Abstract
We examined the impact of methadone maintenance treatment (MMT) on risk behaviors for transmission of bloodborne diseases in polydrug users who had tested positive or negative for hepatitis C (HCV). At intake, HCV-positive participants (n = 362) engaged in more HIV risk behaviors (as measured by the HIV Risk-Taking Behaviour Scale; HRBS) than HCV-negative participants (n = 297) (p<.001). This difference was specific to injection-related behaviors and decreased significantly within the first few weeks of MMT (p<.0001). Where needles continued to be used, HCV-positive participants became more likely over time to engage in safer injecting practices. Furthermore HCV-positive participants became more likely to use condoms than HCV-negative participants. These findings demonstrate that both drug- and sex-related risk behaviors decrease during MMT, and emphasize the benefits of methadone programs for public health and HIV/HCV prevention.
Keywords: Hepatitis C, Risk behaviors, Methadone maintenance, Injection drug use, Substance abuse
1. Introduction
Hepatitis C virus (HCV) is a leading cause of chronic liver disease (Quaglio et al., 2003; Alter, 1995; Mansell & Locamini, 1995) and the most prevalent bloodborne infection in the United States (US; Centers for Disease Control and Prevention, 2006). The World Health Organization estimates that about 180 million people, some 3% of the world's population, are infected with HCV, of which 130 million are chronic HCV carriers at risk for developing liver cirrhosis and/or liver cancer. It is estimated that three to four million persons are newly infected each year (World Health Organization, 2006).
The major risk factor for HCV transmission is injection drug use (IDU; Bollepalli et al., 2006; Maher et al., 2006; Mathei et al., 2006), and with injection drug users (IDUs) accounting for 70% of all HCV cases (Alter et al., 1999), HCV is fast becoming a prominent public health problem among IDUs in the U.S. The seroprevalence of HCV in IDUs generally ranges from 60% to 90% (Hagan, 1998; Patrick et al., 2001), suggesting a very high efficiency of parenteral transmission. Yet HCV prevalence in drug users who do not use intravenously remains higher than that of the general population (5–12% vs 2%; Howe et al., 2005), and several studies examining non-injecting drug users for other risk behaviors have produced conflicting results (Cohen et al., 2006; Howe et al., 2005; Koblin et al., 2003; Turner et al., 2006).
Methadone maintenance treatment (MMT) has been strongly associated with a reduction in HIV-risk behaviors, including reduced injection frequency and needle-related risk behaviors (Kwiatkowski and Booth, 2001; Sorensen and Copeland, 2000; Thiede et al., 2000), presumably as a result, MMT is associated with reduced risk of HIV seroconversion (Metzger et al., 1993; Moss et al., 1994). Less is known about the effect of MMT on HCV transmission, and recent studies have failed to demonstrate significant reductions in HCV seroconversion among methadone treatment patients (Crofts et al., 1997; Selvey et al., 1997; Thiede et al., 2000). Research in this area is divided between studies focusing on seroconversion and studies focusing on behavioral risk factors for infection. It is assumed that decreasing risk behaviors will decrease the spread of HCV. Yet at least one recent study comparing new and long-term IDUs has shown that syringe sharing, while not common in HIV-positive participants, was about as common in HCV-positive participants as in HCV-negative participants (Miller et al., 2003). The authors concluded that there is an increased need to heighten the awareness of the transmission risks and seriousness of HCV infection. Although there have been a few studies exploring HCV risk factors within the context of MMT, they have either focused on seroconversion (as opposed to risk behaviors) as their primary outcome measure (Rezza et al., 1996), have analyzed risk behaviors separately from HCV status (Thiede et al., 2000), or have included no intake measures as a baseline for comparison (Weaver et al., 2005). To our knowledge, no study has (1) examined the effect of MMT on both drug and sex risk behaviors in HCV-positive and HCV-negative individuals, (2) closely examined the time course of any such behavioral change over the first few weeks of methadone maintenance, or (3) assessed, in such individuals, whether risky behaviors such as injection drug use may continue in less-risky ways (such as decreased needle sharing).
We therefore assessed risk behaviors in HCV-positive and HCV-negative drug users, before, during, and after MMT. In doing so, we also hoped to contribute to a growing body of literature reporting upon the demographics associated with risk of HCV infection. Although there is a general consensus that HCV infection can be positively correlated with length of injection drug use and drug risk behaviors (sharing needles etc; (Bollepalli et al., 2006; Maher et al., 2006; Mathei et al., 2006; Quaglio et al., 2003; Roy et al., 2002), there remain discrepancies concerning the association between HCV status and particular demographic variables such as age (Van Beek et al., 1998), race (Kwiatkowski et al., 2002; Ompad et al., 2002), and gender (Crofts et al., 1993; Patrick et al., 2001).
2. Methods
2.1. Participants and setting
This report is based on a retrospective analysis of data from 659 patients consecutively admitted to any of 3 clinical trials evaluating the efficacy of combining contingency management and methadone maintenance for dual cocaine and heroin use (Epstein et al., 2005; Epstein et al., 2003). The trials were conducted at an outpatient treatment research clinic in Baltimore, MD. In brief, eligibility criteria for initial enrollment were the same in all three trials: age 18–65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates.
Screening conducted before admission to the trial and MMT included medical, psychiatric, and drug-use histories, a physical examination, urine and blood screens, and a battery of assessment instruments, including the Addiction Severity Index (ASI; McLellan et al., 1985) and the Diagnostic Interview Schedule (DIS-IV; Robins et al., 1995). Liver function tests were included in the general chemistry screen. Laboratory testing included screening for hepatitis C, hepatitis B and HIV. All participants were told the results of hepatitis and HIV testing; those who tested positive were also given advice by a physician's assistant or nurse practitioner on how to reduce the medical consequences and spread of their infection.
All participants began methadone maintenance upon enrollment in the study and received, without charge, daily methadone and weekly individual counseling (standard treatment) for up to 35 weeks. After a 5-week baseline of this standard treatment, participants began an additional 12-week intervention in which they either earned incentives such as vouchers with monetary value or opportunities to draw for prizes each time they tested negative for heroin and/or cocaine (contingent group) or received vouchers or prize draws independent of their urine results (noncontingent group). The specific method of earning contingency management incentives was the only variation between each of the three clinical studies. At the end of 25 weeks participants transferred to a community treatment program; those who chose not to transfer were given a methadone-taper over the last 10 weeks of the study. The trials were approved by and conducted under the ethical standards of the local Institutional Review Board for human research; each participant gave written informed consent.
2.2 Data collection
Three times a week (each Monday, Wednesday, and Friday), urine specimens were collected from all participants under observation by laboratory technicians. Qualitative testing (EMIT; Syva Corp., Palo Alto, California) was conducted for cocaine (benzoylecgonine), opiates (morphine), benzodiazepines (oxazepam), phencyclidine, barbiturates, and marijuana. Cutoff concentrations for positive specimens were 300 ng/ml for cocaine, opiates, and benzodiazepines, 25 ng/ml for PCP, and 50 ng/ml for marijuana. Breath alcohol levels were determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO). Participants completed the HIV Risk-Taking Behaviour Scale (HRBS; Darke et al., 1991) in written questionnaire form at 2-week intervals, from intake up until week 30. The HRBS is an 11-item scale comprising two subscales: The drug-use scale, with 6 items about injection frequency and sharing and cleaning needles, and the sexual-behavior scale, with 5 items about number of partners, condom use and anal sex. We added a 12th item to assess how often condoms were used during anal sex. Scoring on each subscale ranges from 0 to 30. Adequate reliability of the individual items on this instrument has been established, and subjects' and their sexual partners' responses are highly correlated (Darke et al., 1991).
2.3. Data Analysis
For all analyses, the alpha level was p ≤ .05 (two-tailed). Analyses were carried out using the Statistical Package for the Social Sciences version 13 (SPSS, Chicago, IL, USA) and SAS version 8 (Statistical Analysis System, Cary, NC, USA). Participants were grouped according to their HCV status.
Intake measures were analyzed by ANOVA or t-test (for continuous variables) and Pearson χ2 (for categorical variables). To follow up on these analyses, a four-step mediation analysis and Sobel test (Baron & Kenny 1986) were conducted on the relationship between race, route of drug administration and HCV status. Frequency tables were generated to describe the participant characteristics in terms of demographics and hepatitis C serostatus. Study retention was analyzed with a log-rank test (SAS LIFETEST procedure) of time until provision of the final urine sample.
Scores from the HRBS were analyzed by repeated-measures regression (SAS Proc Mixed), which produces an output like that of a repeated-measures ANOVA but does not require imputation of missing data points. In each of three separate repeated-measures regressions, the dependent variable was each participant’s total HRBS score, drug-subscale score, or sex-subscale score. The independent variables were HCV status (positive or negative) and week of treatment (0 through 30). (These analyses were repeated to control for treatment group, i.e. contingent or noncontingent, but the results were essentially identical and are not reported here.) Groups were compared at each time point with Tukey-Kramer tests. A first-order autoregressive error structure was used.
In order to assess whether changes in drug risk behaviors were due solely to the decrease of injection drug use, a similar repeated-measures regression was performed on each of the 12 individual items on the HRBS. For items 2–6 (regarding injection practices), these analyses were performed only for occasions when participants had reported injecting (item 1); for items 8–12 (regarding sexual practices), the analyses were performed only for occasions when participants had reported sexual activity (item 7); thus, the question addressed in these ten individual-item analyses was the degree of risk associated with each potentially risky behavior when it occurred at all.
Because data were pooled from three sequentially run studies involving slightly different behavioral interventions, the HRBS analyses were repeated with Study as a covariate. This did not appreciably change the results, so the data are reported without the use of this covariate.
Urine-screen results were analyzed by repeated-measures logistic regression (SAS GLIMMIX macro). In each of two separate analyses, the dependent variable was the urine-screen result for either opiates or cocaine (positive or negative) at each of up to 15 time points during the first 5 weeks of treatment (a baseline phase during which no contingency interventions were in place). The independent variables were HCV status (positive or negative) and day. A first-order autoregressive error structure was used.
3. Results
3.1. Demographic characteristics and drug use histories at intake
Among the participants (N = 659), men and women were approximately equally represented, as were African Americans and Caucasians. The mean age at enrollment was 38 years and the median length of time that participants had been injecting heroin at intake was 9 years (range, 1 – 38 years). Participants spent a median time in treatment of 197 days (with an upper and lower quartile range of 75–245 days); rates of retention did not differ across contingency management groups (data not shown) or by HCV status (log-rank chi square = 0.52, p = .47). Over half (N = 361, 54.9%) were HCV positive; prevalences of hepatitis B and HIV were much lower, only 3.9% (N= 26) and 3.2% (N = 21), respectively. Additional demographic and drug-use-history data are reported in Table 1. Data pertaining to several illicit substances, including street methadone, barbiturates, sedatives, amphetamine, hallucinogens, and inhalants, are not reported here, as use of those substances was rarely reported by our participants. HCV-positive and HCV-negative participants did not differ on age, gender, marital status, education, or ASI items assessing medical status, occupation type, employment/support status, legal status, family/social relationships, psychiatric status, and days of alcohol/drug use in the prior 30 days (data not shown).
Table 1.
Distributions of study participants and analysis of ASI data
| Category | Total N (%) | HCV+ N (%) | HCV− N (%) | p |
|---|---|---|---|---|
| Hepatitis C (n=659) | 362 (54.9) | 297 (45.1) | ||
| Ethnicity (n=658) | ||||
| African American | 385 (58.5) | 171 (44) | 214 (56) | 0.001 |
| Caucasian | 263 (40) | 184 (70) | 79 (30) | |
| Other | 10 (1.5) | 7 (70) | 3 (30) | |
| Years of Drug/Alcohol Use | ||||
| (mean ± SD) | ||||
| Alcohol (n=655) | 4.1 ± 7.2 | 4.4 ± 7.2 | 3.7 ± 7.1 | ns |
| Heroin (n=659) | 10.5 ± 7.6 | 11.4 ± 8.1 | 9.4 ± 6.7 | 0.004 |
| Cocaine (n=659) | 8.3 ± 6.8 | 9.1 ± 7.2 | 7.4 ± 6.1 | 0.001 |
| Cannabis (n=656) | 4.1 ± 7.2 | 4 ± 6.1 | 3.9 ± 5.5 | ns |
| Poly Drug Use (n=654) | 6.9 ± 6 | 7.6 ± 6.4 | 6 ± 5.4 | 0.000 |
| Primary Drug Route: Summary (n=659) | ||||
| IV | 406 (61.6) | 322 (79) | 84 (21) | 0.000 |
| Non-IV | 253 (38.4) | 40 (16) | 213 (84) | |
| Primary Drug Route: Heroin (n=658) | ||||
| IV | 406 (61.7) | 281 (79) | 125 (21) | 0.000 |
| Nasal | 251 (38.1) | 81 (32) | 170 (68) | |
| Smoking | 1 (.2) | 0 (0) | 1 (100) | |
| Primary Drug Route: Cocaine (n=656) | ||||
| IV | 325 (49.5) | 233 (72) | 92 (28) | 0.000 |
| Nasal | 282 (43) | 114 (40) | 168 (60) | |
| Smoking | 49 (7.5) | 13 (27) | 36 (73) | |
Race was the only demographic variable significantly associated with HCV infection: Caucasians were significantly overrepresented among HCV-positive participants, χ2(1, N = 659) = 11.24, p < .01. On drug-use-history variables collected as part of the ASI at intake, HCV-positive participants reported significantly more years of heroin, cocaine, and polydrug use than those who were HCV negative (Table 1). HCV infection was significantly more common in participants who reported on the ASI that they had used drugs intravenously.
To clarify the relationship amongst these variables associated with HCV serostatus, we performed a four-step mediation analysis (Baron & Kenny 1986). In the mediation analysis, logistic regression showed that race predicted HCV status (OR = 0.58; 95% CI = 0.42-0.8), but it also showed that race predicted IDU (if participants were Caucasian, they were more likely to use heroin and/or cocaine intravenously) (OR = 0.5; 95% CI = 0.36-0.7), and that IDU completely mediated the initial relationship between race and HCV status (OR = 19.86; 95% CI = 13.1-30.13). A Sobel test (Baron & Kenny 1986) confirmed that the effect of race was mediated through route of administration (p< .001).
3.2. Risk-taking behaviors - total scores
Analysis of total scores from the HRBS (N = 647) showed that HCV-positive participants reported engaging in more HIV-risk behaviors than HCV-negative participants (HRBS; F1, 645 = 86.6, p<.001) during the two weeks preceding intake. Risk behaviors for both groups decreased in the first few weeks of methadone maintenance and stabilized to plateau through the treatment course (Figure 1). This was shown by a significant main effect of time (F15, 6229 = 70.1, p<.0001) and a significant HCV status by time interaction (F15, 6229 = 10.3, p< .0001). In Tukey-Kramer post hoc comparisons, differences in total HRBS between HCV-positive and HCV-negative groups were significant at weeks zero (screening) (p<.001), two (p<.001) and four (p<.05) but not thereafter (p>.05).
Figure 1. Total HIV Risk Behaviors and Subscale Risk Behaviors.
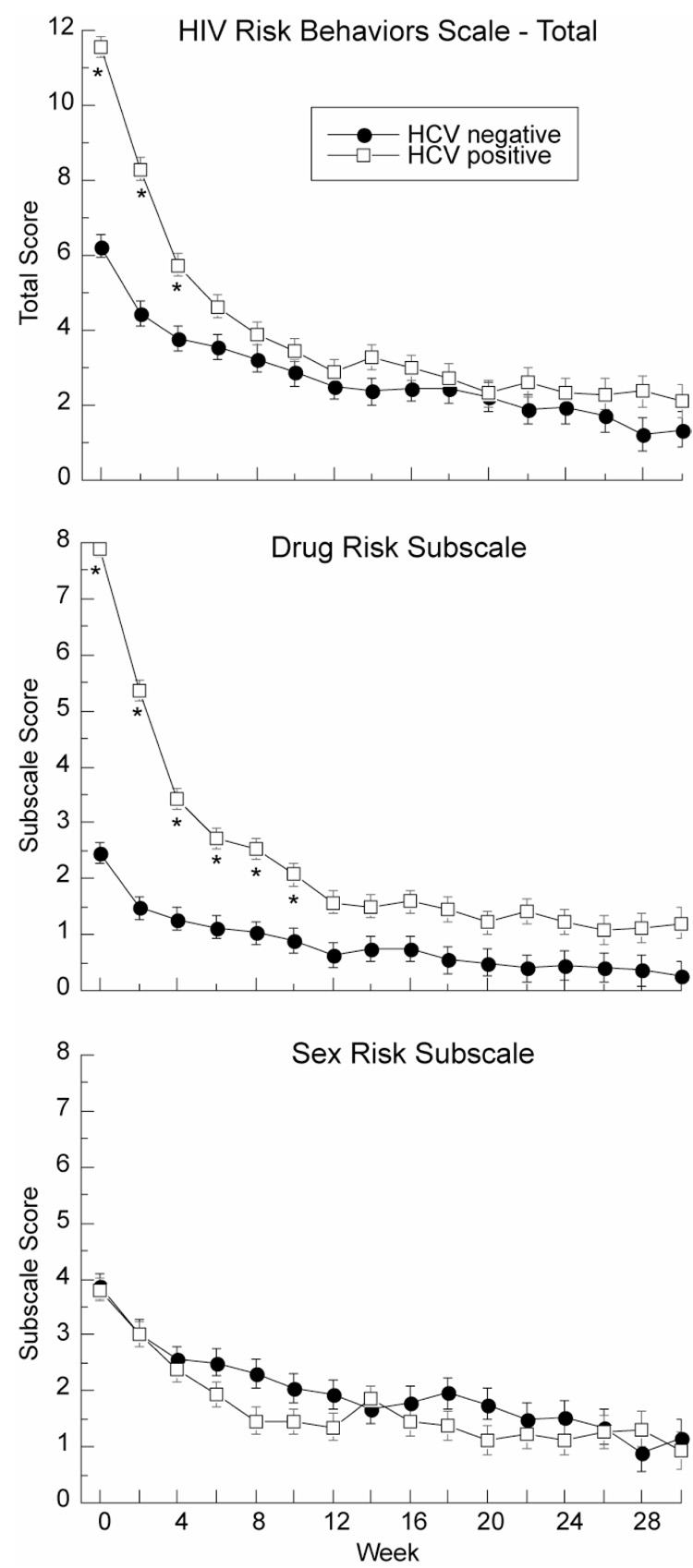
Total HIV risk behavior scores (top panel), drug risk behavior scores (middle panel) and sex risk behavior scores (bottom panel) from the HIV Risk Behavior Scale in HCV-positive (N=356) and HCV-negative (N=291) participants at screening (week 0) and during treatment. Asterisk indicates weeks at which total score for HCV-positive and HCV-negative participants were significantly different (p<.05). Higher scores indicate greater frequency of risky behaviors.
3.3. Drug risk behaviors
On the drug subscale of the HRBS, HCV-positive participants reported more drug risk behaviors than HCV-negative participants (F1, 645 = 359.1, p<.001) during the two weeks preceding intake. Drug risk behaviors reported by HCV-positive participants declined dramatically within the first few weeks of methadone treatment, then stabilized (Figure 2, top panel); there was a significant main effect of time (F15, 6147 = 76.9, p< .0001) and a significant HCV status by time interaction (F15, 6147 = 23.9, p< .0001). In Tukey-Kramer post hoc comparisons, differences in drug risk behaviors between HCV-positive and HCV-negative individuals were significant at weeks zero (p<001), two (p<.001), four (p<.001), six (p<.001), eight (p<.001), and ten (p<.001), but not thereafter (p>.05).
Figure 2. Individual Drug and Sex Risk Items.
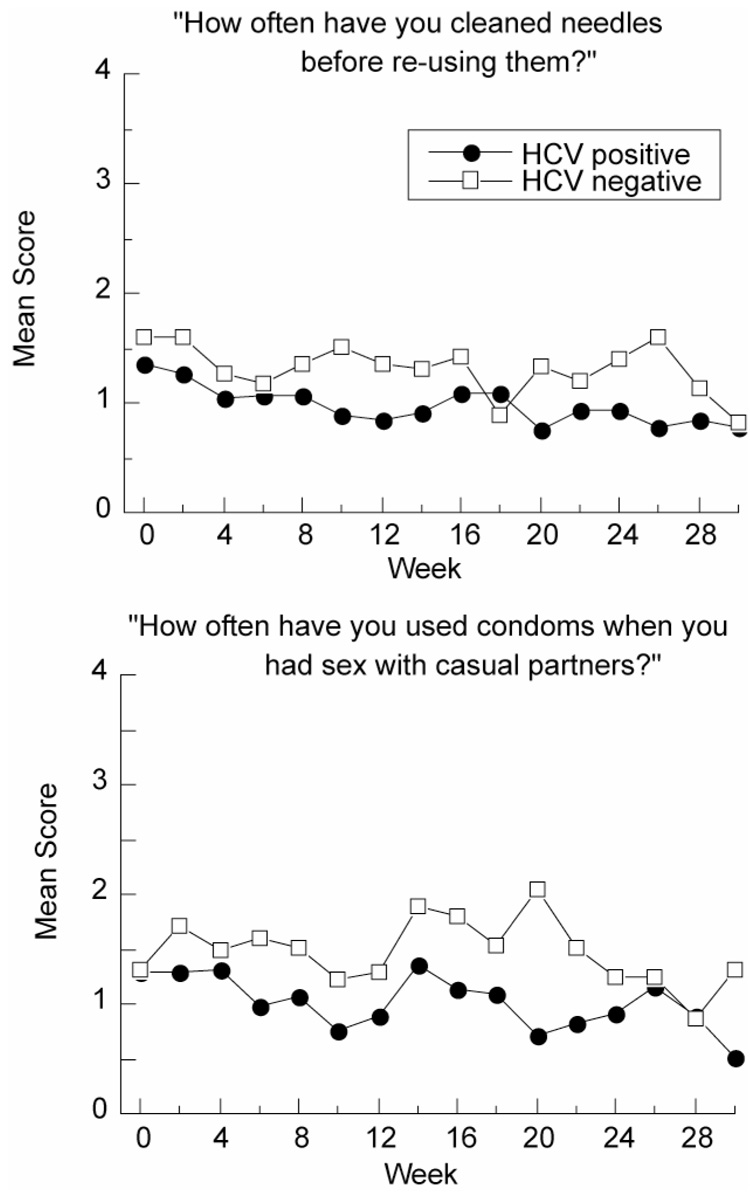
Upper panel: Example item from the drug subscale of the HIV Risk Behavior Scale in HCV-positive (N=322) and HCV-negative (N=96) participants at screening (week 0) and during treatment. Data are shown only for occasions when participants had reported injecting (item 1; drug risk subscale). Possible item responses ranged from 0 (“I didn’t re-use needles in the last 2 weeks”) to 5 (“Never”).
Lower panel: Example item from the sex subscale of the HIV Risk Behavior Scale in HCV-positive (N=265) and HCV-negative (N=215) participants at screening (week 0) and during treatment. Data are shown only for occasions when participants had reported sexual activity (item 7; sex risk subscale). Possible item responses ranged from 0 (“I haven’t had any casual partners in the last 2 weeks”) to 5 (“Never”).
3.4 Drug risk behaviors – individual questionnaire items
During the first several weeks of treatment HCV-positive participants reported more frequent injecting than HCV-negative participants. This was reflected by a significant main effect of HCV status (F1, 645 = 552.5, p<.0001). Injection frequency decreased with treatment for both HCV-positive and HCV-negative participants. This was reflected by a significant main effect of time (F15, 6135 = 132.02, p< .0001). Injection frequency decreased more steeply in HCV-positive participants, compared with HCV-negative participants, and towards the end of treatment both groups were injecting at similarly low frequencies. This was reflected by a significant HCV status by time interaction (F15, 6135 = 41.73, p< .0001).
Among the 418 participants who continued to inject at any time point during treatment (65% of the total sample); HCV-positive participants began to report significantly decreased needle sharing compared to HCV-negative participants (F1, 413 = 5.15, p< .05). There was no HCV status by time interaction, though visual inspection of the data suggested a fluctuating pattern, with group differences more apparent after the first four weeks of treatment. HCV-positive participants who continued to inject significantly increased needle cleaning compared to HCV-negative participants (F1, 412 = 11.31, p< .01) (Figure 2, top panel). Again, there was no main effect of time or HCV status by time interaction, though visual inspection of the data suggested that group differences were more apparent after the first eight weeks of treatment.
3.5. Sex risk behaviors
In contrast to the drug-risk behaviors, HCV-negative participants reported more sex risk behaviors than HCV-positive participants during the course of treatment (F1, 645 = 9.8, p<.01) (Figure 3, bottom panel). Risk behaviors decreased with treatment, as reflected in a significant main effect of time (F15, 6144 = 18.9, p< .0001). There was no HCV status by time interaction (F15, 6144 = 0.8, p = .70).
Figure 3. Opiate and Cocaine Abstinence.
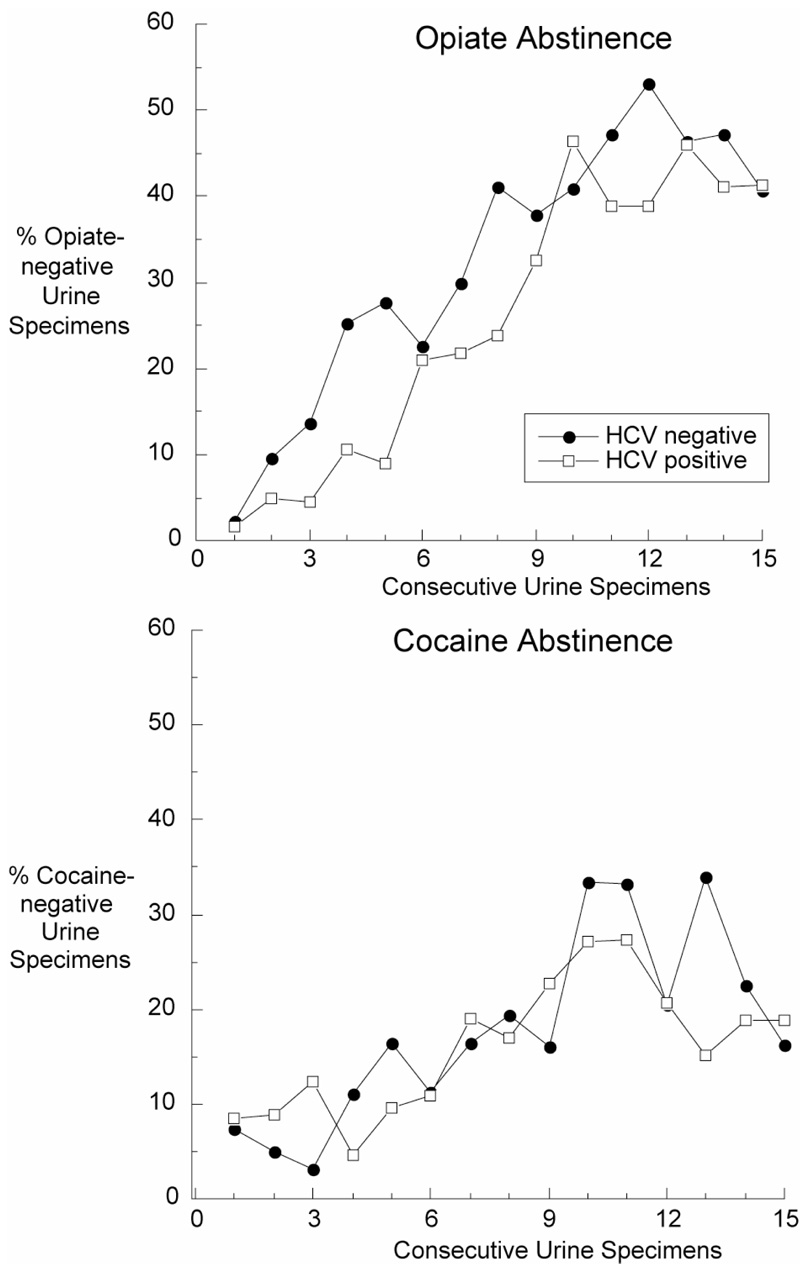
Abstinence from opiates (upper panel) and cocaine (lower panel) in HCV-positive (N=356) and HCV-negative (N=291) participants, based on 15 urine specimens collected three times weekly during the first five weeks of treatment.
3.6 Sex risk behaviors – individual questionnaire items
Among the 480 participants who remained sexually active at any time point during treatment (74% of the total study population); HCV-positive participants began to report significantly more condom use with regular partners than HCV-negative participants (F1, 477 = 17.22, p<.0001). There was no HCV status by time interaction, but visual inspection of the data suggested that group differences were most apparent after the first eight weeks of treatment. HCV-positive participants also reported greater condom use with casual partners (F1, 472 = 19.86, p<.0001) (Figure 2, bottom panel) and with paying clients (F1, 471 = 10.72, p<.01). Again, there was no HCV status by time interaction, but visual inspection of the data suggested that group differences were most apparent after week two.
3.7. Drug use
Opiate abstinence increased with treatment (F14, 2031 = 19.82, p<.0001) in both HCV-positive and HCV-negative participants (Figure 3, top panel); there was no main effect of HCV status (F1,640 = 1.83, p = 0.18), and no significant interaction between HCV status and time (F14, 2031 = 1.29, p = 0.20). Cocaine abstinence also increased with treatment in both groups (F14,2031 = 7.04, p<.0001) (Figure 3 bottom panel); there was no main effect of HCV status (F1, 639 = 0.01, p = 0.90), although there was a significant interaction between HCV status and time (F14, 2031 = 2.01, p = 0.01), most likely reflecting fluctuations of little clinical significance.
4. Discussion
Our principal findings were that participants who tested HCV positive at treatment intake, reported engaging in more drug risk behaviors than HCV-negative participants upon entry into treatment, and that these risk behaviors decreased sharply within the first few weeks of methadone treatment.
Even though the HCV-positive participants entered treatment as high-frequency injectors, they successfully decreased their injection frequency down to the same low level as the HCV-negative participants. Perhaps most importantly, when needles continued to be used, the HCV-positive participants became more likely over time to clean the needles and to avoid sharing them with others. Actual use of drugs (as reflected in urine-screen results) also decreased with treatment, but that decrease did not significantly differ between the two groups.
In a recent study by Millson et al. (2007), an analysis similar to ours was performed (but participants were not categorized by HCV status). They assessed drug risk behaviors in a subcohort of participants who continued to inject after enrollment in MMT. Similar to our results, Millson et al., reported a decrease in specific drug risk behaviors (including sharing needles and using shooting galleries), otherwise unaccounted for by the observed decrease in injection frequency. They conclude that the flexible approach of methadone programs that accept individuals who continue to use drugs, without threat of expulsion from treatment, can be of great benefit for public health through even partial reduction of risky injection behavior.
The finding that risky drug use decreased with MMT is consistent with previous research (Fabris et al., 2006; Stark et al., 1996; Strathdee & Patterson, 2006), but to our knowledge, this is the first time this result has been presented in terms of HCV status. Historically, HIV prevention programs have focused primarily on developing risk reduction interventions for those at high risk for becoming infected with HIV (Ward et al., 1998), yet by the time opioid injectors present for MMT, they have usually been injecting for a number of years and have already been exposed to blood-borne viruses (Ward et al., 1998). High numbers of HIV infections are transmitted by persons already aware of their HIV serostatus, and in recent years, U.S. federal agencies such as the Centers for Disease Control and Prevention (CDC) have begun to emphasize a strategy of “prevention for positives” (Janssen et al., 2001; Ward et al., 1998; Strathdee & Patterson, 2006).
However, few empirical studies have evaluated interventions focused on HCV-seropositive IDUs (Strathdee & Patterson, 2006). At least three recent studies have shown that HCV-positive IDUs are as likely, or indeed more likely, to share drugs and injection paraphernalia than HCV-negative IDUs (Hagan et al., 2006; Ompad et al., 2002; Vidal-Trécan et al., 2000). Whereas we observed a decrease in specific risk behaviors with MMT, Ompad et al (2002) reported similar or increased rates of high-risk injection behaviors 6 months after disclosure of HCV infection (and with no significant interim treatment); suggesting that knowledge of HCV status may not be enough to prompt a change in risk-related behavior (Hagan et al., 2006, Miller et al., 2003). In contrast, Kwiatkowski et al. (2002) showed that those who were aware of their positive status were significantly less likely to engage in risk behaviors; thus underscoring the need for improved access to HCV testing. In the current study, prior HCV knowledge was not recorded, although our clinical impression is that many, perhaps most, of our participants were previously naïve to their status. New studies are clearly required to investigate the behavioral impact of HCV status knowledge on subsequent risk-related behaviors.
Beyond specific drug risk behaviors, the second important finding in our data relates to sexual risk behaviors. HCV-positive participants engaged in less sexual risk behaviors than HCV-negative participants, and they became more likely over time to report greater condom use with both regular and casual partners, and with paying clients. This finding is consistent with results reported recently by Bollepalli et al. (2006), demonstrating that HIV/HCV coinfection (compared to HIV monoinfection) was associated with IDU but not sexual risk factors.
The unexpected result that HCV-positive individuals engaged in fewer sexual risk behaviors over the course of treatment than HCV-negative individuals, may be related to a recent finding by Fabris and colleagues (2006) showing that one in four patients changed their sexual habits after learning they were HCV positive. This is surprising, considering the low rate of sexual transmission of HCV, and may illustrate a lack of understanding by patients of how the hepatitis virus is transmitted. Previous studies of HIV risk reduction in methadone maintained patients report conflicting findings regarding its ability to reduce sexual risk behaviors (Baker et al., 1993, Gottheil et al., 1998). Our results therefore demonstrate importantly the broad beneficial effects of MMT for HCV/HIV prevention (Schroeder et al., 2006).
Our findings supplement existing data documenting associations between demographic variables and HCV status. Longer duration of drug use has reliably been associated with higher HCV prevalence (Crofts et al., 1993; Quaglio et al., 2003), and this relationship is supported by our data. However some previous incidence studies have reported a high prevalence of HCV infection among new and young injectors (Maher et al., 2006; Mathei et al., 2006; Van Beek et al., 1998). Similarly, some studies have reported a higher incidence of HCV in males (Crofts et al., 1993), and others in females (Maher et al., 2006; Patrick et al., 2001). Kwiatkowski et al. (2002) claimed that non-white IDUs are at elevated risk for HCV, whereas in a study by Ompad et al. (2002), participants in the HCV-positive group were significantly less likely to be African American. The lack of concordance across studies may reflect confounding by other risk factors; as in our study, some associations between demographic characteristics may result from underlying drug-use patterns that themselves may reflect cultural and social differences. There is, however, a general agreement among studies (including ours) that route of drug administration (for both heroin and cocaine) is strongly associated with HCV infection.
4.1. Study limitations
It is important to note that this is a retrospective analysis of data from a study designed to investigate behavioral and pharmacologic treatment interventions for polydrug abuse. This study clearly has limitations; for example, as this was not a longitudinal study of risk behaviors preceding seroconversion, no extrapolation can be made about which baseline risk behaviors were predictors of seroconversion. Also, as there was no comparison condition to MMT, no inferences can be made about the causal role of MMT in reducing HIV-risk behaviors. The treatment program, in the context of the research study, combined methadone dosing with counseling and a brief contingency management intervention: it cannot be known to what extent the current data will apply to basic “dose only” programs. As mentioned, participants were not questioned about previous knowledge of HCV status; also, we did not formally assess participants’ understanding of the hepatitis virus and how it is transmitted.
However, our study does have the strengths of a large sample size, data acquired through a combination of self-report and semistructured interview, prospective collection of in-treatment drug use and HIV-risk behaviors, and the use of biological samples to verify HCV serostatus and drug abstinence. In the absence of published studies investigating both drug and sexual risk behaviors in HCV seropositives within the context of MMT, our study provides a valuable insight into HCV risk and intervention.
5. Conclusion
There is a very evident association between unsafe drug practices and HCV infection. Prevention of infection is important, not least because of its toll on health through chronic liver disease and because therapy for HCV is costly and unpleasant. IDUs need to be educated about safer drug injection methods and more importantly provided with the tools to prevent contracting or spreading blood-borne diseases. Interventions that reduce high-risk transmission behaviors among HCV-positive individuals could also have a significant impact on HIV prevention, because HCV is often acquired before HIV among IDUs (Strathdee & Patterson, 2006). There is a need for adequate counseling to highlight the relative importance and dangers of different HCV risk factors. Further work is needed to understand the effect of MMT and knowledge of serostatus on HCV-risk behavior; though our results support the effectiveness of interventions targeted at drug use in HCV-positive individuals and the broad beneficial effects of MMT.
Acknowledgements
This research was supported by NIDA Intramural funds. A preliminary version of these analyses was presented at the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence, Scottsdale, AZ, 2006, and at a conference organized by the National Institute on Drug Abuse (“Drug abuse and risky behaviors: the evolving dynamics of HIV/AIDS”), MD, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter MJ. Epidemiology of hepatitis C in the West. Seminars in Liver Disease. 1995;15:5–14. doi: 10.1055/s-2007-1007259. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Kruszon-Moran MD, Nainan OV, et al. The Prevalence of hepatitis C virus infections in the United States, 1988 through 1994. The New England Journal of Medicine. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- Baker A, Heather N, Wodak A, Dixon J, Holt P. Evaluation of a cognitive-behavioral intervention for HIV prevention among injecting drug users. AIDS. 1993;7:247–256. doi: 10.1097/00002030-199302000-00014. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bollepalli S, Mathieson K, Bay C, Hillier A, Post J, Van Thiel DH, Nadir A. Prevalence of Risk Factors for Hepatitis C Virus in HIV-Infected and HIV/Hepatitis C Virus- Coinfected Patients. Sexually Transmitted Diseases. 2006;33:000. doi: 10.1097/01.olq.0000240295.35457.b1. [DOI] [PubMed] [Google Scholar]
- [Accessed October 19, 2006];Centers for Disease Control and Prevention: National hepatitis C prevention strategy for the prevention and control of hepatitis C virus infection and consequences. Online at: http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/HCV_infection.htm.
- Cohen DE, Russell CJ, Golub SA, Mayer KH. Prevalence of Hepatitis C Virus Infection among Men Who Have Sex with Men at a Boston Community Health Center and Its Association with Markers of High-Risk Behavior. AIDS Patient Care and STDs. 2006;20:557–564. doi: 10.1089/apc.2006.20.557. [DOI] [PubMed] [Google Scholar]
- Crofts N, Hopper JL, Bowden DS, Breschkin AM, Milner R, Locarnini SA. Hepatitis C virus infection among a cohort of Victorian injecting drug users. The Medical Journal of Australia. 1993;159:237–241. doi: 10.5694/j.1326-5377.1993.tb137822.x. [DOI] [PubMed] [Google Scholar]
- Crofts N, Nigro L, Oman K, Stevenson E, Sherman J. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction. 1997;92:999–1005. [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking among intravenous drug users. AIDS. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Schroeder JR, Preston KL. 65th Annual Scientific Meeting of the College on Problems of Drug Dependence. Bal Harbour, FL: 2003. Promoting simultaneous abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Schroeder JR, Preston KL. European Behavioural Pharmacology Society. Alicante, Spain: 2005. Comparison of two schedules of lottery-based abstinence reinforcement in polydrug users in methadone maintenance therapy. [Google Scholar]
- Fabris P, Tositti G, Giordani MT, Baldo V, Grasso A, Pignattari E, Canton S, Rossato S, Floreani A. Assessing patients' understanding of hepatitis C virus infection and its impact on their lifestyle. Alimentary Pharmacology and Therapeutics. 2006;23:1161–1170. doi: 10.1111/j.1365-2036.2006.02882.x. [DOI] [PubMed] [Google Scholar]
- Gottheil E, Lundy A, Weinstein SP, Sterling RC. Does intensive outpatient cocaine treatment reduce AIDS risky behaviors? Journal of Addictive Diseases. 1998;17:61–69. doi: 10.1300/J069v17n04_06. [DOI] [PubMed] [Google Scholar]
- Hagan F. Hepatitis C virus transmission dynamics in injection drug users. Substance Use and Misuse. 1998;33:1197–1212. doi: 10.3109/10826089809062214. [DOI] [PubMed] [Google Scholar]
- Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, Hudson S, Garfein RS. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Reports. 2006;121:710–719. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Anderson B, Stein M. Sexual risk behaviors among substance users: relationship to impulsivity. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2006;20:328–332. doi: 10.1037/0893-164X.20.3.328. [DOI] [PubMed] [Google Scholar]
- Howe CH, Fuller CM, Ompad DC, Galea S, Koblin B, Thomas D, Vlahov D. Association of sex, hygiene and drug equipment sharing with hepatitis C virus infection among non-injecting drug users in New York City. Drug and Alcohol Dependence. 2005;79:389–395. doi: 10.1016/j.drugalcdep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Janssen RS, Holtgrave DR, Valdiserri RO, Sheperd M, Gayle HD, DeCock KM. The serostatus approach to fighting the HIV epidemic: Prevention strategies for infected individuals. American Journal of Public Health. 2001;91:1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblin BA, Factor SH, Wu Y, Vlahov D. Hepatitis C Virus Infection Among Noninjecting Drug Users in New York City. Journal of Medical Virology. 2003;70:387–390. doi: 10.1002/jmv.10407. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Booth RE. Methadone Maintenance as HIV Risk Reduction with Street-Recruited Injecting Drug Users. Journal of Acquired Immune Deficiency Syndromes. 2001;26:483–489. doi: 10.1097/00126334-200104150-00014. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97:1289–1294. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Maher L, Jalaudin B, Chant KG, Jayasuriya R, Sladden T, Kaldor JM, Sargent PL. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Mathei C, Shkedy Z, Denis B, Kabali C, Aerts M, Molenberghs G, Van Damme P. Evidence for a substantial role of sharing of injecting paraphernalia other than syringes/needles to the spread of hepatitis C among injecting drug users. Journal of Viral Hepatitis. 2006;13:560–570. doi: 10.1111/j.1365-2893.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- Mansell CJ, Locarnini SA. Epidemiology of hepatitis C in the East. Seminars in Liver Disease. 1995;15:15–32. doi: 10.1055/s-2007-1007260. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Miller M, Mella I, Moi H, Eskild A. HIV and Hepatitis C Virus Risk in New and Longer-Term Injecting Drug Users in Oslo, Norway. Journal of Acquired Immune Deficiency Syndromes. 2003;33:373–379. doi: 10.1097/00126334-200307010-00012. [DOI] [PubMed] [Google Scholar]
- Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, Shore R, Hopkins S. Reduction in injection-related risk after 6 months in a low-threshold methadone treatment program. AIDS Education and Prevention. 2007;19(2):124–136. doi: 10.1521/aeap.2007.19.2.124. [DOI] [PubMed] [Google Scholar]
- Moss AR, Vranizan K, Gorter R, Bacchetti P, Watters J, Osmond D. HIV seroconversion in intravenous drug users in San Francisco, 1985–1990. AIDS. 1994;8:223–231. doi: 10.1097/00002030-199402000-00010. [DOI] [PubMed] [Google Scholar]
- Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;35:783–788. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- Patrick DM, Tyndall MW, Cornelisse PG, Li K, Sherlock CH, Rekart ML, Strathadee SA, Currie SL, Schechter MT, O’Shaughnessy MV. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. Canadian Medical Association Journal. 2001;165:889–895. [PMC free article] [PubMed] [Google Scholar]
- Quaglio GL, Lugoboni F, Pajusco B, Sarti M, Talamini G, Mezzelani P, Des Jarlais DC, et al. Hepatitis C virus infection: prevalence, predictor variables and prevention opportunities among drug users in Italy. Journal of Viral Hepatitis. 2003;10:394–400. doi: 10.1046/j.1365-2893.2003.00448.x. [DOI] [PubMed] [Google Scholar]
- Rezza G, Sagliocca L, Zaccarelli M, Nespoli M, Siconolfi M, Baldassarre C. Incidence Rate and Risk Factors for HCV Seroconversion among Injecting Drug Users in an Area with Low HIV Seroprevalence. Scandinavian Journal of Infectious Diseases. 1996;28:27–29. doi: 10.3109/00365549609027145. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK. The Diagnostic Interview Schedule, version IV. St Louis, MO: Washington University; 1995. Compton III WM. [Google Scholar]
- Roy K, Hay G, Andragetti R, Taylor A, Goldberg D, Wiessing L. Monitoring hepatitis C virus infection among injecting drug users in the European Union: a review of the literature. Epidemiology and Infection. 2002;129:577–585. doi: 10.1017/s0950268802007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS user’s guide Statistics. Version 6 ed. Cary NC: SAS Institute Inc; 1990. [Google Scholar]
- Schroeder JR, Epstein DH, Umbricht A, Preston KL. Changes in HIV risk behaviors among patients receiving combined pharmacological and behavioral interventions for heroin and cocaine dependence. Addictive Behaviors. 2006;31:868–879. doi: 10.1016/j.addbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Selvey LA, Denton M, Plant AJ. Incidence and prevalence of hepatitis C among clients of a Brisbane methadone clinic: factors influencing hepatitis C serostatus. Australian and New Zealand Journal of Public Health. 1997;21:102–104. doi: 10.1111/j.1467-842x.1997.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. A comparison of injection and non-injection methamphetamine-using HIV positive men who have sex with men. Drug and Alcohol Dependence. 2004;76:203–212. doi: 10.1016/j.drugalcdep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug and Alcohol Dependence. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- Stark K, MÜller R, Bienzle U, Guggenmoos-Holzmann I. Methadone maintenance treatment and HIV risk-taking behavior among injecting drug users in Berlin. Journal of Epidemiology and Community Health. 1996;50:534–537. doi: 10.1136/jech.50.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Patterson TL. Behavioral interventions for HIV positive and HCV positive drug users. AIDS and Behavior. 2006;10:115–130. doi: 10.1007/s10461-005-9055-5. [DOI] [PubMed] [Google Scholar]
- Thiede H, Hagan H, Murrill CS. Methadone Treatment and HIV and Hepatitis B and C Risk Reduction among Injectors in the Seattle Area. Journal of urban health: bulletin of the New York Academy of Medicine. 2000;77:331–345. doi: 10.1007/BF02386744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Rider AT, Imrie J, Copas AJ, Edwards SG, Dodds JP, Stephenson JM. Behavioural predictors of subsequent hepatitis C diagnosis in a UK clinic sample of HIV positive men who have sex with men. Sexually Transmitted Infections. 2006;82:298–300. doi: 10.1136/sti.2005.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek I, Dwyer R, Dore GJ, Luo K, Kaldor JM. Infection with HIV and Hepatitis C virus among injecting drug users in a prevention setting: retrospective cohort study. BMJ. 1998;317:433–437. doi: 10.1136/bmj.317.7156.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Trécan G, Coste J, Varescon-Pousson I, Christoforov B, Boissonnas A. HCV status knowledge and risk behaviors amongst intravenous drug users. European Journal of Epidemiology. 2000;16:439–445. doi: 10.1023/a:1007622831518. [DOI] [PubMed] [Google Scholar]
- Ward J, Mattick RP, Hall W. The effectiveness of methadone maintenance treatment 2: HIV and infectious hepatitis. In: Ward J, Mattick RP, Hall W, editors. Methadone Maintenance Treatment and Other Opioid Replacement Therapies. Amsterdam: Harwood Academic Publishers; 1998. pp. 59–73. [Google Scholar]
- Weaver MF, Cropsey KL, Fox SA. HCV Prevalence in Methadone Maintenance: Self-report Versus Serum Test. American Journal of Health Behavior. 2005;29:387–394. doi: 10.5555/ajhb.2005.29.5.387. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. [Accessed October 19, 2006]; Online at: http://www.who.int/vaccine_research/diseases/viral_cancers/en/index2.html.
- Wright N, Tompkins C. A review of the evidence for the effectiveness of primary prevention interventions for hepatitis C among injecting drug users. Harm Reduction Journal. 2006;3:27–36. doi: 10.1186/1477-7517-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


