Abstract
To examine the sex-specific contributions of the metabolic syndrome and microalbuminuria to cardiovascular disease (CVD) and coronary heart disease (CHD) mortality in community-dwelling older adults, between 1992–1995, 869 women and 575 men aged 40–96 years (mean 71) completed questionnaires, physical examinations, and fasting laboratory tests. Participants were followed over an average of 8 years. CVD and CHD mortality were analyzed using Cox proportional hazards models. At baseline, 267 participants had the Adult Treatment Panel III metabolic syndrome, 151 had microalbuminuria, and 34 had both. During follow up, there were 180 CVD deaths, including 83 CHD deaths. In women, microalbuminuria was associated with a 2-fold increased risk of CVD and CHD mortality (p ≤ 0.01). Women with both microalbuminuria and the metabolic syndrome (n = 18) had a 3-fold increased risk of CVD mortality and a 5-fold increased risk of CHD mortality compared with women without either (n = 657). A significant interaction existed between microalbuminuria and the metabolic syndrome in the prediction of both CVD and CHD (p = 0.021). In men, neither the combination of the metabolic syndrome and microalbuminuria (n = 16), nor either alone, significantly increased the risk of CVD or CHD mortality. In conclusion, in this cohort, microalbuminuria and the metabolic syndrome together were a more powerful predictor of CVD mortality than either alone in women but not in men. Screening for microalbuminuria in older women may identify women at high risk for CVD mortality, beyond that conferred by risk factors included in the metabolic syndrome.
Keywords: Cardiovascular disease, Elderly, Metabolic syndrome, Microalbuminuria
Background
The 1998 World Health Organization definition of the metabolic syndrome differs from Adult Treatment Panel III definition in its emphasis on insulin resistance and its inclusion of microalbuminuria 1,2. Microalbuminuria is an independent risk factor for CVD, and cardiovascular and all-cause mortality in diabetics 3, 4, hypertensives, 5, 6, 7, 8 and the general population 4, 9, 10, but it is unclear whether microalbuminuria adds to the prediction of CVD mortality in those with the metabolic syndrome. The Adult Treatment Panel III definition of metabolic syndrome is more widely used in the USA, therefore we used this definition. We examined the sex-specific separate and joint contributions of the metabolic syndrome and microalbuminuria to CVD and CHD mortality and explored whether including microalbuminuria might improve the utility of the Adult Treatment Panel III metabolic syndrome in identifying in older, community-dwelling men and women at risk for CVD death.
Methods
The Rancho Bernardo Study, a cohort of largely Caucasian, middle to upper middle class, community-dwelling adults in southern California, was established in 1972; the details of the initial study have been described previously 11, 12. Between 1992 and 1995, 80% (n = 1778) of surviving local residents participated in a research clinic visit focused on diabetes. The research protocol was approved by the institutional review board of the University of California, San Diego; all participants gave written informed consent.
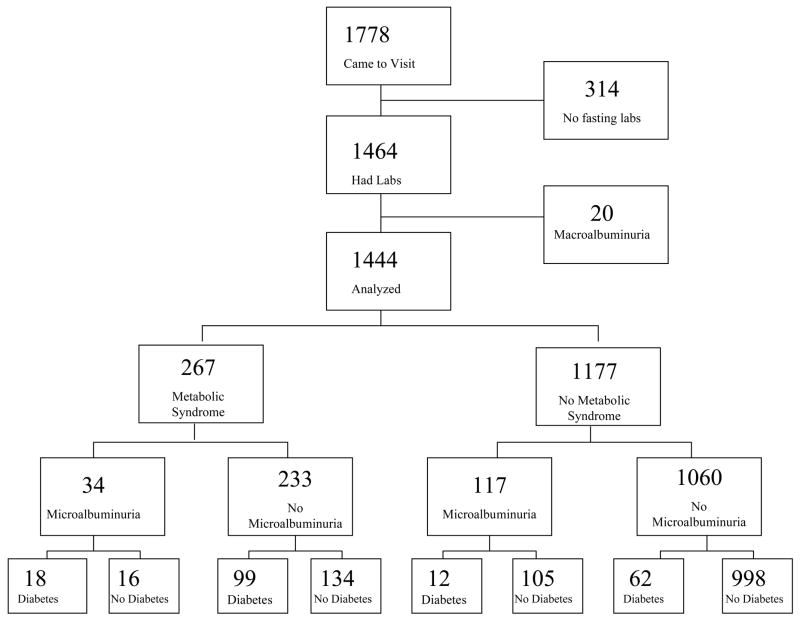
A total of 879 women and 585 men, ages 40 to 96, completed a 2-hour oral glucose tolerance test, and had levels of fasting lipids, and urine albumin and creatinine measured. Only 20 participants (1.4%) had macroalbuminuria (urine albumin/creatinine ratio >300mg/g); these 10 men and 10 women were excluded as our aim was to examine the risk conferred by microalbuminuria (a urine albumin/creatinine ratio 30–300mg/g) 13 an earlier marker of kidney dysfunction; the remaining 869 women and 575 men form the basis for this report (Figure 1).
Figure 1.
Cohort Flow Diagram
At the 1992–1995 visit, participants also completed standardized questionnaires about medical history including cardiovascular disease and medication use; the latter was validated by examination of pills and prescriptions brought to the clinic for that purpose. Information about current cigarette smoking, alcohol consumption (number of drinks per day during the last 2 weeks), and exercise was also obtained using standard questionnaires. Participants were asked about a history of physician-diagnosed myocardial infarction, angina, stroke, and claudication, and completed the Rose cardiovascular questionnaire 14.
Height and weight were measured using a calibrated stadiometer and balance-beam scale with participants wearing light clothing and no shoes. Systolic and diastolic blood pressures were measured twice in seated subjects after a five minute rest, using the Hypertension Detection and Follow-up Program protocol 15. Body mass index was calculated as kg/m2. Diabetes was defined according to the 1999 World Health Organization criteria 13--fasting plasma glucose ≥ 7 mmol/L (126mg/dL) or 2-hour glucose after 75 g oral glucose tolerance test ≥ 11.1 mmol/L (200 mg/dL)--or if he or she was using diabetes medication (oral or insulin). People with missing information on the 2-hour glucose test and diabetes drug use were classified as having diabetes if they reported a history of physician-diagnosed diabetes even in the absence of an abnormal glucose. Fasting and 2-hour glucose was measured by the glucose oxidase method in a clinical laboratory. Fasting plasma cholesterol, triglyceride, and high density lipoprotein and low density lipoprotein cholesterol levels were measured in a Center for Disease Control Certified Lipid Research Clinic laboratory. Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, Texas). High density lipoprotein was measured after precipitation of the other lipoproteins with heparin and manganese chloride according to the standardized procedures of the Lipid Research Clinics manual 16, low density lipoprotein was estimated using the Friedewald formula 17.
The metabolic syndrome was defined using the 2001 Adult Treatment Panel III criteria 2 as three or more of the following: 1) abdominal obesity--waist circumference > 102 cm in men and > 88 cm in women; 2) hypertriglyceridemia--serum triglycerides ≥ 1.69 mmol/L (150 mg/dL); 3) low high density lipoprotein cholesterol--high density lipoprotein < 1.04 mmol/L (40 mg/dL) in men and < 1.29 mmol/L (50 mg/dL) in women; 4) hypertension--systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg; 5) hyperglycemia--fasting plasma glucose ≥ 6.1 mmol/L (110 mg/dL). Participants reporting use of anti-hypertensive medications, and those with diabetes by World Health Organization 1999 criteria 13 were also categorized as meeting the hypertension and hyperglycemia criteria, respectively.
A single, clean-catch, untimed morning urine sample (usually second void of the day) was obtained, frozen, and shipped to the National Institute of Health laboratory (Phoenix, Arizona) of Dr. Peter Bennett. Urine albumin was measured using the Behring Nephelometer BNA. The inter-assay coefficient of variance was 4.5%. Urine creatinine was measured by the kinetic alkaline picrate method using the Ciba-Corning Express.
Vital status, determined annually by mailed questionnaire until 2003, was known for 94% of participants. Death certificates, available for 89% of decedents, were coded for the underlying cause of death by a certified nosologist using the International Classification of Disease—9th Revision. CHD deaths were those assigned codes between 410 and 414 (ischemic heart disease), including acute myocardial infarction (410), other acute and subacute forms of ischemic heart disease (411), old myocardial infarction (412), angina pectoris (413), and other forms of chronic ischemic heart disease (414). CVD deaths included all deaths assigned codes between 410 and 414 (above), and included conduction disorders (426), cardiac dysrhythmias (427), heart failure (428), cerebrovascular disease (430–438), and diseases of arteries, arterioles, and capillaries (440–448).
Most measures were normally distributed; serum triglycerides and the urine albumin/creatinine ratio were skewed; transformation was not necessary as categorical cutpoints were used. Sex-specific means were compared using ANalysis Of VAriance or Wilcoxon rank sum (if skewed) for continuous variables, and sex-specific prevalence was compared using the chi-squared statistic for categorical variables. Cox proportional hazards models were used to generate sex-specific hazard ratios (HR) with adjustment for: 1) age alone; 2) age plus prevalent CVD; 3) age plus microalbuminuria or metabolic syndrome; and 4) age plus prevalent CVD plus microalbuminuria or metabolic syndrome. Receiver operating curves using unadjusted logistic regression were performed to test whether adding microalbuminuria to metabolic syndrome improved prediction of CVD and CHD death. Statistical tests were 2-tailed, with statistical significance defined as p<0.05. SPSS (SPSS Inc. SPSS Base 11.0 for Windows) was used for all analyses.
Results
Baseline sex-specific characteristics and the sex-specific prevalence of metabolic syndrome and its components and microalbuminuria are summarized in Table 1. The mean age was 71 years. Hypertension was present in nearly 70% of men and women--more than twice as prevalent as any other component. Men were more likely than women to have the metabolic syndrome (22% vs. 16%, p = 0.01). Microalbuminuria was present in 151 participants (10%) and did not differ significantly by sex--89 women (10%) and 62 men (11%) (p = 0.79). Although microalbuminuria was more common in those with metabolic syndrome (13% vs. 10%, respectively) and metabolic syndrome was more common in those with microalbuminuria (23% vs. 18%, respectively), these differences were not statistically significant (p = 0.18 for both).
Table 1.
Baseline Characteristics: The Rancho Bernardo Study 1992–1995
| Characteristic | Women(n=869) | Men(n=575) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p value | |
| Age (yrs) | 70 (12) | 71 (11) | 0.50 |
| Body Mass Index (kg/m2) | 25 (4) | 26 (4) | <0.001 |
| Waist Circumference (cm) | 80 (11) | 95 (11) | <0.001 |
| Systolic Blood Pressure (mmHg) | 136 (23) | 134 (20) | 0.06 |
| Diastolic Blood Pressure (mmHg) | 75 (9) | 77 (9) | <0.001 |
| Fasting Plasma Glucose (mg/dL) | 96 (21) | 102 (22) | <0.001 |
| Triglycerides (mg/dL) † | 102 (75–144) | 104 (70–153) | 0.96 |
| High Density Lipoprotein (mg/dL) | 65 (17) | 49 (13) | <0.001 |
| Low Density Lipoprotein (mg/dL) | 129 (33) | 125 (30) | 0.03 |
| Urine Albumin/Creatinine (g/mg) † | 6 (4–13) | 6 (3–13) | 0.06 |
| Blood Urea Nitrogen (mg/dL) | 16 (6) | 18 (6) | <0.001 |
| Serum Creatinine (mg/dL) | 0.9 (0.2) | 1.1 (0.2) | <0.001 |
| Alcohol (g/week) † | 20 (0–70) | 53 (0–126) | <0.001 |
| Hemoglobin A1C (%) | 4.2 (0.6) | 4.3 (0.8) | 0.64 |
|
| |||
| Abdominal obesity | 22% | 20% | 0.47 |
| Hypertriglyceridemia | 22% | 27% | 0.04 |
| Low High Density Lipoprotein | 20% | 26% | <0.01 |
| Hypertension | 66% | 66% | 0.96 |
| Hyperglycemia | 15% | 21% | <0.01 |
| Metabolic Syndrome | 16% | 22% | 0.01 |
| Microalbuminuria | 10% | 11% | 0.79 |
| Exercise >3x/week | 70% | 76% | 0.02 |
| Current smoking | 7% | 7% | 0.92 |
| Diabetes | 11% | 16% | 0.01 |
| Current ERT Use | 45% | n/a | n/a |
Values for continuous variables are mean if normally distributed or
median (25–75%ile) if skewed
Values for categorical variable are percentages within each level
p value for ANOVA (normally distributed) or Wilcoxon rank sum (if skewed) for continuous variables and chi-square for categorical variables
Over a follow-up of 0.03 to 12 (mean 8) years, there were 180 CVD deaths (99 women, 81 men), including 83 (37 women and 46 men) CHD deaths; 11% of women versus 14% of men died due to CVD (p = 0.14 for sex difference), and 4% of women versus 8% of men died due to CHD (p = 0.004 for sex difference).
Metabolic syndrome was not significantly associated with CVD mortality in either sex (Table 2), nor was any individual metabolic syndrome component (data not shown). The metabolic syndrome was significantly associated with age-adjusted CHD mortality in women HR (95% CI) 1.99 (1.06–3.97), but not in men (sex interaction p = 0.03) (Table 2); the association in women was no longer significant in analyses adjusting for prevalent CVD (Table 2). No individual metabolic syndrome component was associated with age-adjusted CHD mortality in either sex (data not shown).
Table 2.
Age and Prevalent Cardiovascular Disease Adjusted Hazard Ratios (95% CI) for Metabolic Syndrome and Microalbuminuria as Predictors for Cardiovascular Disease and Coronary Heart Disease Death
| Women HR (95% CI) | Men HR (95% CI) | |
|---|---|---|
| Cardiovascular Disease Death | ||
|
| ||
| Metabolic Syndrome | ||
|
| ||
| Age adjusted | 1.40 (0.89–2.21) | 1.04 (0.58–1.86) |
| +Adjusted for Prevalent Cardiovascular Disease | 1.38 (0.88–2.18) | 1.15 (0.64–2.06) |
| +Adjusted for Microalbuminuria | 1.42 (0.90–2.23) | 1.02 (0.56–1.87) |
| +Adjusted for Prevalent Cardiovascular Disease & Microalbuminuria | 1.34 (0.85–2.12) | 1.14 (0.62–2.07) |
|
| ||
| Microalbuminuria | ||
|
| ||
| Age adjusted | 2.31 (1.45–3.67) | 1.06 (0.60–1.88) |
| +Adjusted for Prevalent Cardiovascular Disease | 2.19 (1.37–3.50) | 1.07 (0.61–1.89) |
| +Adjusted for Metabolic Syndrome | 2.32 (1.46–3.70) | 1.06 (0.59–1.91) |
| +Adjusted for Prevalent Cardiovascular Disease & Metabolic Syndrome | 2.17 (1.36–3.46) | 1.04 (0.58–1.86) |
|
| ||
| Coronary Heart Disease Death | ||
|
| ||
| Metabolic Syndrome | ||
|
| ||
| Age adjusted | 1.99 (1.06–3.97) | 1.06 (0.49–2.28) |
| +Adjusted for Prevalent Cardiovascular Disease | 1.96 (0.98–3.90) | 1.19 (0.55–2.60) |
| +Adjusted for Microalbuminuria | 2.02 (1.01–4.02) | 0.98 (0.44–2.17) |
| +Adjusted for Prevalent Cardiovascular Disease & Microalbuminuria | 1.89 (0.94–3.78) | 1.13 (0.51–2.49) |
|
| ||
| Microalbuminuria | ||
|
| ||
| Age adjusted | 2.40 (1.15–5.02) | 1.32 (0.65–2.69) |
| +Adjusted for Prevalent Cardiovascular Disease | 2.26 (1.08–4.74) | 1.34 (0.66–2.72) |
| +Adjusted for Metabolic Syndrome | 2.43 (1.16–5.08) | 1.33 (0.64–2.78) |
| +Adjusted for Prevalent Cardiovascular Disease & Metabolic Syndrome | 2.20 (1.04–4.64) | 1.32 (0.64–2.70) |
In contrast, microalbuminuria was associated with age-adjusted CVD mortality HR (95% CI) 2.31 (1.45–3.67), before and after adjustment for prevalent CVD, metabolic syndrome, or both (Table 2), but only in women (sex interaction p = 0.04) (Table 2). Microalbuminuria was also associated with age-adjusted CHD mortality in women HR (95% CI) 2.40 (1.15–5.02), but not in men (sex interaction p = 0.04), before and after adjustment for prevalent CVD, metabolic syndrome, or both (Table 2).
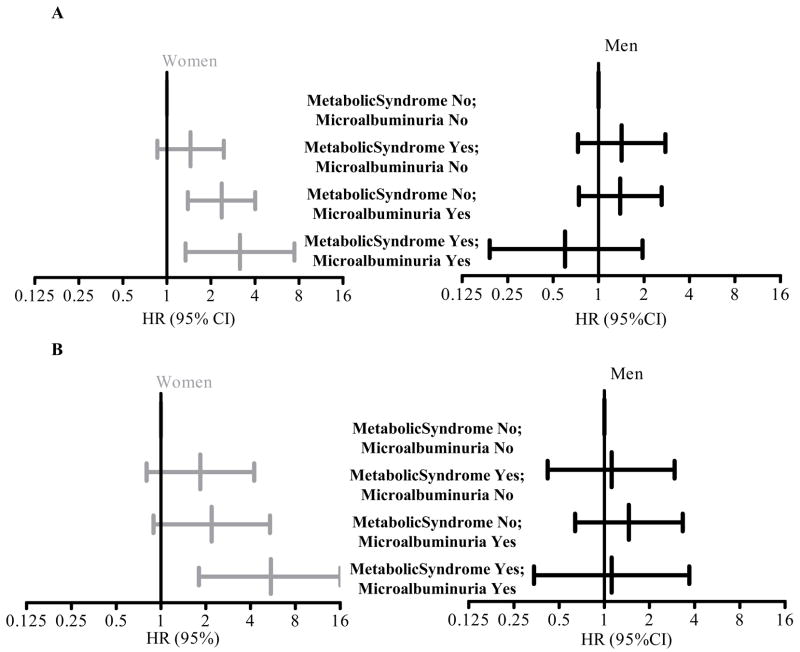
Women with both microalbuminuria and the metabolic syndrome had a 3-fold risk of CVD mortality HR (95% CI) 3.16 (1.34–7.43) (Figure 2A) and a 5-fold risk of CHD mortality HR (95% CI) 5.48 (1.80–16.64) (Figure 2B) compared to those with neither condition. A significant interaction existed between microalbuminuria and metabolic syndrome in the prediction of both CVD and CHD (p = 0.02). Associations did not differ by prevalent CVD (interaction p > 0.05 in each case), but tests did reveal a significant interaction between diabetes and microalbuminuria in the risk for CVD and CHD death (p = 0.02). Excluding women currently using estrogen did not materially change results. These associations were not seen in men.
Figure 2.
Age Adjusted Hazard Ratios (95% CI) for Cardiovascular Disease (A) and Coronary Heart Disease (B) Mortality by Metabolic Syndrome and Microalbuminuria Group
Receiver operating curves using logistic regression were performed to test whether adding microalbuminuria to metabolic syndrome improved prediction of CVD and CHD death. In women only, the area under the curve (AUC) for the ability of both metabolic syndrome and microalbuminuria together to predict CVD death AUC (95% CI) 0.629 (0.575–0.683) was greater than either the metabolic syndrome alone AUC (95% CI) 0.551 (0.506–0.596) (p for difference < 0.001) or microalbuminuria alone AUC (95% CI) 0.590 (0.546–0.635) (p for difference = 0.03). In men, the AUC for the ability of the metabolic syndrome alone, microalbuminuria alone, or both together to predict CVD death did not differ significantly. For CHD death, results were similar in women, with the AUC for the ability of both metabolic syndrome and microalbuminuria together to predict CHD death showing a trend towards being greater than either metabolic syndrome or microalbuminuria alone, but the differences were not statistically significance (p for difference = 0.07 and 0.08, respectively), probably based on the smaller sample size. In men, the AUC for the ability of metabolic syndrome and microalbuminuria together to predict CHD death also suggested a trend towards being greater than metabolic syndrome alone (p for difference = 0.09).
Conclusions
Microalbuminuria is used by clinicians as both a screening and diagnostic test, primarily for diabetic nephropathy. The spot test for microalbuminuria is useful because of the simplicity of its collection, a reduced collection error compared with timed urine specimens, and a strong correlation with twenty-four hour urinary albumin excretion rates 18, 19. Age is an important predictor of albumin/creatinine ratio; the age-related decline in urine creatinine excretion results in a higher albumin/creatinine ratio in older people 20.
The findings of our study are consistent with many prior prospective studies in which microalbuminuria has been shown to be a powerful, graded, predictor of CVD 3, 4, 5, 6, 7, 8, 9, 10, 21, 22, 23. Few studies have explored whether microalbuminuria adds to the risk of CVD mortality differentially in those with or without metabolic syndrome. In the only study of which we are aware, Ko and colleagues followed 5202 Chinese men and women with diabetes, and found that the World Health Organization definition was better than the Adult Treatment Panel III for predicting cardiovascular death, a difference they attributed to the inclusion of microalbuminuria in the World Health Organization definition 24.
The significant interaction (p = 0.02) between microalbuminuria and metabolic syndrome for the prediction of CVD or CHD in women explains the disproportionately increased risk in those who have both conditions. This observation may lend support to adding microalbuminuria to traditional cardiovascular risk factors, such as those in the metabolic syndrome, for improved risk stratification, at least in women. Further, because diabetics were included in our metabolic syndrome definition, the microalbuminuria and metabolic syndrome interaction in women may be driven by diabetes (which showed the same interaction); microalbuminuria may identify those with the most severe metabolic dysfunction and thus the highest mortality rate. The improved ability of microalbuminuria to predict CVD mortality may stem from its role as a marker for the diffuse endothelial injury that is associated with impaired arterial reactivity, endothelial activation and impaired fibrinolytic capacity--critical precursors in the pathogenesis of vascular disease 25, 26. This endothelial dysfunction may confer a greater risk for CVD because it is a marker of a more advanced stage in the atherosclerotic pathway than the traditional risk factors captured by the metabolic syndrome alone.
The reasons for the sex difference in the ability of microalbuminuria to predict CHD and CVD mortality are unexplained; it was not explained by estrogen use. Tests revealed significant interactions between sex and both metabolic syndrome and microalbuminuria in the risk of both CVD and CHD death. One possible explanation for the absent association in men may be survival bias; it is likely that men at increased risk of CVD had died prior to this study visit, leaving men at lower risk in the studied cohort. Because women develop CVD at an older age than men, they would have been more likely to have survived to be in this cohort. Although the sex difference could be due to chance, Ko and colleagues also found a significant association between microalbuminuria and CVD mortality only in women (RR; 95% CI 6.10; 2.62–15.19 in women vs. 1.77; 0.91–3.44 in men) 24. It may be that some components of the metabolic syndrome (such as waist girth, hyperglycemia, and hypertriglyceridemia) convey a greater risk for CVD mortality in older women than they do in men 27, 28, 29.
We note a few limitations of the present study. Our population was homogeneous (largely Caucasian and middle class), therefore the results may not be generalizable to other populations. The average age of participants was 71 at baseline, so they were potentially at higher risk of fatal CVD than younger cohorts, although the observed mortality rates were quite low for this age group. The prevalence of the metabolic syndrome was much lower in our Caucasian cohort (20%) than that predicted for US adults of this age by the ethnically diverse third National Health and Nutrition Examination Survey (40%), which oversampled members of underrepresented minority groups 30. Women in our cohort were also leaner at baseline than those of similar age from the Health and Nutrition Examination Survey 12. Urine albumin and creatinine were measured only once using a spot urine test, increasing the possibility of misclassification 19. However, the urine albumin/creatinine ratio has been shown to be a good surrogate for a 24 hour urine albumin excretion 19, and misclassification usually results in bias towards the null, reducing the observed association.
Acknowledgments
Funding sources: Grant AG07181 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Geneva: World Health Organization; 1999. [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160:1093–100. doi: 10.1001/archinte.160.8.1093. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 5.Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristianson K, Lederballe-Pedersen O, Nieminen MS, Okin PM, Omvik P, Oparil S, Wedel H, Snapinn SM, Aurup P. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 6.Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlof B, Gerdts E, Wright JT, Jr, Papademetriou V, Mogensen CE, Borch-Johnsen K, Ibsen H, Devereux RB. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J. 2002;143:319–326. doi: 10.1067/mhj.2002.119895. [DOI] [PubMed] [Google Scholar]
- 7.Ravera M, Ratto E, Vettoretti S, Viazzi F, Leoncini G, Parodi D, Tomolillo C, Del Sette M, Maviglio N, Deferrari G, Pontremoli R. Microalbuminuria and subclinical cerebrovascular damage in essential hypertension. J Nephrol. 2002;15:519–524. [PubMed] [Google Scholar]
- 8.Bianchi S, Bigazzi R, Campese VM. Microalbuminuria in essential hypertension: significance, pathophysiology, and therapeutic implications. Am J Kidney Dis. 1999;34:973–995. doi: 10.1016/S0272-6386(99)70002-8. [DOI] [PubMed] [Google Scholar]
- 9.Yuyun MF, Khaw KT, Luben R, Welch A, Bingham S, Day NE, Wareham NJ. A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: the EPIC-Norfolk study. Am J Epidemiol. 2004;159:284–293. doi: 10.1093/aje/kwh037. [DOI] [PubMed] [Google Scholar]
- 10.Diercks GF, van Boven AJ, Hillege HL, Janssen WM, Kors JA, de Jong PE, Grobbee DE, Crijns HJ, van Gilst WH. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J. 2000;21:1922–1927. doi: 10.1053/euhj.2000.2248. [DOI] [PubMed] [Google Scholar]
- 11.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108:367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 12.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–12. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Rose GABH, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. 2. Geneva: World Health Organization; 1982. pp. 162–165. [Google Scholar]
- 15.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 16.NIH UDoH, Education and Welfare. Lipid Research Clinics Program Manual of Laboratory Operations. US Govt Printing Office; 1974. pp. 75–628. [Google Scholar]
- 17.Friedewald WTLR, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1976;18:499–502. [PubMed] [Google Scholar]
- 18.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 19.Dyer AR, Greenland P, Elliott P, Daviglus ML, Claeys G, Kesteloot H, Ueshima H, Stamler J. Evaluation of measures of urinary albumin excretion in epidemiologic studies. Am J Epidemiol. 2004;160:1122–1131. doi: 10.1093/aje/kwh326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houlihan CA, Tsalamandris C, Akdeniz A, Jerums G. Albumin to creatinine ratio: a screening test with limitations. Am J Kidney Dis. 2002;39:1183–1189. doi: 10.1053/ajkd.2002.33388. [DOI] [PubMed] [Google Scholar]
- 21.Weiner DE, Sarnak MJ. Microalbuminuria: a marker of cardiovascular risk. Am J Kidney Dis. 2003;42:596–598. doi: 10.1016/s0272-6386(03)00825-4. [DOI] [PubMed] [Google Scholar]
- 22.Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: An angiographic study. Am J Kidney Dis. 1999;34:918–925. doi: 10.1016/S0272-6386(99)70051-X. [DOI] [PubMed] [Google Scholar]
- 23.Zandbergen AA, Sijbrands EJ, Lamberts SW, Bootsma AH. Normotensive women with type 2 diabetes and microalbuminuria are at high risk for macrovascular disease. Diabetes Care. 2006;29:1851–1855. doi: 10.2337/dc06-0287. [DOI] [PubMed] [Google Scholar]
- 24.Ko GT, So WY, Chan NN, Chan WB, Tong PC, Li J, Yeung V, Chow CC, Ozaki R, Ma RC, Cockram CS, Chan JC. Prediction of cardiovascular and total mortality in Chinese type 2 diabetic patients by the WHO definition for the metabolic syndrome. Diabetes Obes Metab. 2006;8:94–104. doi: 10.1111/j.1463-1326.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 25.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 26.Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet. 1989;1:461–463. doi: 10.1016/s0140-6736(89)91365-2. [DOI] [PubMed] [Google Scholar]
- 27.Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3:131–158. doi: 10.1016/s1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- 28.Ulmer H, Kelleher C, Diem G, Concin H. Why Eve is not Adam: prospective follow-up in 149650 women and men of cholesterol and other risk factors related to cardiovascular and all-cause mortality. J Womens Health (Larchmt) 2004;13:41–53. doi: 10.1089/154099904322836447. [DOI] [PubMed] [Google Scholar]
- 29.Tanko LB, Bagger YZ, Qin G, Alexandersen P, Larsen PJ, Christiansen C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111:1883–1890. doi: 10.1161/01.CIR.0000161801.65408.8D. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]