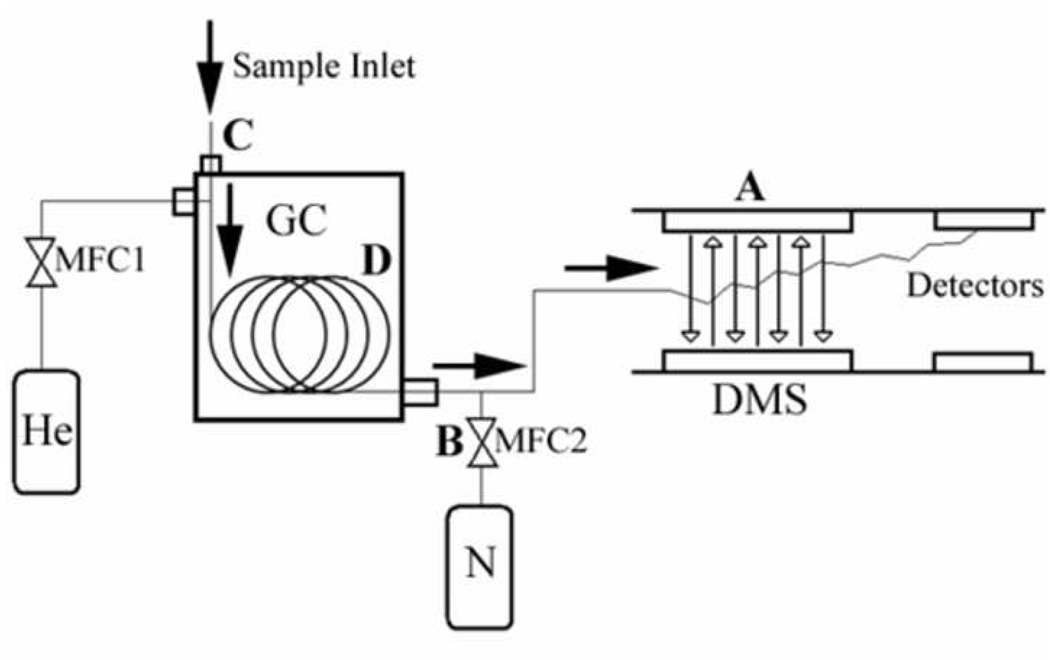
As shown in figure 1, we identified four important parameters from our system to test in this set of DOE experiments. Each of these four variables were found to have significant effects on the system output, but with varying degrees of importance. What is rather more interesting is that we were able to determine the optimum settings for our particular analytical application of this instrument system for breath analysis. We also found significant parameter interaction effects which were sometimes unexpected.
Figure 1. Experimental setup of GC-DMS system.
Chemical analysis is performed using gas chromatography differential mobility spectrometry (GC/DMS). Several user-defined parameters were selected factorial experiments: (A) the RF voltage of the DMS sensor. (B) nitrogen carrier gas flow rate through the DMS, (C) solid phase microextraction (SPME) filter type, (D) GC cooling profile.
Since RF Voltage is the main filtering component of the DMS [37], it is not surprising that this variable is the most significant in determining the number of peaks in an output spectra. As expected, a high RF voltage value resulted in increased resolution of chemicals that had co-eluted from the GC column. A high RF value, thus increased the number of identifiable peaks in the spectra.
Carrier gas flow rate proved to be the most significant single factor in the investigation of acetone peak amplitude, and it was also significant in the analysis of number of peaks. As the chemicals from the exhaled breath condensate elute off of the GC column, the carrier gas sweeps them into the DMS detection sensor. High values of flow rate correlated with lower numbers of measured chemicals, possibly due to dilution of low abundance chemicals. At the higher flow rate, the chemicals passed through the sensor very rapidly, which resulted in a lower acetone signal amplitude. This is potentially due to a lower probability of striking the capacitive detector plate in the DMS as they rapidly progress through the sensor system.
Cryogenic cooling is a common method used in conjunction with gas chromatography. After the sample is injected into the inlet of the analysis system, the interior of the GC oven is cooled as the chemicals are focused onto the entrance of the GC column. When the GC oven is cycled over its normal operating temperatures, the sample chemicals enter the column as a more focused “plug” of material. This often results in higher amplitude peaks, sharper peaks, and potentially a higher number of distinct trace chemical peaks that can be identified. The data from our system unexpectedly shows that the maximum number of peaks is attained with a non-cryogenic cooling profile, which was the only difference between configurations 5 and 6 in our DOE. Configuration 6 showed the maximum number of peaks for the system, and from Table 1 we see that adding cryogenic cooling (configuration 5) resulted in an output of 143 less peaks than that of configuration 6; this is a negative effect. It is possible that a latent variable is not being adequately controlled in this experimental configuration, such as time the EBC sample spends in residence in the sample inlet of the system. These so called “lurking variables” have the potential to obscure parameter effects, which may have been the case in this test. The acetone amplitude signal was positively effected by the cryogenic cooling, which is seen in configurations 9 and 10.
The only variable found to be statistically insignificant was SPME type, and then only in the case of the acetone peak amplitude. In analytical chemistry analysis, different SPME polymer chemistries are frequently selected for different sample types. Polyacrylate coated SPME are optimized to absorb molecules of molecular weights between 80–300 amu, while PDMS-DVB coated SPME are most compatible with molecules of molecular weights between 50–300 amu [38]. With acetone at a molecular weight of 58 amu, it would be assumed that a PDMS-DVB coated SPME would be optimal for such an experiment. The data, however, shows that in both configurations 6 and 9, a polyacrylate coated SPME was used. Values differed only slightly when the same configurations were used with a PDMS-DVB coated SPME. This is potentially due to the fact that the length of time we left the SPME fibers exposed to the EBC sample allowed both to reach maximal acetone concentration.
Another benefit of using a DOE is the ability to see any possible significant interactions between variables. Although SPME type may not have been significant as a single variable in our study, as seen in the evaluation of statistical significance of each individual parameter, it is evident from Table 2B, that SPME type does play a role in the significance of its interactions with the other three variables. This significance suggests that changes in SPME type may not affect the end results based on acetone peak amplitude; however, results may differ when there are changes in any of the other parameters simultaneously.
Table 2. Effects and interactions of all tested parameters including signal-to-noise t-ratios for each case, based on acetone peak amplitude.
(A) Effects for each parameter were calculated using the average acetone peak amplitude found for each of the 16 configurations. Signal-to-noise-ratios were then found by dividing each effect by the calculated standard deviation. (B) Similarly, interactions and their respective t-ratios were also calculated by using the average acetone peak amplitude in accordance with the standard deviation. The critical t value for a statistically significant interaction was t=2.038.
| A. Main effects | ||||
|---|---|---|---|---|
| Factors of Interest | ||||
| A | B | C | D | |
| Main effects | −3.0·10−4 | 8.0·10−4 | 4.0·10−4 | 4.0·10−4 |
| signal-to-noise t-ratio | −3.1088 | 8.0829 | 3.997 | 4.53 |
| significance (|te|>t95) | YES | YES | YES | YES |
| B. Interaction effects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interactions Between Factors | ||||||||||
| AB | AC | AD | BC | BD | CD | ABC | ABD | BCD | ABCD | |
| Interaction effects | −1.7·10−5 | −3.0·10−3 | −5.0·10−5 | 2.2·10−4 | 3.7·10−4 | 6.7·10−5 | 8.3·10−6 | −1.6·10−4 | 2.6·10−4 | −3.3·10−5 |
| signal-to-noise t-ratio | −0.178 | −3.375 | −0.533 | 2.309 | 3.908 | 0.711 | 0.089 | −1.688 | 2.754 | −0.355 |
| significance (|te|>t95) | NO | YES | NO | YES | YES | NO | NO | YES | YES | NO |
On the contrary, SPME type did not play a role at all in the significance of the interactions found in the results based on number of peaks, as seen in Table 3B. As expected, by the significance of their own individual changes, interactions between RF voltage, carrier gas flow rate, and GC cooling, when changed together may affect the results. From the data we can see that the most significant of all interactions was between RF voltage and carrier gas flow rates, both parameters found to be extremely influential in both number of peaks and peak amplitude.
It must be noted that statistical significance in this experiment is found relative to the other variables involved under the conditions specified here. Results found to be “insignificant” may not mean that the data is not significant from a macroscopic standpoint. Insignificance, as described by the DOE, is defined as compared to all other variables in the experiment, including any noise due to insufficiently controlled testing methods. The repeated acetone peak amplitude measurements all had variances that were very low across all configurations. However, the number of peaks measured under identical replicate conditions sometimes had higher variability, which is common with biological samples. It is also possible that some of this variance was due to lurking variables in the system, or could be due to slight variations in experimental methods which were beyond our control.
The DOE experiment optimization is important, because it allows us to optimize our parameters in a systematic way. The statistical significance of specific variables also determines which factors must be further investigated in follow-on experiments. It is possible that the optimum configurations found here may be generalized for analysis of other biological sample types, i.e. blood or urine, as a starting point for defining an analytical protocol; however, further refinement of the system configurations for those different biological sample types may or may not be necessary.
ACKNOWLEDGMENTS
This work was partially supported by grant number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The authors would also like to acknowledge and thank the Defense Advanced Research Projects Agency, Microsystems Technology Office for partially supporting this work (PM Dennis Polla). M. Molina was partially supported through a fellowship (HRD 0603239) from the NSF-funded California Alliance for Minority Participation (CAMP), and she received a travel grant to attend and present a poster on this work at the 2007 Society for Advancement of Chicanos and Native Americans in Science (SACNAS) National Meeting (Kansas City, MO). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Manolis A. Clinical Chemistry. 1983;29:5. [PubMed] [Google Scholar]
- 2.Cao WQ, Duan YX. Clinical Chemistry. 2006;52:800. doi: 10.1373/clinchem.2005.063545. [DOI] [PubMed] [Google Scholar]
- 3.Miekisch W, Schubert JK, Noeldge-Schomburg GFE. Clinica Chimica Acta. 2004;347:25. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Pauling L, Robinson AB, Teranish R, Cary P. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:2374. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao WQ, Duan YX. Critical Reviews in Analytical Chemistry. 2007;37:3. [Google Scholar]
- 6.Phillips M, Cataneo RN, Condos R, Erickson GAR, Greenberg J, La Bombardi V, Munawar MI, Tietje O. Tuberculosis. 2007;87:44. doi: 10.1016/j.tube.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Gaston B. American Journal of Respiratory and Critical Care Medicine. 2003;167:292. doi: 10.1164/rccm.2211005. [DOI] [PubMed] [Google Scholar]
- 8.Montuschi P. Clinica Chimica Acta. 2005;356:22. doi: 10.1016/j.cccn.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Rooth G, Ostenson S. Lancet. 1966;2:1102. doi: 10.1016/s0140-6736(66)92194-5. [DOI] [PubMed] [Google Scholar]
- 10.Zihlif N, Paraskakis E, Tripoli C, Lex C, Bush A. Pediatric Pulmonology. 2006;41:509. doi: 10.1002/ppul.20344. [DOI] [PubMed] [Google Scholar]
- 11.Mazzone PJ, Hammel J, Dweik R, Na J, Czich C, Laskowski D, Mekhail T. Thorax. 2007;62:565. doi: 10.1136/thx.2006.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs MD, Zapata AM, Nazarov EG, Miller RA, Costa IS, Sonenshein AL, Davis CE. Ieee Sensors Journal. 2005;5:696. [Google Scholar]
- 13.Morgan JT. Differential mobility spectrometry applications in homeland security, clinical diagnostics and drug discovery, 2006 ASME International Mechanical Engineering Congress and Exposition. ASME; Chicago, Illinois, USA: 2006. [Google Scholar]
- 14.Eiceman GA, Krylov EV, Nazarov EG, Miller RA. Analytical Chemistry. 2004;76:4937. doi: 10.1021/ac035502k. [DOI] [PubMed] [Google Scholar]
- 15.Shnayderman M, Mansfield B, Yip P, Clark HA, Krebs MD, Cohen SJ, Zeskind JE, Ryan ET, Dorkin HL, Callahan MV, Stair TO, Gelfand JA, Gill CJ, Hitt B, Davis CE. Analytical Chemistry. 2005;77:5930. doi: 10.1021/ac050348i. [DOI] [PubMed] [Google Scholar]
- 16.Davis CE. Spore biomarker detection using a MEMS differential mobility spectrometer, IEEE Transducers Proceedings. 2003:1233. [Google Scholar]
- 17.Krebs MD, Mansfield B, Yip P, Cohen SJ, Sonenshein AL, Hitt BA, Davis CE. Biomolecular Engineering. 2006;23:119. doi: 10.1016/j.bioeng.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Rearden P, Harrington PB, Karnes JJ, Bunker CE. Analytical Chemistry. 2007;79:1485. doi: 10.1021/ac060527f. [DOI] [PubMed] [Google Scholar]
- 19.Sankaran Microfabricated differential mobility spectrometers for breath analysis, Ieee Sensors Journal. 2007 [Google Scholar]
- 20.Vicente G, Coteron A, Martinez M, Aracil J. Industrial Crops and Products. 1998;8:29. [Google Scholar]
- 21.Daroux M, Zamani H, Greffe JL, Bordet J. Chemical Engineering Journal and the Biochemical Engineering Journal. 1981;22:125. [Google Scholar]
- 22.Martin C, Cuellar J. Industrial & Engineering Chemistry Research. 2004;43:2093. [Google Scholar]
- 23.Molina R, Martinez F, Melero JA, Bremner DH, Chakinala AG. Applied Catalysis B-Environmental. 2006;66:198. [Google Scholar]
- 24.Dante RC, Escamilla JL, Madrigal V, Theuss T, Calderon JD, Solorza O, Rivera R. International Journal of Hydrogen Energy. 2003;28:343. [Google Scholar]
- 25.Gotti R, Furlanetto S, Andrisano V, Cavrini V, Pinzauti S. Journal of Chromatography A. 2000;875:411. doi: 10.1016/s0021-9673(99)01303-5. [DOI] [PubMed] [Google Scholar]
- 26.Shukla AA, Sorge L, Boldman J, Waugh S. Biotechnology and Applied Biochemistry. 2001;34:71. doi: 10.1042/ba20010019. [DOI] [PubMed] [Google Scholar]
- 27.Baillargeon MW, Ross DA, Eisenhauer D, Lundell EO. Abstracts of Papers of the American Chemical Society. 2003;225:U232. [Google Scholar]
- 28.Gullberg J, Jonsson P, Nordstrom A, Sjostrom M, Moritz T. Analytical Biochemistry. 2004;331:283. doi: 10.1016/j.ab.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Cano A, Moschou EA, Daunert S, Coello J, Palet C. Bioprocess and Biosystems Engineering. 2006;29:261. doi: 10.1007/s00449-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YP, Zhang YJ, Gong WJ, Wang SM, Xue HY, Lee KP. Journal of Liquid Chromatography & Related Technologies. 2007;30:215. [Google Scholar]
- 31.Lendrem D, Owen M, Godbert S. Organic Process Research & Development. 2001;5:324. [Google Scholar]
- 32.Tye H. Drug Discovery Today. 2004;9:485. doi: 10.1016/S1359-6446(04)03086-7. [DOI] [PubMed] [Google Scholar]
- 33.Seto C, Bateman KP, Gunter B. Journal of the American Society for Mass Spectrometry. 2002;13:2. doi: 10.1016/S1044-0305(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 34.Cole DC, Pagano N, Kelly MF, Ellingboe J. Journal of Combinatorial Chemistry. 2004;6:78. doi: 10.1021/cc034013v. [DOI] [PubMed] [Google Scholar]
- 35.Riter LS, Vitek O, Gooding KM, Hodge BD, Julian RK. Journal of Mass Spectrometry. 2005;40:565. doi: 10.1002/jms.871. [DOI] [PubMed] [Google Scholar]
- 36.Yusa V, Pardo O, Pastor A, de la Guardia M. Analytica Chimica Acta. 2006;557:304. [Google Scholar]
- 37.Eiceman GA, Wang M, Prasad S, Schmidt H, Tadjimukhamedov FK, Lavine BK, Mirjankar N. Analytica Chimica Acta. 2006;579:1. doi: 10.1016/j.aca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Sigma-Aldrich [Google Scholar]