Abstract
Vaccinia virus (VV) is an effective vaccine and vector but has evolved multiple mechanisms for evading host immunity. We characterized the interactions of VV (TianTan and New York City Board of Health strains) with human γδ T cells because of the role they play in immune control of this virus. Exposure to VV failed to trigger proliferative responses in γδ T cells from unprimed individuals, but it was an unexpected finding that VV blocked responses to model antigens by the Vγ2Vδ2 T cell subset. Infectious or ultraviolet light–inactivated VV inhibited proliferative Vγ2Vδ2 T cell responses to phosphoantigens and tumor cells, prevented cytolysis of Daudi B cells, and reduced cytokine production. Inhibiting Vγ2Vδ2 T cells may be a mechanism for evading host immunity and increasing VV virulence. Increased VV replication or expression in the absence of γδ T cell responses might contribute to its potency as a vaccine against poxvirus and recombinant antigens.
Vaccinia virus (VV), a member of the poxvirus family, has been used successfully as a vaccine to eradicate human smallpox [1]. With DNA replication occurring exclusively in the cytosol and the ability to induce both cellular and humoral immunity, VV has also been championed as a live recombinant vaccine vector that promotes immunity against tumors and infectious diseases [2]. Although VV can induce strong humoral and cellular immune responses to viral and recombinant antigens, it is also known that poxviruses employ many mechanisms to evade host immunity. VV gene products block complement, cytokines, and chemokines; they also prevent apoptosis and interfere with intracellular signaling [3, 4]. VV modulates the function of NK cells [5], inhibits the maturation of human dendritic cells [6], and disrupts major histocompatibility complex (MHC) class I or II–mediated antigen presentation [7–12]. The effects that VV has on another important cell type, γδ T cells, is poorly understood.
Human γδ T cells comprise, on average, 1%–10% of the total peripheral-blood T cell population. Of these γδ T cells, a majority express the Vγ2Vδ2 receptor, and ~75% have the Vγ2-Jγ1.2 rearrangement [13]. In contrast to αβ T cells, the γδ T cell subset generally lacks CD4 or CD8 expression and recognizes antigens independently of conventional MHC-restricted presentation [14]. A rapid expansion and activation of γδ T cells has been noted in infections of human beings and nonhuman primates, including mycobacterium [15–17], malarial [18–20], and simian immunodeficiency virus [21] infections. In mice, copious interferon (IFN)–γ production by γδ T cells is key for resistance to lethal VV challenge [22].
A recent report on human experiments using VV described memory responses in the circulating γδ T cell population in vaccine recipients [23]. To our knowledge, this was the first report of γδ T cell responses being elicited by immunization after not being present in the starting T cell population. In all other examples of human γδ T cell reactions to mycobacteria, malarial parasites, infected cells, and tumors, circulating γδ T cells from naive donors recognized these immunogens and generated strong proliferative and cytotoxic responses [24]. Accordingly, we were interested in this unique example of VV-induced γδ T cell responses. In the mouse, γδ T cell responses to VV fit the regular pattern in that they were already strong in the unprimed host and were cytotoxic for VV-infected cells with high levels of IFN-γ secretion [22].
γδ T cells have been variously associated with innate and adaptive immunity or proposed as a link between the 2 systems [25]. Confusion arises because the starting γδ T cell repertoire reflects a bias for the Vγ2-Jγ1.2Vδ2 T cell receptor (TCR) [13], which is a consequence of chronic stimulation by self-antigens or ubiquitous antigens and selective expansion of 1 subset [26]. This self-selected repertoire is dominated by a single TCR rearrangement and encodes the response to pathogens and some tumors, meaning that γδ T cells from naive or unprimed individuals generally have the same responses. Thus, it was surprising to learn of de novo responses to VV that appear only after vaccination [23], and we wanted to study γδ T cell responses to VV using in vitro models. In this study, we address the host-pathogen interaction between VV and human γδ T cells, to better understand the absence of γδ T cell responses among unprimed individuals.
MATERIALS AND METHODS
Cells and virus
Whole blood was obtained from healthy human volunteers, and total lymphocytes were separated from heparinized peripheral blood by density gradient centrifugation (Ficoll-Paque; Amersham Biosciences). Peripheral-blood mononuclear cells (PBMCs) and CEM cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; GIBCO), 2 mmol/L L-glutamine, and penicillin–streptomycin (100 U/mL and 100 μg/mL, respectively); for Daudi B cells (CCL-213; ATCC), 4.5 g/L glucose, 1.5 g/L NaHCO3, 10 mmol/L HEPES, and 1 mmol/L sodium pyruvate were added. HeLa cells were cultured in MEM (GIBCO) supplemented with 10% FBS, 2 mmol/L L-glutamine, and penicillin-streptomycin (100 U/mL and 100 μg/mL, respectively).
The TianTan VV strain carrying the HIV gag gene (VVTT) [27] and the nonrecombinant New York City Board of Health (NYCBH) strain (VVNY) [28] were propagated and titered in HeLa cells. VV was released from infected cells by 3 cycles of freezing/thawing, sonication, and clarification by centrifugation and then was purified by ultracentrifugation on a 36% sucrose gradient. Similarly treated lysates from uninfected HeLa cells were used for mock infections. Where indicated, purified VV was inactivated with UV light (UV-VV) for 3 min (GS Gene Linker UV chamber; Bio-Rad). UV-VV was no longer able to form plaques. In some proliferation experiments, CEM cells or Daudi cells were infected with VV (MOI of 1) for 2 h, washed twice, and cultured for an additional 20 h.
In vitro proliferation assays
PBMCs (5 × 105 cells/well) were cultured in 12-well plates in the presence of UV-VV, irradiated (120 Gy) VV-infected CEM cells (CEM/VV), or CEM cells at varying ratios. In some experiments, cocultures were stimulated by a single addition of isopentyl pyrophosphate (IPP; Sigma) at a final concentration of 15 μmol/L or by adding either irradiated (120 Gy) Daudi B cells or VV-infected Daudi B cells (Daudi/VV) at a 2:1 Daudi:PBMC ratio on day 0 along with 100 U/mL human recombinant interleukin (IL)–2 (Tecin; Biological Resources Branch, National Institutes of Health). Fresh medium that included IL-2 was added every 3 days, and PBMCs were incubated at 37°C in 5% CO2. Expansion of γδ T cells was evaluated by double staining for CD3/PANγδ or CD3/Vδ2 and defining the percentage of γδ T cells within the total CD3+ cell population at day 14. For phytohemagglutinin (PHA) stimulation, PBMCs were first stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; CellTrace CFSE cell proliferation kit; Invitrogen), in accordance with the manufacturer’s directions. CFSE-labeled PBMCs (1 × 105 cells/well) were cultured in 96-well round-bottomed plates in the presence of corresponding virus or cells as indicated above and then stimulated with 5 μg/mL PHA and 100 U/mL IL-2 for 4 days before staining with anti–CD4 clone SK3 antibody (BD Biosciences) for flow cytometry assays.
Calcein-release cytotoxicity assay
A nonradioactive fluorometric cytotoxicity assay using calcein-acetoxymethyl (calcein-AM; Molecular Probes) [29, 30] was used to evaluate the cytotoxicity of Vγ2Vδ2 T cells. Expanded γδ T cells (effector cells) either were treated with VV or UV-VV (MOI of 1) or were mock treated for 2 h at 37°C. During this time, Daudi B cells (target cells) were labeled for 15 min with 2 μmol/L calcein-AM at 37°C and then washed once with PBS. Cells were combined at various effector-to-target (E:T) ratios in 96-well round-bottomed microtiter plates (Corning) and incubated at 37° C in 5% CO2 for 4 h; assays were performed in triplicate. After incubation, supernatants were carefully transferred to a 96-well flat-bottomed microtiter plate, and calcein content was measured using a Wallac Victor2 1420 multichannel counter (λ485/535 nm). Percentage of specific lysis was calculated as (test release – spontaneous release)/(maximumrelease – spontaneous release)×100.
RNA extraction, reverse-transcription polymerase chain reaction (RT-PCR), and PCR
Total RNA was extracted from cells by use of the RNeasy Mini Kit (Qiagen). One microgram of total RNA was converted into cDNA by use of a reverse-transcription system kit (Promega). Each reaction was incubated at 42°C for 2 h and then cDNAs were diluted to 100 μL by adding 80 μL of deionized H2O to the reaction. PCR amplification and gel analysis of products have been described elsewhere [13].
Spectratype analysis
Primer extension reactions were performed as described elsewhere [13]. After heat denaturation of the products (5 min at 95°C followed by immediate quenching on ice), products were loaded on an Applied Biosystems 3130 four-microcapillary genetic analyzer (Hitachi) and run on a performance-optimized polymer (POP-7). Molecular size and relative frequency of extension products were determined using GeneMapper software (version 3.6; Applied Biosystems). To standardize the data irrespective of the runoff primer position, CDR3 length variation was expressed in terms of the total Vγ2 coding-region lengths. Runoff product lengths were corrected by adding the length of the known Vγ2 mRNA coding regions outside the runoff product.
Flow cytometry
Unless noted otherwise, expanded Vγ2Vδ2 T cells were stained for cell-surface markers with fluorophore-conjugated monoclonal antibodies from BD Biosciences. Generally, 3 × 105 –5 × 105 cells were washed, resuspended in 50–100 μL of RPMI 1640, and stained with mouse anti–human PANγδ–phycoerythrin (PE) clone 5A6.E9 (Pierce Biotechnology), mouse anti–human Vδ2–PE clone B6, mouse anti–human CD3–fluorescein isothiocyanate (FITC) clone UCHT1, mouse anti–human CD3–allophycocyanin (APC) clone UCHT1, mouse anti–human CD107a–FITC clone H4A3, and isotype controls, including rabbit anti–mouse IgG1–FITC clone X40, IgG1-PE clone X40, and IgG1-APC clone X40. For detection of intracellular IFN-γ or tumor necrosis factor (TNF)–α, expanded cells were stained with Vδ2-PE, fixed, permeabilized, and incubated for 45 min at 4°C with mouse anti–human IFN-γ–APC clone B27 or mouse anti–human TNF-α–APC clone MAb11. Intracellular staining solutions were obtained from the Cytofix/Cytoperm Kit (BD Biosciences). Data for at least 1×104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were acquired for each sample on a FACSCalibur flow cytometer (BD Biosciences). All samples were analyzed using FCS Express software (version 3; De Novo Software).
For stimulation before staining, Vγ2Vδ2 T cells were treated with VV or UV-VV (MOI of 1) for 2 h, washed twice, and added to 96-well plates (Corning). In some experiments, to stimulate Vγ2Vδ2 cells, wells were coated with the anti–human γδ TCR antibody, clone B1.1 (eBiosciences).
Statistical analysis
Differences among groups were analyzed by Student’s t test. P < .05 was considered to be significant.
RESULTS
Expansion of γδ T cells not stimulated by VV in vitro
PBMCs from 2 healthy, unrelated donors were stimulated with IPP, UV-VVTT (MOI of 1, 0.1, or 0.01), CEM/VVTT (CEM/VVTT:PBMC ratio of 1, 0.1, or 0.01), or appropriate controls plus IL-2. Fresh medium containing IL-2 was added every 3 days. After 2 weeks, we observed strong γδ T cell proliferative responses to IPP stimulation (figure 1). γδ T cells were not expanded after exposure to UV-VVTT or CEM/VVTT (figure 1).
Figure 1.
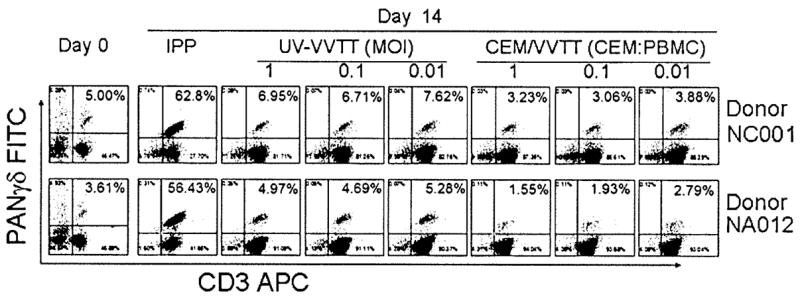
Lack of stimulation of γδ T cell expansion in vitro by vaccinia virus (VV). Peripheral-blood mononuclear cells (PBMCs) from 2 healthy, unrelated donors were stained for PANγδ and CD3 on day 0 and 14 days after stimulation with isopentyl pyrophosphate (IPP), UV light–inactivated TianTan strain carrying the HIV gag gene (UV-VVTT; MOI of 1, 0.1, or 0.01), or irradiated VV-infected CEM cells (CEM/VVTT; CEM/VVTT:PBMC ratio of 1, 0.1, or 0.01). Although IPP stimulated obvious expansion of γδ T cells, UV-VVTT and CEM/VVTT did not induce any expansion of γδ T cells. APC, allophycocyanin; FITC, fluorescein isothiocyanate.
Inhibition of IPP- or Daudi cell–stimulated expansion of human γδ T cells by VV
We next wanted to know whether VV affects IPP-stimulated expansion of Vγ2Vδ2 T cells. UV-VVTT (MOI of 1, 0.1, or 0.01), CEM/VVTT (CEM/VVTT: PBMC ratio of 1:1, 1:10, or 1:100), and appropriate controls were added to the IPP/IL-2–treated cultures, and cells were cultured for 14 days. IPP-driven expansion was inhibited by both UV-VVTT and CEM/VVTT in a dose-dependent manner, compared with that observed in mock-treated or CEM cell controls (figure 2A). At an MOI of 1 for UV-VVTT or a 1:1 CEM/VVTT:PBMC ratio for CEM/VVTT, the frequency of Vδ2+ T cells on day 14 was indistinguishable from that in the starting cultures, and IPP-stimulated expansion was inhibited completely. We repeated the experiment using 3 additional donors. At an MOI of 1 for UV-VVTT or a 1:1 CEM/VVTT: PBMC ratio, we obtained similar results for all donors, finding complete suppression of Vγ2Vδ2 T cell proliferation (figure 2B). We also detected an effect of VV on Daudi cell–stimulated expansion of γδ T cells. Either UV-VVTT was added to a Daudi cell/IL-2–treated culture or Daudi B cells were preinfected with VVTT for 20 h before being added to the culture system. The results indicated that Daudi cell–stimulated expansion of γδ T cells was completely inhibited by VV (figure 2B). Importantly, VVTT had little inhibitory effect on PHA-stimulated proliferation of CD4+ or CD4− cells in PBMCs (figure 2C). There was a trend toward a lower proliferative response among CD4− cells that were exposed to CEM/VV or UV-VV (figure 2C), but the differences were small compared with the effects on Vγ2Vδ2 T cells. This indicates that IPP/Daudi cell–driven expansion of γδ T cells is much more sensitive to VV-induced negative signaling and that the effect is not likely due to random destruction of T cells.
Figure 2.
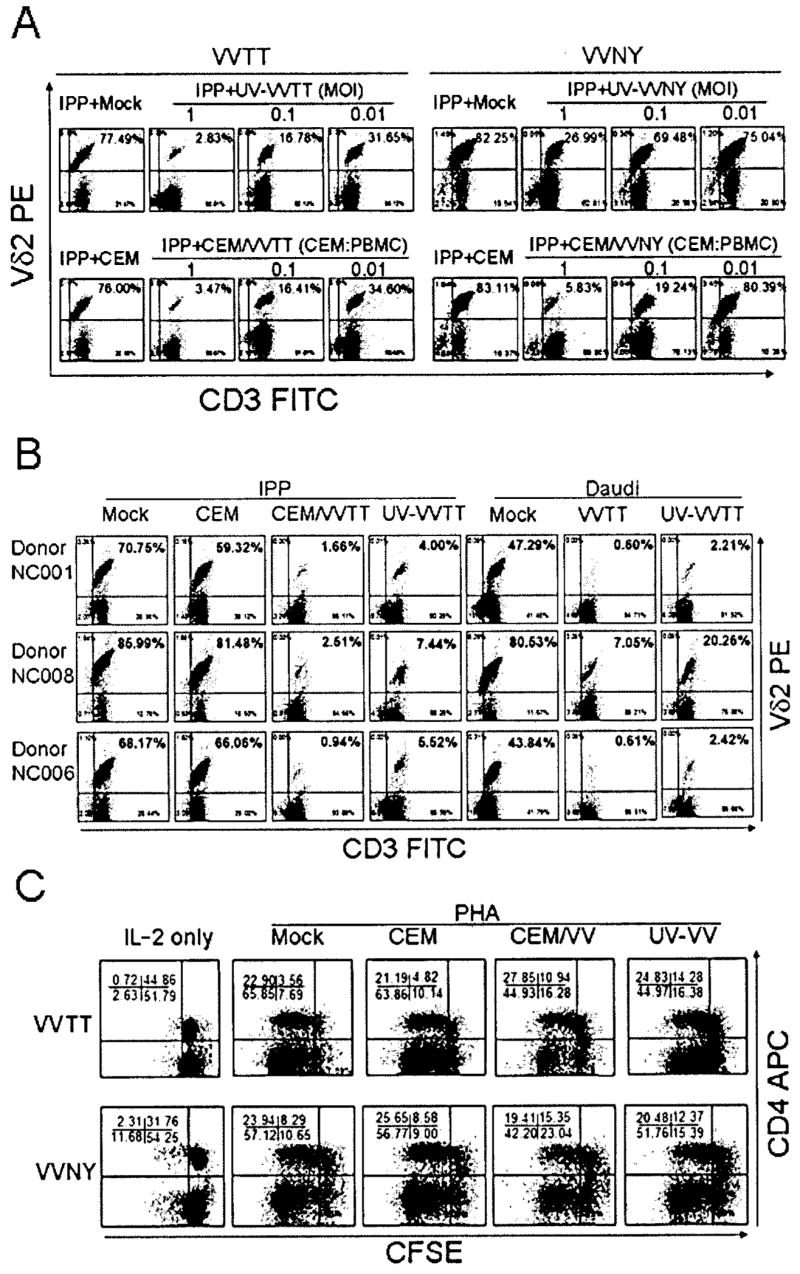
Inhibition of isopentyl pyrophosphate (IPP)– or Daudi cell–stimulated expansion of human γδ T cells by vaccinia virus (VV). A, Dose dependency of inhibition. UV light–inactivated VV (UV-VV; either the TianTan strain carrying the HIV gag gene [VVTT] or the nonrecombinant New York City Board of Health strain [VVNY]; MOI of 1, 0.1, or 0.01), irradiated VVTT- or VVNY-infected CEM cells (CEM/VV; CEM/VV:peripheral-blood mononuclear cell [PBMC] ratio of 1:1, 1:10, or 1:100), or appropriate controls were added to IPP/interleukin (IL)–2–treated cultures. On day 14, the expansion was measured by flow cytometry. IPP-driven expansion of γδ T cells was inhibited by both UV-VV and CEM/VV in a dose-dependent manner, compared with that in mock-treated or CEM cell controls. B, Repetition of experiment in 3 additional donors. At an MOI of 1 for UV-VVTT or a CEM/VVTT:PBMC ratio of 1:1, we obtained similar results for all donors, showing complete suppression of Vγ2Vδ2 T cell proliferation. An effect of VV on Daudi cell–stimulated expansion of γδ T cells was also detected. UV-VVTT (MOI of 1) was added to Daudi/IL-2–treated cultures or Daudi B cells were preinfected with VV (MOI of 1) for 20 h before being added to the culture system. The results indicated that Daudi cell–stimulated expansion of γδ T cells was completely inhibited. C, Little inhibitory effect of VVTT on phytohemagglutinin (PHA)–stimulated proliferation of CD4+ or CD43 cells in PBMCs. PBMCs were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE), cultured in 96-well round-bottomed plates in the presence of either UV-VV (VVTT or VVNY) at an MOI of 1 or CEM/VV (VVTT or VVNY) at a CEM/VV:PBMC ratio of 1:1, stimulated with 5 μg/mL PHA and 100 U/mL IL-2 for 4 days, and stained with anti-CD4 antibody for the flow cytometry assay. APC, allophycocyanin; FITC, fluorescein isothiocyanate.
We hypothesized 2 models for inhibition: either VV proteins blocked a signaling pathway that prevented γδ T cell activation or VV killed γδ T cells that were activated by exposure to IPP or Daudi cells. To discriminate between these 2 mechanisms, we analyzed the Vγ2 chain repertoire before and after stimulation in the presence of VV, and we tested Vγ2Vδ2 T cell viability. It is known that IPP stimulation expands a subset of γδ T cells that express the Vγ2-Jγ1.2/Vδ2 TCR [13]. This selective expansion skews the Vγ2 repertoire toward longer chain lengths, and the change can be detected by spectratyping. If the first hypothesis is correct, the spectratype would remain unchanged after IPP or Daudi cell stimulation in the presence of VV. If the second hypothesis is correct, the proportion of Vγ2-Jγ1.2/Vδ2 T cells will be reduced sharply, and the bias toward longer Vγ2+ chains will be relaxed. Even though UV-VVTT, CEM/VVTT, and infectious VVTT completely inhibited the IPP-driven expansion of γδ T cells, there was little impact on the length distribution of Vγ2 chains (figure 3A–3B). In addition, we incubated a Vγ2Vδ2 T cell line (obtained by expanding IPP-responsive γδ T cells from PBMCs for 10 days) with VV. There were no changes in the frequency of cells expressing the Vγ2-Jγ1.2 chain over 35 h after VV exposure (figure 3C), and there were only slight effects on cell viability (figure 3D). Because there were only small changes in the Vγ2 repertoire and because incubation with VV did not cause widespread cell death, we favor the first model hypothesizing that VV blocks a signaling pathway in Vγ2Vδ2 T cells and prevents their proliferative response to IPP stimulation. However, it is clear that additional studies are needed to confirm this mechanism.
Figure 3.
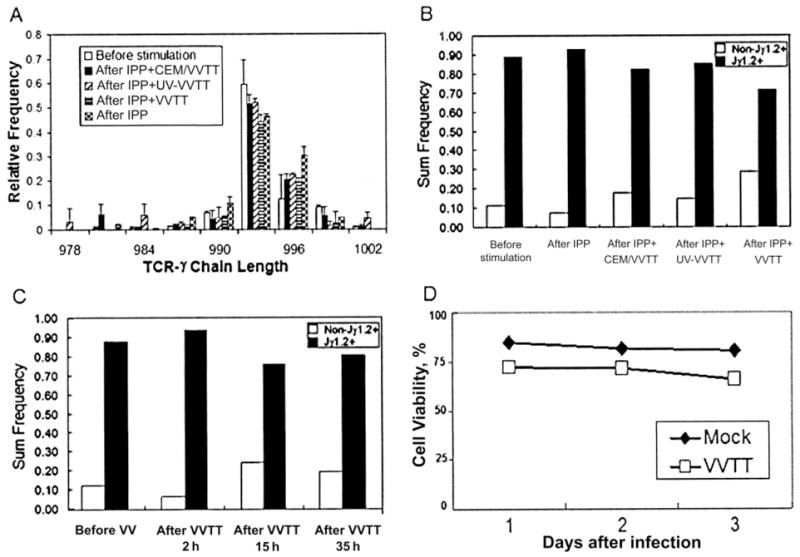
Alteration of the length distribution of Vγ2 chains or reduction in viability not induced by vaccinia virus (VV) in a Vγ2Vδ2 T cell line. Total RNA was extracted from cells before stimulation or 14 days after stimulation with isopentyl pyrophosphate (IPP), IPP plus irradiated CEM cells infected with the TianTan VV strain carrying the HIV gag gene (CEM/VVTT; CEM/VVTT:peripheral-blood mononuclear cell [PBMC] ratio of 1:1), UV light–inactivated VVTT (UV-VVTT; MOI of 1), or live VVTT (MOI of 1) (A). cDNA was synthesized, and a spectratype assay was performed. The proportion of Vγ2-Jγ1.2 chains was not significantly changed after VV exposure (B). A Vγ2Vδ2 T cell line was generated by IPP stimulation of PBMCs and proliferation of the γδ T cell subset (C). These cells were exposed to VV and samples were collected for 35 h, to measure the frequency of Vγ2-Jγ1.2 chains. There were no significant changes over this time interval. The Vγ2Vδ2 T cell line was cultured with VVTT for 3 days without substantial loss of cell viability (D).
Inhibition of the cytotoxicity function of γδ T cells by VV
Unlike NK cells, which exhibit increased lysis of VV-infected targets in vitro [31], γδ T cells did not kill VV-infected CEM cells (data not shown). We measured the effect of VV infection on γδ T cell cytotoxity for Daudi cells. IPP-expanded γδ T cells from different donors were exposed to purified VVTT or UV-VVTT at an MOI of 1 for 2 h and then were tested for lysis of Daudi B target cells in a calcein-release cytotoxicity assay. Infection reduced the effectiveness of γδ T cells from all donors (figure 4). Exposure to UV-VV had less effect on the level of lysis by γδ T cells, indicating that active virus infection was more potent for inhibiting γδ T cell killing of Daudi cells.
Figure 4.
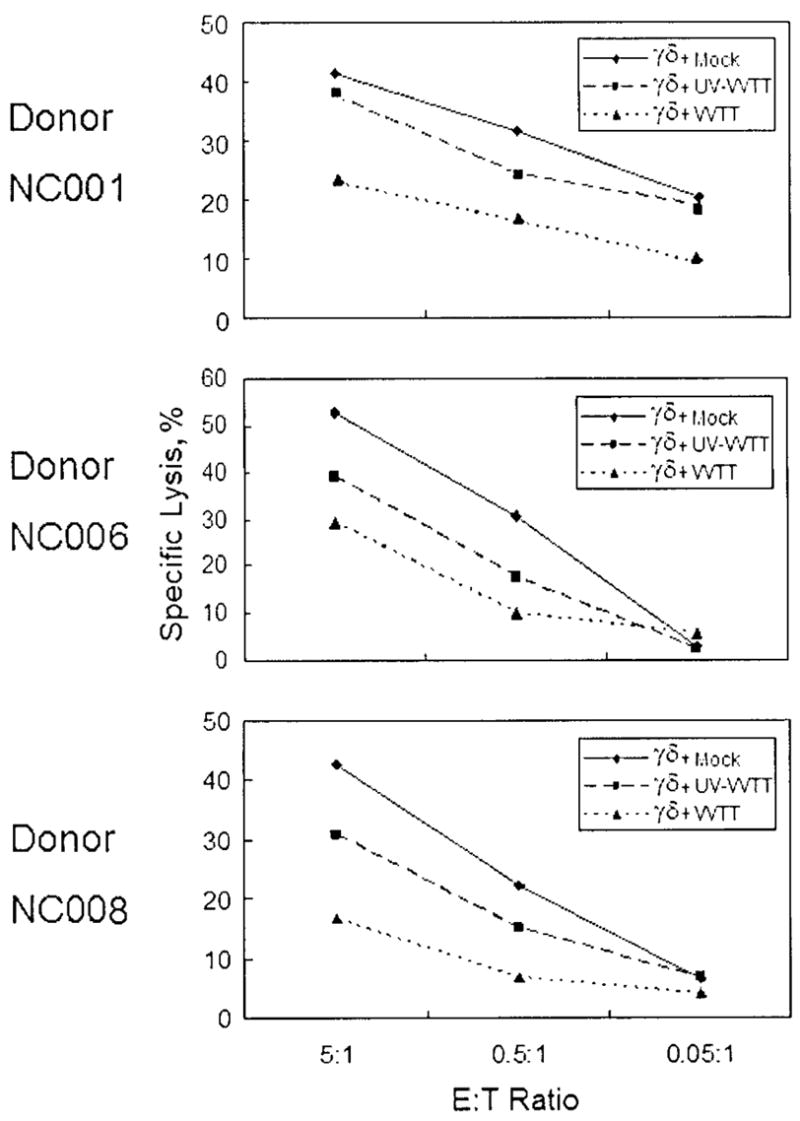
Inhibition of Daudi cell cytotoxicity of γδ T cells by vaccinia virus (VV). Isopentyl pyrophosphate (IPP)–expanded γδ T cells from several donors were incubated with purified TianTan strain carrying the HIV gag gene (VVTT) or UV light–inactivated VVTT (UV-VVTT) at an MOI of 1 or with mock control for 2 h, and then the ability of these cells to lyse Daudi B target cells was determined by calcein-release cytotoxicity assay. E:T ratio, effector-to-target cell ratio.
Cell-surface CD107a not reduced by VV after stimulation by anti–human γδ TCR antibody
CD107a (lysosomal-associated membrane protein 1) is present in membranes of cytotoxic granules and is transiently expressed on cell surfaces as a result of degranulation [32, 33]. We know that CD107a appears on the surface of γδ T cells from ~10 to 60 min after stimulation through the TCR [34]. We observed that VVTT infection (MOI of 1) did not alter CD107a expression after brief stimulation with anti–human γδ TCR antibody (figure 5). This indicates that VV inhibition of γδ T cell cytotoxicity is not a direct result of altered degranulation.
Figure 5.
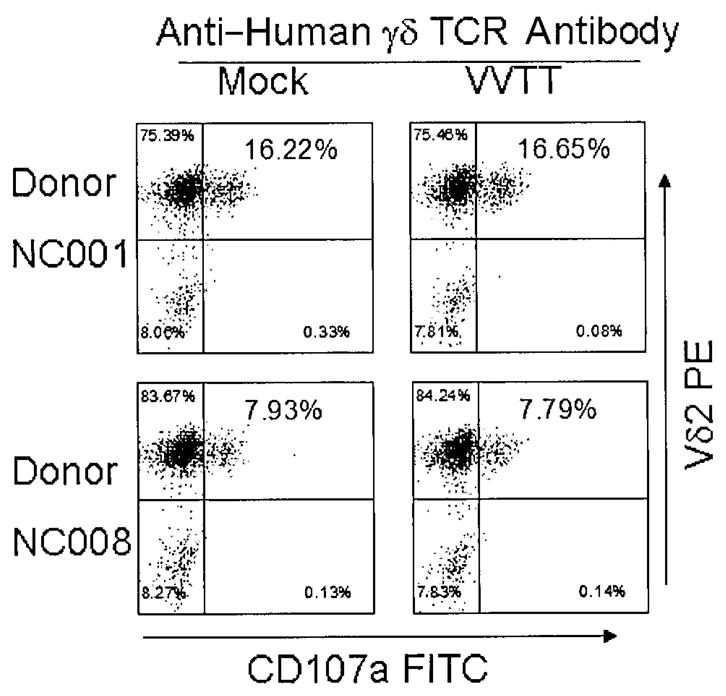
Reduction in cell-surface CD107a expression not induced by vaccinia virus (VV) after stimulation with anti–human γδ T cell receptor (TCR) antibody. Isopentyl pyrophosphate (IPP)–expanded γδ T cells from 2 donors were incubated with purified VVTT at an MOI of 1 as well as with mock control for 2 h and then stimulated with anti–human γδ TCR antibody for 2 h and stained for Vδ2 and CD107a. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Inhibition of anti–human γδ TCR antibody–stimulated γδ T cell production of IFN-γ and TNF-α by VV
We measured the effect of VV infection on TNF-α and IFN-γ production by γδ T cells. VVTT infection (MOI of 1) significantly inhibited TNF-α and IFN-γ production by γδ T cells after 2 h of stimulation with anti–human γδ TCR antibody (figure 6).
Figure 6.
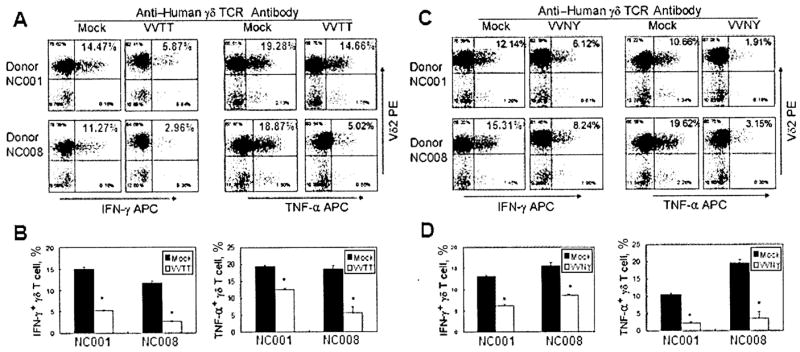
Inhibition of γδ T cell interferon (IFN)–γ and tumor necrosis factor (TNF)–α production by vaccinia virus (VV) after stimulation with anti–human γδ T cell receptor (TCR) antibody. Isopentyl pyrophosphate (IPP)–expanded γδ T cells from 2 donors were incubated with purified VV (either the TianTan strain carrying the HIV gag gene [VVTT] or the nonrecombinant New York City Board of Health strain [VVNY]) at an MOI of 1 as well as with mock control for 2 h, stimulated with anti–human γδ TCR antibody for 2 h, and stained for Vδ2 and intracellular IFN-γ or TNF-α (A and C ). Intracellular staining for IFN-γ and TNF-α was performed in triplicate (B and D ). Statistically significant differences (P < .05) are indicated by asterisks. APC, allophycocyanin; PE, phycoerythrin.
Observation of similar effects with VVNY
We repeated some of the previous experiments using the VVNY strain. We observed similar inhibition of IPP-stimulated γδ T cell expansion (figure 2A) and reduced TNF-α and IFN-γ production in the presence of VVNY (figure 6C–6D). Overall, the VVNY strain was less potent with respect to inhibition of γδ T cell responses but still produced significant reduction of activity compared with the controls. We concluded that the effects on γδ T cell functions are not unique to the VVTT strain or to the recombinant antigens expressed therein but seemed to be a general property of VV, although we do expect there to be differences in the magnitudes of effects for other VV strains.
DISCUSSION
We observed a strong, VV-mediated inhibition of human γδ T cells. Infectious VV, VV particles inactivated with UV light, and VV-infected cells all produced a specific and potent suppression of γδ T cell proliferation without substantially inhibiting αβ T cell responses in the same cultures. The TianTan strain carrying the HIV gag gene [27] and the nonrecombinant NYCBH VV strain [28] showed similar inhibition of Vγ2Vδ2 T cell proliferation and cytokine expression. In previous experiments using HIV-infected CEM cells as stimulators (the same cells used for VV infection here), we observed Vγ2Vδ2 proliferation [35]. HIV virions themselves stimulate Vγ2Vδ2 T cells [36], and there was no evidence for inhibition of proliferation by HIV particles or by HIV-infected cells. The mechanism for suppressing γδ T cells is specific to VV (either TianTan or NYCBH strains) and was not due to retroviral Gag antigens produced by the recombinant TianTan strain.
The mechanism for VV suppression of γδ T cells likely involves a blocking of T cell activation. Treatment with VV blocked the production of cytokines known to be expressed soon after γδ T cell activation. We measured cytokine production by intracellular staining, to avoid problems with the VV decoy receptors for TNF-α and IFN-γ, which have confused some assays for soluble cytokine [3, 4]. Cytokine responses are strong within 2 h after TCR-dependent stimulation and were suppressed by VV. However, another γδ T cell response (cell-surface expression of CD107a) was not affected, even though exposure to VV produced a marked reduction in γδ T cell cytotoxicity. Tumor cell cytotoxicity by γδ T cells is a combination of perforin/granzyme and FasL/TNF-related apoptosis-inducing ligand (TRAIL) mechanisms [37], and the incomplete block of tumor cell killing may indicate a greater effect on FasL/TRAIL, compared with perforin/granzyme, release. VV also inhibits Fas-mediated apoptosis [38], and this may also be important in our cytotoxicity studies.
We attempted to rule out VV destruction of activated γδ T cells. Cell viability was preserved during brief exposures to VV, and the extent of γδ T cell suppression was similar for both infectious and inactivated VV particles. Spectratyping measures changes in the subset of γδ T cells that respond to model antigens [13] and showed that the Vγ2 repertoire was not changed greatly by VV exposure. These data argue that VV inhibits Vγ2Vδ2 T cell proliferation by blocking an activation signal or by arresting cell proliferation, but the effect is not explained by killing the responding cells. It is important to note the minor impact of VV on PHA-induced αβ T cell proliferation, because there may be similarities between the αβ T cell response to PHA and the γδ T cell response to IPP or Daudi cells.
Other viruses have been reported to block lymphocyte responses. For example, measles virus glycoprotein has a broad antiproliferative effect on lymphocyte proliferation [39]. Quite unlike the example for VV suppression of γδ T cell responses, the measles virus inhibits proliferation of all lymphocyte subsets without apparent specificity.
What is the biological importance for VV suppression of γδ T cell responses? Our results and the emerging literature suggest 2 working hypothesis. First, inhibition of γδ T cells may increase VV replication or persistence in the host. Selin et al. concluded that γδ T cells were necessary for resistance to lethal VV infection in the mouse [22], and a mechanism for overcoming this host response may contribute to VV replication in human beings with a consequent impact on immunity to VV and expressed recombinant antigens.
Second, we know that γδ T cells have important regulatory functions. Mice that were orally tolerized to sheep red blood cells (SRBCs) suppressed this response and recovered the ability to recognize SRBCs after adoptive transfer of γδ T cells from a naive donor [40]. Murine γδ T cells are believed to be critical for the suppression of pathological inflammatory reactions [41, 42] via a mechanism involving cell-surface FasL [43, 44]. Immune suppression was also implicated when γδ T cell knockout mice were challenged with Listeria monocytogenes. The γδ T cell deficient mice were unable to develop protective immunity to L. monocytogenes and, surprisingly, developed a lymphoproliferative disorder that was lethal [45] because of the absence of γδ T cell suppressor activity. VV inhibition of γδ T cells may reduce immune suppression and allow for greater immune responses to VV and recombinant antigens.
VV inhibition of human γδ T cell responses is another of the viral mechanisms for evading host immunity. By blocking this important T cell subset, it is likely that the magnitude, duration, and quality of immune response to poxvirus and recombinant antigens are altered. In future studies, we hope to identify and inactivate the viral and host proteins responsible for γδ T cell inhibition and to reexamine VV immunity in the presence of functional γδ T cell immunity.
Acknowledgments
We are grateful to Drs. Robert C. Gallo, Bruce Gilliam, Robert R. Redfield, and Mika Popovic, for helpful comments.
Financial support: US Public Health Service (grants AI51212 and CA 113621 to C.D.P.); International Clinical Operational and Health Services Research and Training Awards for AIDS and Tuberculosis in China (grant to H.L.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Fenner F. History of international public health. 6. Geneva: World Health Organization; 1988. World Health Organization. Smallpox and its eradication. [Google Scholar]
- 2.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–8. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130(Suppl):S11–25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- 4.Seet BT, Johnston JB, Brunetti CR, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 5.Kirwan S, Merriam D, Barsby N, McKinnon A, Burshtyn DN. Vaccinia virus modulation of natural killer cell function by direct infection. Virology. 2006;347:75–87. doi: 10.1016/j.virol.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Engelmayer J, Larsson M, Subklewe M, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–8. [PubMed] [Google Scholar]
- 7.Coupar BE, Andrew ME, Both GW, Boyle DB. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur J Immunol. 1986;16:1479–87. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- 8.Townsend A, Bastin J, Gould K, et al. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–24. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brutkiewicz RR, Klaus SJ, Welsh RM. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat Immun. 1992;11:203–14. [PubMed] [Google Scholar]
- 10.Freer G, Senesi S. No recognition of MHC class II+ cells infected with a vaccinia virus encoding influenza type A nucleoprotein by class II-restricted T cells. Immunol Lett. 1993;36:305–12. doi: 10.1016/0165-2478(93)90105-b. [DOI] [PubMed] [Google Scholar]
- 11.Domanico SZ, Pierce SK. Virus infection blocks the processing and presentation of exogenous antigen with the major histocompatibility complex class II molecules. Eur J Immunol. 1992;22:2055–62. doi: 10.1002/eji.1830220815. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Wang N, Zhou D, et al. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J Immunol. 2005;175:6481–8. doi: 10.4049/jimmunol.175.10.6481. [DOI] [PubMed] [Google Scholar]
- 13.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/Vdelta2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann SH. Gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueta C, Tsuyuguchi I, Kawasumi H, Takashima T, Toba H, Kishimoto S. Increase of gamma/delta T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–41. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito M, Kojiro N, Ikeda T, Ito T, Funada J, Kokubu T. Increased proportions of peripheral blood gamma delta T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–7. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 17.Balbi B, Valle MT, Oddera S, et al. T-lymphocytes with gamma delta+ V delta 2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–90. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 18.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–5. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz E, Shapiro R, Shina S, Bank I. Delayed expansion of V delta 2+ and V delta 1+ gamma delta T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol. 1996;97:1387–92. doi: 10.1016/s0091-6749(96)70208-7. [DOI] [PubMed] [Google Scholar]
- 20.Roussilhon C, Agrapart M, Ballet JJ, Bensussan A. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis. 1990;162:283–5. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 21.Gan YH, Pauza CD, Malkovsky M. Gamma delta T cells in rhesus monkeys and their response to simian immunodeficiency virus (SIV) infection. Clin Exp Immunol. 1995;102:251–5. doi: 10.1111/j.1365-2249.1995.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–94. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 23.Abate G, Eslick J, Newman FK, et al. Flow-cytometric detection of vaccinia-induced memory effector CD4+, CD8+, and γδTCR+ T cells capable of antigen-specific expansion and effector functions. J Infect Dis. 2005;192:1362–71. doi: 10.1086/444423. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZW, Letvin NL. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes Infect. 2003;5:491–8. doi: 10.1016/s1286-4579(03)00074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modlin RL, Sieling PA. Immunology. Now presenting: gammadelta T cells. Science. 2005;309:252–3. doi: 10.1126/science.1115264. [DOI] [PubMed] [Google Scholar]
- 26.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francois-Bongarcon V, Feng Y, Lee SK, et al. Cross-clade CD8 T-cell responses to HIV(IIIB) and Chinese B′ and C/B′ viruses in North American and Chinese HIV-seropositive donors. J Acquir Immune Defic Syndr. 2004;37:1435–44. doi: 10.1097/01.qai.0000145220.81304.b0. [DOI] [PubMed] [Google Scholar]
- 28.Yin C, Wu MS, Pauza CD, Salvato MS. High major histocompatibility complex-unrestricted lysis of simian immunodeficiency virus envelope-expressing cells predisposes macaques to rapid AIDS progression. J Virol. 1999;73:3692–701. doi: 10.1128/jvi.73.5.3692-3701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–39. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang XM, Terasaki PI, Rankin GW, Jr, Chia D, Zhong HP, Hardy S. A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol. 1993;37:264–70. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 31.Baraz L, Khazanov E, Condiotti R, Kotler M, Nagler A. Natural killer (NK) cells prevent virus production in cell culture. Bone Marrow Transplant. 1999;24:179–89. doi: 10.1038/sj.bmt.1701825. [DOI] [PubMed] [Google Scholar]
- 32.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 33.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 34.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–11. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gan YH, Malkovsky M. Mechanisms of simian gamma delta T cell cytotoxicity against tumor and immunodeficiency virus-infected cells. Immunol Lett. 1996;49:191–6. doi: 10.1016/0165-2478(96)02508-4. [DOI] [PubMed] [Google Scholar]
- 36.Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clin Exp Immunol. 1996;103:177–84. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pennington DJ, Vermijlen D, Wise EL, Clarke SL, Tigelaar RE, Hayday AC. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv Immunol. 2005;87:27–59. doi: 10.1016/S0065-2776(05)87002-6. [DOI] [PubMed] [Google Scholar]
- 38.Heinkelein M, Pilz S, Jassoy C. Inhibition of CD95 (Fas/Apo1)-mediated apoptosis by vaccinia virus WR. Clin Exp Immunol. 1996;103:8–14. doi: 10.1046/j.1365-2249.1996.927619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bieback K, Breer C, Nanan R, ter Meulen V, Schneider-Schaulies S. Expansion of human gamma/delta T cells in vitro is differentially regulated by the measles virus glycoproteins. J Gen Virol. 2003;84:1179–88. doi: 10.1099/vir.0.19027-0. [DOI] [PubMed] [Google Scholar]
- 40.Fujihashi K, Taguchi T, Aicher WK, et al. Immunoregulatory functions for murine intraepithelial lymphocytes: gamma/delta T cell receptor-positive (TCR+) T cells abrogate oral tolerance, while alpha/beta TCR+ T cells provide B cell help. J Exp Med. 1992;175:695–707. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu YX, Roark CE, Kelly K, et al. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 42.Girardi M, Lewis J, Glusac E, et al. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–67. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 44.Roessner K, Wolfe J, Shi C, Sigal LH, Huber S, Budd RC. High expression of Fas ligand by synovial fluid-derived gamma delta T cells in Lyme arthritis. J Immunol. 2003;170:2702–10. doi: 10.4049/jimmunol.170.5.2702. [DOI] [PubMed] [Google Scholar]
- 45.DiTirro J, Rhoades ER, Roberts AD, et al. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–9. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


