Abstract
Rapid, progestin actions initiated at the cell surface that are often nongenomic have been described in a variety of reproductive tissues, but until recently the identities of the membrane receptors mediating these nonclassical progestins actions remained unclear. Evidence has been obtained in the last 4-5 years for the involvement of two types of novel membrane proteins unrelated to nuclear steroid receptors, progesterone membrane receptors (mPRs) and progesterone receptor membrane component one (PGMRC1 [also known as progesterone receptor membrane component 1, PGRMC1]), in progestin signaling in several vertebrate reproductive tissues and in the brain. The mPRs, (MW ∼40 kDa) initially discovered in fish ovaries, comprise at least three subtypes, α, β and γ and belong to the seven-transmembrane progesterone adiponectin Q receptor (PAQR) family. Both recombinant and wild type mPRs display high affinity (Kd ∼5 nM), limited capacity, displaceable and specific progesterone binding. The mPRs are directly coupled to G proteins and typically activate pertussis-sensitive inhibitory G proteins (Gi), to down regulate adenylyl cyclase activity. Recent studies suggest the alpha subtype (mPRα) has important physiological functions in variety of reproductive tissues. The mPRα is an intermediary in progestin induction of oocyte maturation and stimulation of sperm hypermotility in fish. In mammals, the mPRαs have been implicated in progesterone regulation of uterine function in humans and GnRH secretion in rodents. The single-transmembrane protein PGMRC1 (MW 26 ∼28 kDa) was first purified from porcine livers and its cDNA was subsequently cloned from porcine smooth muscle cells and a variety of other tissues by different investigators. PGMRC1 and the closely related PGMRC2 belong to the membrane-associated progesterone receptor (MAPR) family. The PGMRC1 protein displays moderately high binding affinity for progesterone which is 2-10-fold greater than that for testosterone and glucocorticoids, and also can bind to other molecules such as heme, cholesterol metabolites and proteins. The signal transduction pathways induced by binding of progesterone to PGMRC1 have not been described to date, although motifs for tyrosine kinase, kinase binding, SH2 and SH3 have been predicted from the amino acid sequence. Evidence has been obtained that PGMRC1 mediates the antiapoptotic affects of progesterone in rat granulosa cells. The PGMRC1 protein may also be an intermediary in the progesterone induction of the acrosome reaction in mammalian sperm. Despite these recent advances, many aspects of progestin signaling through these two families of novel membrane proteins remain unresolved. Biochemical characterization of the receptors has been hampered by rapid degradation of the partially purified proteins. A major technical challenge has been to express sufficient amounts of the recombinant receptors on the plasma membranes in eukaryotic systems to permit investigations of their progestin binding and signal transduction characteristics. Additional basic information on the molecular and cellular mechanisms by which mPRs and PGMRC1 interact with progestins, signal transductions pathways and other proteins will be required to establish a comprehensive model of nontraditional progestin actions mediated through these novel proteins.
Introduction
Many effects of steroids cannot be readily explained by the classic genomic mechanism of steroid action involving new mRNA and protein synthesis which is relatively slow, typically occurring over a time scale of hours to days. Steroids have been shown to initiate rapid actions through activation of intracellular signaling pathways, resulting in alterations in ion fluxes and intracellular free calcium concentrations occurring within seconds [6], and of other second messengers such as cyclic nucleotides and extracellular-regulated kinase 1 and 2 (erk 1/2), within a few minutes [30,150]. The majority of studies have shown that these rapid steroid actions are initiated at or near the cell surface and evidence has accumulated that they are mediated by binding to specific receptors in the plasma membranes of the target cells [28, 75,102,103,118,142]. However, steroids readily diffuse into cells where they can also activate intracellular steroid receptors to rapidly initiate signal transduction pathways [10,111]. The biological response mediated through steroid membrane receptors can be rapid, such as the stimulation of sperm hypermotility within 1-5 minutes of progestin treatment, or can occur over a prolonged period of 6-18hrs in the case of oocyte maturation in fish and amphibians after treatment with the same steroids [118,121]. Progestins induction of oocyte maturation through progestin membrane receptors is via a nongenomic mechanism since it is not blocked by inhibitors of transcription and translation [20,128]. However, progestin binding to progestin membrane receptors in oocytes also activates MAPkinases [82] which is likely to ultimately result in alterations in gene transcription. In addition, progestin membrane receptor-mediated pathways can also regulate transactivation of the nuclear progesterone receptor resulting in alteration in gene transcription [41]. Thus the only characteristic common to all these nonclassical steroid actions is rapid activation of intracellular signaling pathways.
The receptors mediating these rapid steroid actions have been studied extensively in many laboratories over the past 20 years. For example binding moieties with the characteristics of progestin membrane receptors have been demonstrated in fish and amphibian oocytes [55, 84] in bovine ovaries [97,98]), in rat Leydig cells [106] in human and fish sperm membranes [6, 53, 26, 120,127], in pig liver microsomes [63] and for progesterone and progesterone metabolites in breast cancer cells [23, 144]. However, the identities of the receptors mediating many nonclassical actions of progestins and other steroids, their precise locations in the cell and their mechanisms of action remain unclear and are surrounded by controversy. A variety of receptor proteins have been proposed as intermediaries in nonclassical steroid actions in various cell models. Candidates for these receptors include nuclear steroid receptors or steroid nuclear receptor-like forms that are membrane–bound [101, 143] or can be activated intracellularly [66], receptors for other ligands that also bind steroids [70,72, 105], novel receptor proteins unrelated to any previously described receptors [104,123], and receptors with different characteristics from those of any known receptors [49,63]. For example, a diverse range of receptor proteins have been implicated in nonclassical progestin actions in different vertebrate and cell models, including nuclear receptors that can activate intracellular signaling cascades [3, 10,111, 130] GABAA and oxytocin receptors that can bind progesterone [32, 34] and two novel receptor families which are unrelated to any previously described receptor proteins, membrane progestin receptors (mPRs) and progesterone membrane receptor component 1 [27,150].
The identification of the genes encoding mPRs and PGMRC1 and a knowledge of their protein structures has generated broad interest amongst researchers resulting in recent advances in our understanding of their likely physiological functions. The characteristics of these two novel putative progestin membrane receptors and their roles in mediating rapid progestin actions in a variety of target tissues and cells are reviewed in this paper. Evidence that mPR alpha (mPRα) and PGMRC1 function as membrane progestin receptors is critically evaluated. In addition, methodological problems in progestin membrane receptor measurement and expression of recombinant receptors in plasma membranes are discussed. The involvement of nuclear progesterone receptors in mediating rapid progestin actions involving activation in second messengers will not be discussed because there is an excellent recent review on this topic [9].
Membrane progestin receptor alpha-mPRα
1. Progestin membrane receptors in fish and amphibian oocytes
Meiotic maturation of fish and amphibian oocytes is one the most extensively studied and well-characterized rapid, nonclassical progestin actions mediated by activation of membrane bound steroid receptors at both the biochemical and cytological levels [55, 75, 146]. The presence of progesterone binding sites on the cell surface of amphibian oocytes was demonstrated in the early 1980s, and specific progestin binding to the membrane receptors in Xenopus and Rana oocytes has been well characterized [8, 45, 50, 71, 109]. The Xenopus membrane receptor displays high affinity progesterone binding (Kd 10-9M) [50], whereas lower progesterone binding affinity has been reported for the Rana receptor (Kd 10-7 M) [45]. The nuclear progestin receptor agonist, R5020, also binds to the Xenopus membrane receptor, but with much lower affinity than progesterone (10-5-10-6M) [8, 109], which suggests that these receptors are unrelated to nuclear receptors [50]. Association of [H-3]-progesterone binding to Xenopus plasma membranes is rapid, similar to that of other steroid membrane receptors, reaching equilibrium within 15 min [50]. Finally, oocyte receptor abundance changes during the ovarian cycle. Receptor concentrations in small oocytes (stage IV) incapable of undergoing maturation in response to progesterone are about one tenth of those in full grown stage V/VI oocytes which are responsive to the steroid [50]. Moreover, incubation of ovarian fragments with a concentration of gonadotropin that promotes oocyte maturation causes receptor concentrations to increase several-fold to reach peak levels at the beginning of oocyte maturation [50]. These changes in the receptor abundance on Xenopus oocytes are consistent with a role for the receptor as the intermediary in progesterone induction of oocyte maturation in this species.
Fish membrane progestin receptors were first characterized extensively in spotted seatrout ovaries [84]. Spotted seatrout ovarian membranes exhibit a single class of high affinity (Kd 5 nM), low capacity, saturable and displaceable binding sites for 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S), the progestin hormone in this species that initiates oocyte maturation (maturation inducing steroid, MIS). The binding is specific for progestins and 20β-S, which has hydroxyls at the 17, 20 and 21 positions, has the highest binding affinity for the receptor. The relative binding affinities of progesterone and the other major teleost progestin hormone that stimulates oocyte maturation, 17, 20β-dihydroxy-4-pregnen-3-one (17,20βDHP), are 6% and 0.6%, respectively, of the affinity of 20β-S [121]. There is a close correlation between the relative binding affinities of various progestins and 11-deoxycorticosteroids for the spotted seatrout progestin membrane receptor and their agonist or antagonist activities in an in vitro oocyte maturation bioassay, suggesting the receptor is the likely intermediary in 20β-S induction of oocyte maturation in this species [121]. The rates of association and dissociation of 20β-S binding to ovarian membranes are rapid (t 1/2 2-5 min). The binding kinetics and steroid specificity of the membrane progestin receptor in spotted seatrout differs markedly from those of the nuclear progestin receptor that controls ovulation in this species [96], suggesting that the membrane receptor is a novel protein unrelated to nuclear receptors. Membrane progestin receptors have also been identified in ovaries and oocytes of striped bass, rainbow trout, arctic char, yellow tail and goldfish [5, 44, 99, 132, 149]. The membrane progestin receptors of rainbow trout, arctic char and goldfish show higher binding affinities for 17,20βDHP than for 20β-S, which is consistent with its identity as the likely MIS in these species. Similar to the observations in amphibians, membrane progestin receptor concentrations increase in fish ovaries during oocyte maturation. In spotted seatrout, ovarian receptor concentrations increase two- to three-fold in vivo during oocyte maturation in fish collected on their spawning grounds which is accompanied by elevated plasma levels of LH and 20β-S and also in captive fish induced to undergo oocyte maturation by LHRHa injection [125]. Tissue culture experiments show that receptor concentrations are also upregulated more than three-fold in vitro in ovarian fragments containing fully grown seatrout ovarian follicles incubated with gonadotropin which is associated with the development of oocyte maturation competence [85,125]. Progestin membrane receptor concentrations are also increased in rainbow trout, arctic char and yellow tail ovaries after gonadotropin treatment [5, 99,149]. This increase in oocyte receptor concentrations is physiologically important because it is associated with the oocytes becoming responsive to the MIS (oocyte maturational competence) and being able to complete oocyte maturation.
2. Cloning, identification and characterization of spotted seatrout membrane progestin receptor alpha (mPRα)
Until recently, despite intensive research in several laboratories, efforts to characterize MIS progestin receptors in lower vertebrates at the molecular level had been unsuccessful [56]. Several features of the seatrout receptor made it an attractive candidate for membrane receptor structure identification. The results from the binding studies suggested that the receptor protein is unrelated to the nuclear progesterone receptor and may be the product of a novel gene. In addition, the seatrout ovary is an abundant source of material for protein purification, since it comprises as much as 15% of the body weight and can contain up to half a million ovarian follicles with high levels of receptor expression undergoing oocyte maturation. Finally, extensive information was available of the biochemical characteristics of the receptor, its fluctuations during the reproductive cycle and its hormonal regulation. Most previous attempts to obtain highly purified preparations of steroid membrane receptors for amino acid sequencing using classical protein purification approaches had been unsuccessful due to instability of the purified receptors. Therefore, a combination of partial receptor purification, antibody generation and expression library screening was used to identify likely cDNA candidates for the membrane progestin receptor [128]. Solubilization procedures that extracted most of the receptor from membranes but did not interfere with the radioreceptor assay were developed to track the receptor protein through the subsequent purification steps. The solubilized receptor preparation was fractionated on a DEAE anion exchange column and eluted with a step-wise NaCl gradient (Fig.1). Most of the [3 H]20βS binding activity eluted in the first fraction (fraction I) which contained only a single major protein band on SDS-PAGE with a molecular weight of ∼40kDa and only minor amounts of other proteins, whereas fractions II and III contained many membrane proteins (Fig.1). Practically all of the receptor binding activity in fraction I was lost upon further fractionation by column chromatography. Therefore, DEAE fraction I was used to immunize mice, spleenocyte hybridomas were established, and the monoclonal antibodies secreted into the culture medium by the hybridomas were subsequently screened for their ability to detect the membrane progestin receptor in a novel double-antibody receptor-capture assay [128]. One positive antibody, PR10-1, which detected a single 40kDa band in DEAE fraction I, was used to screen a spotted seatrout cDNA expression library prepared from ovaries containing fully grown follicles primed with gonadotropin to enhance progestin membrane receptor expression. A positive clone, a 1.4 Kb fragment novel, was detected and completely sequenced and appeared to be a novel cDNA, unrelated to any previously described gene, which encoded a protein of ∼40kDa with 352 amino acids [150]. Subsequent comprehensive studies revealed that the characteristics of this novel gene and its protein are consistent with its identity as the membrane progestin receptor mediating meiotic maturation of spotted seatrout oocytes.
Figure 1.
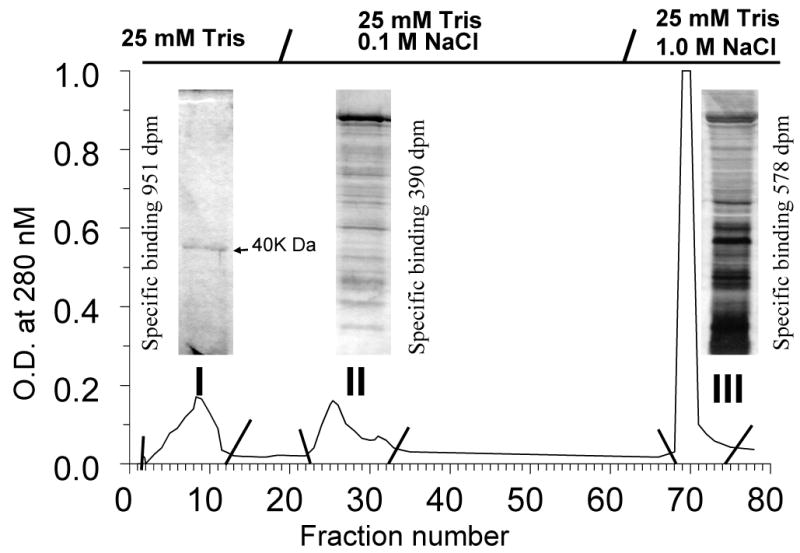
DEAE chromatography of solubilized seatrout ovarian membranes with step wise elution of protein with NaCl. Three fractions (I,II, III) were collected and PAGE was performed on aliquots from each fraction and silver stained. Reproduced from Zhu et al. [150], with permission.
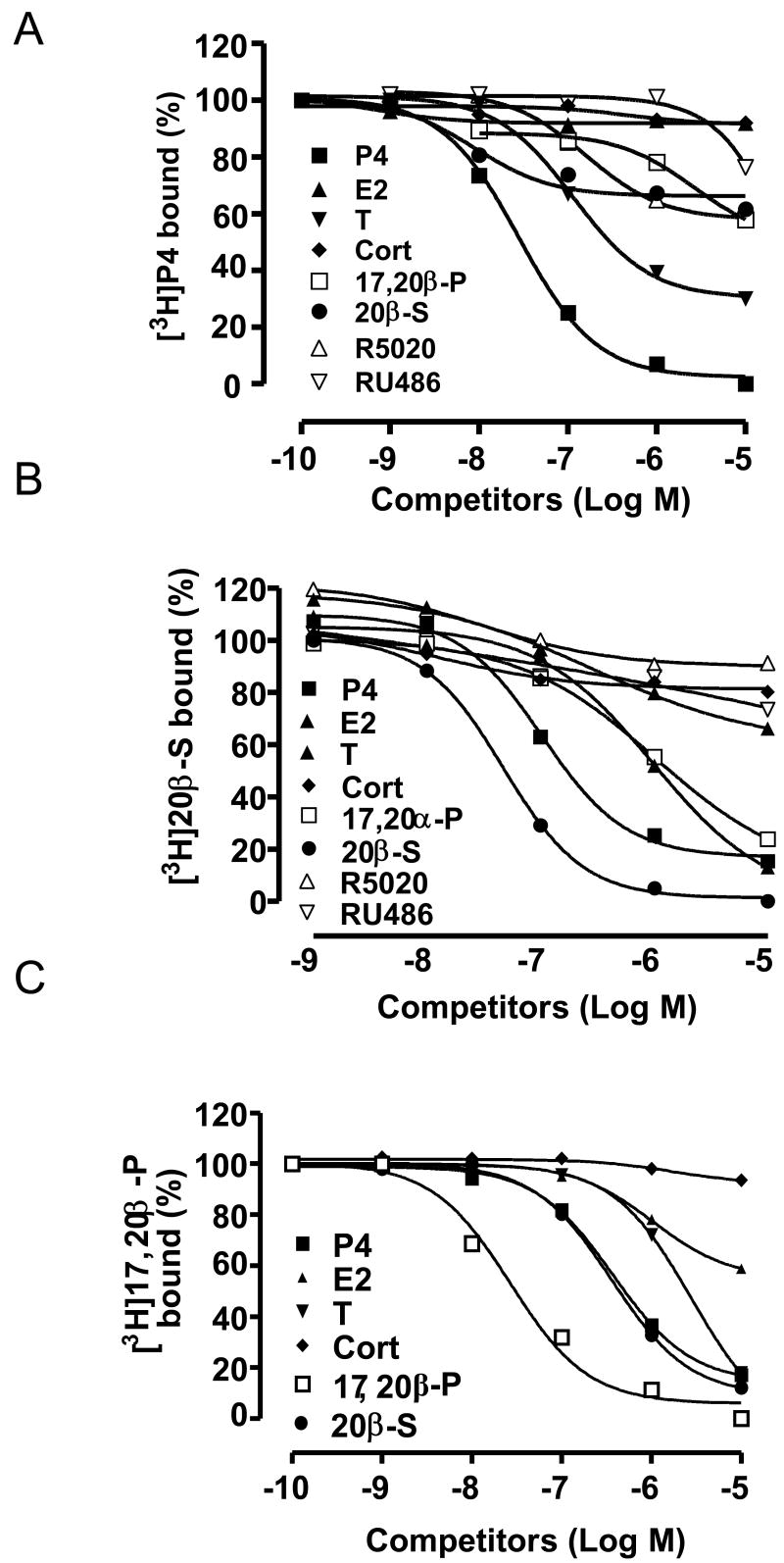
Seven criteria were satisfied for designation of the novel seatrout gene and its protein as a membrane progestin receptor [150]. Plausible structure: hydrophilicity and trans-membrane analysis of the deduced amino acid sequence predicts the protein has seven transmembrane domains, which is characteristic of the largest class of membrane receptors, G protein coupled receptors. Tissue specificity: Northern blot analysis showed that the mRNA of the novel seatrout receptor (mPRα) mRNA (4.0kb) was abundant in the ovaries, testes, pituitary and brain, consistent with the known regulatory roles of progestins in reproductive and endocrine functions. Subcellular localization: Immunocytochemistry of ovarian follicles and Western blot analysis of seatrout tissues using a polyclonal antibody directed against a peptide fragment in the N-terminal region of seatrout mPRα showed that the 40kDa protein is localized peripherally in the region of the oocyte plasma membrane, with practically no staining intracellularly and lower expression in the follicle cells surrounding the oocytes. Strong immunoreactive bands (∼40kDa and 80kDa) have also detected using the seatrout mPRα antibody in Western blots of seatrout sperm plasma membranes, where a progestin membrane receptor has previously been characterized using radioreceptor assay techniques [120]. This localization of the seatrout mPRα on the plasma membranes of oocytes and sperm is consistent with previous biochemical binding and functional studies demonstrating the presence of membrane progestin receptors on these germ cells mediating progestin induction of oocyte maturation and sperm hypermotility, respectively. Steroid binding: Evidence was obtained that the recombinant seatrout mPRα protein can bind progestins with the characteristics of a hormone receptor. High affinity, low capacity, displaceable and specific binding for progestins typical of steroid membrane receptors was initially demonstrated with the recombinant protein produced in a bacterial (E.coli) expression system [150], and subsequently confirmed in membranes prepared from human breast cancer cells (MDA-MB-231 cells, nuclear progesterone receptor negative) transfected with the seatrout cDNA [122]. Recombinant seatrout and human mPRαs produced in this eukaryotic expression system display high affinity (Kd 4-8 nM), limited capacity (Bmax 0.03-0.3 nM), displaceable progestin binding, with rapid rates of association and dissociation (T1/2: 2-5 min.) of [3 H]progestin binding to the receptor proteins [122]. Competition studies with a variety of natural and synthetic steroids demonstrate that binding to seatrout, human and zebrafish mPRαs is specific for progestins, androgens display moderate affinity for the receptors, whereas cortisol and estradiol-17β do not bind to the receptors, even at high concentrations (Fig.2A-C). The steroid specificity of the mPRαs differs from that of the nuclear progesterone receptor because the nuclear progesterone agonist and antagonist, R5020 and RU486, show negligible binding to the seatrout and human mPRαs (Fig.2A, 2B). Importantly, the human, seatrout and zebrafish mPRαs show the highest binding affinities for the physiologically important progestin hormone in each species, progesterone in humans, 20β-S in seatrout and 17,20βDHP in zebrafish. The binding of the other vertebrate progestin hormones was at least ten-fold lower than that of the endogenous progestin hormone for each of these receptors in transfected MDA-MB-231 cells (Fig.2). The finding that these three recombinant mPRαs produced in the same expression system have different progestin specificities suggests that specific progestin binding is an intrinsic property of these proteins. The demonstration that recombinant mPR proteins produced in both eukaryotic and prokaryotic systems display similar high affinity, specific progestin binding is also consistent with this hypothesis. Different research groups have shown that recombinant zebrafish mPRβ, Xenopus mPRβ, goldfish mPRα and ovine mPRα also have the binding characteristics of progestin membrane receptors [1, 4, 37, 132]. Signal transduction: Physiological concentrations of progestins, but not estrogens and androgens, cause rapid activation of signal transduction pathways in the transfected MDA-MB-231 cells. Treatment with 20β-S causes a decrease cAMP levels which is reversed by pretreatment with activated pertussis toxin (PTX), but not after pretreatment with inactive PTX, suggesting seatrout mPRα activates a PTX-sensitive inhibitory G protein [150] which has been confirmed in a subsequent study [122], consistent with its identity as the MIS receptor. Similarly, evidence has been obtained that progesterone treatment down-regulates adenylyl cyclase activity through an inhibitory G protein via both recombinant and wild type human mPRα and mPRβ in several cell models [21, 41, 122] Hormonal regulation: The upregulation of the seatrout mPRα protein observed in response to hormonal treatment further suggests an involvement of the receptor in oocyte maturation. Induction of oocyte maturation in seatrout ovarian fragments incubated with gonadotropin was accompanied by an increase in seatrout mPRα protein expression, in agreement with the earlier radioreceptor studies showing membrane progestin receptor concentrations are increased by this treatment and are associated with the development of oocyte maturational competence [125]. Biological relevance: The pattern of seatrout mPRα expression during the reproductive cycle also suggests it is the MIS receptor. The concentrations of seatrout mPRα are elevated in fully grown oocytes and show a further increase during oocyte maturation in fish captured on their spawning grounds [150], similar to the pattern of changes in 20βS receptor binding activity during oocyte maturation described previously [125]. Spotted seatrout oocytes are relatively small and fragile and do not survive for extended period (16-18hrs) after microinjection. Consequently, experiments to examine the requirement for seatrout mPRα in oocyte maturation by microinjection of antisense oligonucleotides could not be conducted. Instead, antisense oligonucleotides to the homologous mPRα identified in zebrafish, were injected into zebrafish oocytes which are larger and can survive for an extended period after microinjection. Injection of both phosphothioate and morpholino antisense oligonucleotides, but not sense or mis-sense ones, during gonadotropin induction of priming blocked the subsequent response of zebrafish oocytes to the zebrafish MIS, 17,20βDHP, most of them failing to mature [150]. The involvement of mPRα in oocyte maturation has also been demonstrated goldfish oocytes using the same experimental approach [133]. It is concluded from all these studies that mPRα has all the characteristics of a membrane progestin receptor and is the MIS receptor in spotted seatrout.
Figure 2.
Competition curves of steroid binding to plasma membranes of MDA-MB-231 (PR-) cells stably transfected with (A) human mPRα, (B) seatrout mPRα, (C) zebrafish mPRα, expressed as a percentage of maximum [3 H]progestin binding (A: [3 H]P4; B:[3 H]20β-S; C: [3 H]17α,20β-P). P4, progesterone; 20β-S, 17,20β,21-trihydroxy-4-pregnen-3-one; 17α,20β-P, 17,20β-dihydroxy-4-pregnen-3-one; RU486, mifepristone; R5020, promegestone; cort, cortisol; E2, estradiol-17β; T, testosterone. (A and B reproduced from Thomas et al., 2007 [122], C reproduced from Hanna et al., 2005 [37] with permission).
3. Identification and characteristics of mPRs in other species
Soon after the discovery of the seatrout mPRα cDNA, thirteen closely-related genes were identified in other fish, amphibian and mammalian species [151]. The mPRs have been classified into three subtypes, α, β, and γ, all of which are present in both fish and mammals, including humans [151] The subcellular localization, progestin binding, G protein coupling and signaling characteristics of recombinant human mPRα produced in an eukaryotic expression system are very similar to those of seatrout mPRα [122]. Recombinant zebrafish, goldfish and ovine mPRαs expressed in mammalian cell lines have also been shown to have the binding characteristics of membrane progestin receptors [1, 37,132]. Equivalent information on the steroid binding and signaling characteristics of the β and γ subtypes in most of these species is lacking, although the limited data currently available for human, zebrafish and Xenopus mPRβs suggest they are similar to those of the mPRαs [4, 37,41]. However, the finding that the α, β and γ subtypes have different tissue distributions and expression patterns throughout the reproductive cycle in a variety of animal models [15, 29, 42, 79] suggests they may have different physiological functions to those described in the following section for mPRα. The mPRs belong to the seven-transmembrane progesterone adiponectin Q receptor (PAQR) family which has a different origin than that of the GPCR superfamily [115, 122]. Phylogenetic and sequence analyses show that another type of PAQR, PAQR 6, is closely related to the mPRs and may be an additional member of this progestin receptor subfamily [122]. Another PAQR type, PAQR 9, may also be a member of the mPR subfamily because it is clustered with the mPRs [122].
4. Tissue distribution of mPRα
The broad distribution of mPRα mRNAs in reproductive and non-reproductive tissues suggests they have diverse physiological functions in vertebrates. In the initial publication mPRα mRNA was only identified in spotted seatrout ovaries, testes, brain and pituitary by Northern blot analysis [150]. Similarly, mPRα expression was found to be restricted to the gonads in zebrafish [42]. However, it was shown in channel catfish with a more sensitive real-time PCR procedure that in addition to high expression in these tissues, mPRα transcripts were also present on all the nonreproductive tissues examined, with relatively high expression throughout the brain and in the muscle and heart, and low levels of expression in gill, intestine, liver, kidney, interrenal, muscle and spleen [42]. Similarly human mPRα mRNA was initially identified primarily in reproductive tissues, particularly in the ovary, testis, placenta and uterus, and also in the kidney and adrenal by dot blot hybridization [150]. Recent studies have also identified mPRα in human myometrium [15, 29, 41], amnion, chorion and endometrium [29], breast tissues [23], and lymphocytes [21] by RT-PCR. Expression of mPRα mRNA has also been demonstrated in the sheep hypothalamus, pituitary, ovary and corpus luteum [1], the corpus luteum of rats [13] and in the mouse brain, testis and uterus [119]. Moreover, changes have been observed in the expression of mPRα mRNA in many of these tissues during the reproductive cycle and in malignant human breast tissues, which is consistent with important physiological roles for the receptor in a wide variety of reproductive functions as well as its potential involvement in tumorigenesis. However, to date only a few of these possible functions of mPRα have been investigated and are discussed below.
Functions of mPRα
1. Progestin induction of oocyte maturation in fish and amphibian oocytes
Fully grown oocytes of fish and other vertebrates cannot be fertilized because the first meiotic division is incomplete and is arrested at the G2/M border of prophase 1. Meiosis is arrested by high intra-oocyte levels of cyclic AMP which are maintained by adenylyl cyclases [19, 36]. A surge in gonadotropin secretion induces the resumption of meiosis in fish and amphibian oocytes through an intermediary, a MIS, which is secreted from the ovarian follicle cells in response to gonadotropin stimulation [59, 75, 146]. The MIS in turn binds to a specific receptor on the oocyte plasma membrane to initiate a process called oocyte maturation via a nongenomic mechanism, resulting in a fully mature oocyte capable of being ovulated and fertilized [75, 117]. As mentioned previously, the hormonal control of oocyte maturation in these two vertebrate groups has been one of the most thoroughly investigated models of nongenomic steroid actions, since it was first described more than twenty years ago. The MISs in fishes have been positively identified as novel progestin hormones with multiple hydroxyls on the sidechain, 17,20βDHP in salmonids and cyprinid fishes such as goldfish and zebrafish [74], and 20β-S in advanced marine perciform fishes such as Atlantic croaker and spotted seatrout [126,134]. In contrast, considerable controversy surrounds the identity of the MIS in amphibians such as Xenopus, because in addition to progesterone, androgens are also potent inducers of oocyte maturation in this species [54]. Several lines of evidence indicate that MIS induction of oocyte maturation in fish is via a nonclassical steroid mechanism because it is rapid, nongenomic and initiated at the cell surface by binding to progestin membrane receptors [20, 73, 121] which have been characterized in several fish species (see earlier section). The MIS initiates oocyte maturation in fish by causing a decline in intracellular cAMP levels [35, 39], which is associated with activation of a PTX-sensitive inhibitory G protein pathway [128, 148]. Direct evidence was obtained in spotted seatrout that MIS binding to the progestin membrane receptor causes activation of a PTX-sensitive inhibitory G protein pathway which is necessary for the completion of oocyte maturation [83]. Cyclic AMP production in seatrout oocyte membranes, an indirect measure of adenylyl cyclase activity, was specifically down-regulated by 20β-S, whereas cortisol and testosterone were ineffective, and this action of 20β-S was blocked by PTX. The finding that 20β-S induction of oocyte maturation was blocked by microinjection of PTX into the oocytes is also consistent with 20β-S activation of a Gi protein. Confirmation that 20β-S activates a G protein was shown by increased binding of [35S]GTPγS to ovarian membranes after treatment with the steroid, and a follow up immunoprecipitation experiment demonstrated that the increased [35S]GTPγS binding was associated with the alpha subunits of inhibitory G (Gi 1-3) proteins. Uncoupling of G proteins from GPCRs with excess guanine nucleotides such as GTPγS and inhibitory G proteins with PTX decreases ligand affinity of the receptors [16,137]. Binding of [3 H]20β-S to seatrout ovarian membranes was decreased after treatment with either GTPγS or PTX [83], suggesting that the seatrout membrane progestin receptor is directly coupled to an inhibitory G-protein. On the basis of these results it was proposed that 20β-S activation of an inhibitory G protein results in down regulation of adenylyl cyclase activity in spotted seatrout oocytes through the α subunit of the G protein leading to a reduction in protein kinase A (PKA) activation [83]. In Atlantic croaker, a marine fish belonging to the same family as spotted seatrout, 20β-S induction of oocyte maturation is also associated with a decrease in cAMP levels [82] However, the cell permeable PKA inhibitors were ineffective in inducing or enhancing 20β-S-induced oocyte maturation in this species [82] On the other hand inhibitors of phosphodiesterase activity partially blocked 20β-S induction of oocyte maturation in this species and inhibitors of the phosphatidylinositol 3-kinase (PI3K)/Akt signal transduction pathway completely blocked the response to 20β-S [82]. Pace and Thomas proposed that the βγ subunits of the heterotrimeric G protein recruit PI3Kγ to the plasma membrane to catalyze the formation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), which in turn binds to serine/threonine kinase Akt resulting in its activation and subsequent alteration of phosphodiesterases (PDEs), which breakdown cAMP [82]. Therefore, binding of the MIS, 20β-S to its receptor and activation of an inhibitory G protein could result in a decline in intraoocyte cAMP levels through multiple signaling pathways to release the oocytes from meiotic arrest and complete maturation.
Considerable evidence has been obtained in the past few years which strongly suggest that mPRα is the primary progestin membrane receptor mediating MIS induction of oocyte maturation in fishes. The initial study on mPRα expression in seatrout showing the protein is localized mainly on the oocyte plasma membrane and increases in abundance during the reproductive cycle and during natural and gonadotropin-induced oocyte maturation is consistent with its involvement in this process [150] The signal transduction pathway activated by recombinant seatrout mPRα is identical to that described in seatrout oocytes, because it has been shown that the recombinant seatrout mPRα protein activates an inhibitory G protein and is directly coupled to it, resulting in down-regulation of cAMP production (adenylyl cyclase activity) via a PTX-sensitive pathway [150, 122]. Moreover, a co-immunoprecipitation experiment confirms that the mPRα protein on seatrout plasma membranes is coupled to an inhibitory G protein (Fig. 3). More direct evidence for a role for mPRα in oocyte maturation in the original study was obtained in zebrafish, in which microinjection of antisense oligonucleotides into oocytes during the initial gonadotropin priming period blocked the completion of oocyte maturation in response to the MIS, 17,20βDHP [150]. An important role for mPRα in oocyte maturation was subsequently confirmed in another fish model, goldfish, using the same experimental approach [133]. Microinjection experiments into zebrafish oocytes with antisense oligonucleotides to mPRβ suggest it is also involved in oocyte maturation in this species [124]. Finally, a close correlation between the binding affinity of a wide range of steroids to recombinant mPRs and their ability to induce oocyte maturation has been observed. For example, an unusual ligand for goldfish mPRα, diethylstilbestrol, was shown to be effective in inducing oocyte maturation in this species, and the relative binding affinities of diethylstilbestrol analogs for the receptor correlated well with their oocyte maturation inducing activities in an in vitro bioassay [132]. Taken together, these studies on the role of mPRα in oocyte maturation in fish provide the most compelling evidence obtained to date for an important function for mPRs in the hormonal control of a physiological process.
Figure 3.

Co-immunoprecipitation of mPRα coupled to G protein α subunits in spotted seatrout oocyte membranes with specific inhibitory G protein (Gi 1,2, Go) antibodies followed by immunodetection of seatrout mPRα by Western blot analysis. Membranes (10 μg) were incubated for 45 min. with vehicle or 290 nM 20 β-S, solubilized and then incubated overnight with 5 μl of Gαi1,2 or Gαo antibody or nonimmune rabbit serum (niR) and 50 μl of Protein A/G Plus-agarose beads. Immunoprecipitated seatrout mPRα protein was detected using the monoclonal PR10-1 antibody described in Zhu et al. (2003). Representative immunoblot is shown. There was a strong and specific association of Gαi1,2 with seatrout mPRα, whereas the mPRα protein was not detected in the Gαo or nonimmune rabbit serum immunoprecipitated samples. The amount of seatrout mPRα associated with Gαi1,2 was decreased after treatment with 20β-S (reproduced from Pace, 2005 [81] with permission).
Interestingly, attempts to clone the mPRα from Xenopus laevis cDNA libraries have been unsuccessful which suggests this mPR subtype may absent in this species [4, 150]. A recent study suggests the β homolog, which is expressed on the plasma membrane of Xenopus oocytes and shows specific progesterone binding when expressed in mammalian cells, has a similar role in the progestin induction of oocyte maturation in this amphibian model as that demonstrated for mPRα in teleosts [4]. Microinjection of a specific mPRβ antibody into Xenopus oocytes inhibited progesterone-induced oocytes maturation, whereas oocyte maturation was accelerated after microinjection Xenopus mPRβ mRNA [4].
2. Progestin stimulation of sperm hypermotility in fish and humans
Nongenomic steroid actions can be examined alone in vertebrate sperm because they are considered to be transcriptionally inactive. Rapid actions of progesterone to induce both the acrosome reaction and stimulate hypermotility in mammalian sperm have been demonstrated in several laboratories and a variety of receptors have been proposed as potential mediators of these multiple progesterone effects [6, 11, 103, 108, 138]. The mechanism of progestin stimulation of sperm hypermotility can be studied in isolation in fish sperm without the complication of progestins activating pathways involved with the acrosome reaction which does not occur in fish. Rapid induction of sperm hypermotility by progestin hormones and the presence of specific progestin receptors on their sperm membranes have also been demonstrated in several fish species. The fish progestin, 20β-S, causes a concentration-dependent (10 to 200nM) increase in the motility of croaker sperm after a 5-minute incubation [124] which is associated rapid increases in intrasperm free calcium and cAMP concentrations [118]. High-affinity (Kd ∼ 20 nM), low capacity (Bmax ∼0.08 nM) single binding sites specific for 20β-S have been demonstrated on croaker and spotted seatrout sperm membranes with binding characteristics similar to that of recombinant seatrout mPRα [120,122,127]. Moreover, mPRα proteins have been identified on the sperm membranes of these two species and localized to their midpieces, consistent with an involvement of the receptor in sperm motility [127,136,150]. Recent studies on both species demonstrating that sperm with low motility show decreased expression of mPRα protein in their sperm membranes further support a physiological role for mPRα in progestin–induced sperm hypermotility [135]. In agreement with the results of experiments in other animal models and cell types expressing mPRα, progestin treatment causes G protein activation in sperm membranes and the receptor co-immunoprecipitates with a G protein, suggesting mPRα functions as a GPCR in sperm [135]. However, in contrast to these other studies, progestins increase cAMP production in croaker sperm membranes, suggesting activation of a stimulatory G protein. This signaling pathway is critical for progestin induction of sperm hypermotility because it is completely blocked by pretreatment with two specific inhibitors of membrane adenylyl cyclases [135]. Immunoprecipitation studies with specific G protein α subunit antibodies showed an olfactory G protein (Golf) was specifically activated by progestins, and immunocytochemical analysis localized Golf on the midpiece, the same region of sperm where mPRα is expressed. Finally, it was demonstrated that mPRα is co-immunoprecipitated with the Golf antibody, suggesting the receptor is coupled to this G protein. It is concluded from these studies that mPRs can couple to a variety G proteins in different tissues.
Currently, only limited information is available on the physiological and signaling functions of mPRα in mammalian sperm. The mRNA for mPR is present in the human and mouse testis [151, 119] and the protein has been detected on human sperm membranes [unpubl. obs.]. Progesterone effects on the acrosome reaction in mammalian sperm can occur independently from its effects on hyperactive motility and involve different intracellular mechanisms [38, 58], suggesting they may be mediated via different receptors. Immunofluorescence analysis of human sperm with a mPR antibody shows that the receptor the mPR is localized primarily to the midpiece and is not detected on the acrosome, consistent with a role for the receptor in sperm motility. Furthermore, sperm from human donors with low motility had significantly lower mPRα protein concentrations than that in normal motile sperm. Finally, it was demonstrated that G proteins were activated in human sperm in response to progesterone treatment [unpubl. obs.]. Taken together, these preliminary results suggest that mPRα may have a similar function in promoting hypermotility in mammalian sperm to that proposed for fish sperm.
3. Progesterone signaling in human myometrial cells
Although one of the characteristics of parturition in most mammals is the dramatic drop of progesterone levels prior to labor, maternal progestin levels do not decrease during parturition in humans and some primates. Instead, progesterone production by the human placenta gradually increases with advancing pregnancy, reaching 300mg per day at term [140, 147]. Given the relaxant effects of progesterone, for human labor to proceed there must be some form of progestin withdrawal. The expression of nPR-responsive genes is decreased in the primate uterus at term [17], which suggests “functional” progestin withdrawal involves repression of nPR transcriptional activity, although the mechanism remains unclear. One possible intermediary in this withdrawal is the truncated nPR isoform, PR-A, which is upregulated in laboring myometria and can function as a “dominant negative” to repress the transcriptional activity of the PR-B [94,139]. Karteris and coworkers examined another possibility, that progestins also activate mPRs at the cell surface to alter PR-B transcriptional activity via a nonclassical mechanism of steroid action. Their investigation on the signaling and functions of mPRα and mPRβ in human myometrial cells in culture is the most comprehensive investigation to date on the physiological roles of mPRs in a mammalian model [41].
Transcripts for mPRα and mPRβ were identified by real-time PCR in lower myometrial tissue from pregnant women in labor and in subcultured myometrial cells and both mPR proteins were detected on myometrial cell plasma membranes by western blotting and immunocytochemistry [41]. The presence of specific progestin binding on the cell surface of the myocytes was confirmed with a fluorescent probe, progesterone-BSA-FITC, in the presence of steroid competitors and also by radioreceptor assay. This specific progesterone binding was attributed to the presence of mPRα and mPRβ, because it was significantly reduced in cell membranes prepared from myocytes that had been transfected with siRNAs to both receptors. Steroid competition assays and signal transduction studies confirmed that mPRα and mPRβ are functional progestin receptors on the cell surface of human myocytes. The progesterone binding was not due to the presence of nuclear progesterone and glucocorticoid receptors, since R5020 and dexamethasone did not displace [3 H]-progesterone binding from the membrane preparations. Progesterone treatment of myocyte membranes caused activation of inhibitory G proteins and the resulting decline cAMP production through a pertussis toxin- sensitive pathway. Interestingly, a variety of inhibitory G proteins, Gi1/2, Gi3 and Go, were activated in response to progesterone treatment, whereas there was no activation of Gs and Gq/1. In agreement with the steroid binding studies, G protein activation by steroids was specific for progesterone and R5020, dexamethasone and cortisol were ineffective. Finally, both mPRα and mPRβ were shown to directly interact with inhibitory G proteins in two co-immunoprecipitation assays, suggesting they function as GPCRs. The results are identical to those obtained with recombinant mPRα in a eukaryotic expression system [122].
Possible cross-talk between the mPRs and nPR was examined by assessing the binding of the nPR to a glucocorticoid-responsive element (GRE) linked to a luciferase reporter vector, which was transiently transfected into myocytes [41]. As predicted, progesterone treatment with progesterone, dexamethasone and the nPR agonist, R5020, caused transactivation of PR-B in myocytes with high levels of PR-B expression. However, pertussis toxin treatment, which prevents inhibitory G protein signaling through mPRs, differentially affected the PR-B transactivation response to the three steroids. Pretreatment with the toxin partially blocked the response to progesterone, whereas the responses to dexamethasone and R5020, which do not activate mPRs, were unaltered. Additional stronger evidence of an involvement of mPRs in progesterone transactivation of PR-B was obtained from experiments in which myocytes were also transiently transfected with siRNAs for mPRα and mPRβ. The transactivation responses to progesterone were greatly attenuated in myocytes treated with siRNAs for each of the mPRs compared to that in myocytes with normal mPR levels. It was concluded from these studies that progesterone acts through a mPR-dependent pathway as well as through PR-B to transactivate the nPR. Moreover, the results showed that the potentiation of PR-B transactivation through mPRs involves activation of a pertussis-sensitive inhibitory G protein. On the basis of these results, Karteris et al. proposed that during early pregnancy progesterone activation of mPRs results in an amplification of the PR-B action to maintain the myometrium in a quiescent state [41]. Importantly, these results also provided the first evidence that novel steroid membrane receptors can influence the transactivation activities of nuclear receptors.
The mPRs can also potentially influence the transactivation of PR-B in human myometrial cells collected at term through altering the expression of co-activators. The decrease observed in co-activator expression in human myometria during labor has been proposed to contribute to the initiation of partition by impairing nPR function [17]. Expression of the mPRs appears to be elevated at this stage of pregnancy which is likely associated with increases in circulating progesterone and estradiol-17β levels, since mPR mRNA and protein levels are upregulated in myocytes treated with these steroids in vitro. Interestingly, the two mPRs are differentially regulated by these steroids; both are upregulated by estradiol-17β, whereas only mPRα is upregulated by progesterone. Treatment of these myocytes with a progesterone-BSA conjugate in vitro caused transient downregulation of SRC2 mRNA and protein expression which had returned to pretreatment values 1-2 days later, whereas the levels of other co-activators were not significantly altered (Fig.4). This response to progesterone-BSA was attenuated in cells that had been transfected with siRNA for mPRα but not mPRβ, which suggests the progesterone-induced downregulation of the SRC2 gene is at least partially mediated through mPRα via an unknown mechanism This downregulation of SRC2 expression through mPRα could potentially contribute to the functional progesterone withdrawal in human myometria during labor [41].
Figure 4.

Down regulation of coactivator SRC2 mRNA expression in human pregnant myometrial cells treated with 100 nM progesterone-bovine serum albumen conjugate (P4-BSA) for 3hrs. * P<0.05 compared to no supplement (NS) controls, N=4. SRC2 protein expression (insert) was also decreased after 16hr treatment (reproduced from Karteris et al., 2006, with permission). CBP: cAMP response element binding protein coactivator. SRC: steroid receptor (PR) coactivator (reproduced from Karteris et al., 2006 [41] with permission).
Other progesterone signaling pathways initiated at the cell surface of human myocytes through the mPRs were identified that could also facilitate myometrial contraction. Phosphorylation of myosin light chain (MLC) at Ser 19 is the primary mechanism regulating the contractile status of smooth muscle cells [25]. Cyclic AMP inhibits phosphorylation in these cells causing muscle relaxation. Activation of inhibitory G proteins though the mPRs, as predicted, caused rapid decreases in basal and isoproterenol-induced cAMP production in vitro by cultured myocytes and membranes prepared from pregnant myometrial biopsies, which was blocked by pretreatment with pertussis toxin. Therefore, progesterone via the mPRs could potentially remove an inhibitory influence of cAMP on MLC phosphorylation by decreasing cAMP levels. Clear evidence of an involvement of mPRs in MLC phosphorylation was obtained from experiments showing that treatment of myocytes with progesterone-BSA induced phosphorylation of MLC at Ser 19 and that this progesterone action was blocked by pretreatment with pertussis toxin and by transfecting the cells with siRNA for mPRα [41]. Evidence for the presence of a stimulatory progesterone pathway to phosphorylate MLC involving phosphorylation of p38 MAPK was obtained in a subsequent study. The demonstration that pretreatment with pertussis toxin blocked the phosphorylation of p38 MAPK in response to progesterone-BSA suggests this action is mediated through mPR/Gi. Moreover, the finding that treatment with a specific inhibitor of p38 MAPK, SC-68376, almost completely blocked phosphorylation of MLC, suggests that phosphorylation of p38 MAPK is the primary mechanism regulating MLC phosphorylation. Taken together, these studies suggest that progesterone signaling through mPRs could promote myometrial contraction at the onset of labor through three mechanisms; by down-regulating SRC2, which together with a decreased PR-B/PR-A ratio, results in a decrease in PR-B transactivational activity; by inhibiting adenylyl cyclase; and by inducing phosphorylation of MLC [41].
4. Progesterone signaling in human T lymphocytes and Jurkat cells
There is a growing body of evidence that suggests progesterone modulates immune function in mammals [21]. For example, changes in the symptoms of autoimmune diseases such as rheumatoid arthritis, in the cellular immune response to infection, and in the expression of interferon-related genes have been observed in women during the menstrual cycle and pregnancy in [22, 40, 76]. Progesterone has also been shown to promote T cell differentiation in vitro resulting in alterations in the production of cytokines associated with humoral immunity [93]. However, the majority of studies have failed to detect the nPR in immune cells [2, 57, 114], which have led researchers to propose alternative mechanisms of progesterone action [24].
Transcripts for mPRα and mPRβ were recently identified in peripheral blood leukocytes and T lymphocytes of reproductive age women and in immortalized human T (Jurkat) cells, whereas nPR and mPRγ were not detected in any of these cell types [21]. Western blot analyses confirmed expression of mPRα and mPRβ proteins on the plasma membranes of human T cells and Jurkat cells. Interestingly, mPRα mRNA expression was upregulated more than two-fold during the luteal phase of the menstrual cycle in the CD8+ T lymphocytes, but not in the CD4+ T lymphocytes. Dosiou et al., proposed that this increase in mPRα expression could potentially contribute to the immunomodulatory effects of progesterone during the second half of the menstrual cycle and in pregnancy. In support of this they cited a study in mice which showed that CD8+ T lymphocytes mediate the protective effects of progesterone against stress-induced abortion by altering the cytokine profile to the production of those associated with humoral immunity [7].
Clear evidence was obtained for the presence of functional membrane progesterone receptors and progesterone activation of G proteins on human T cell and Jurkat cells [21]. Saturation and Scatchard analyses of [3 H]-progesterone binding to Jurkat membrane showed the presence of a high affinity, single binding site with a Kd of 4.6 nM, very similar to the Kd (4.17 nM) reported for recombinant mPRα in human breast cancer cells [122]. Progesterone caused G protein activation in Jurkat cell membranes in a concentration-dependent manner, whereas the nPR agonist R5020 and a GR agonist, dexamethasone, were ineffective. An immunoprecipitation experiment with antibodies directed against the alpha subunit of different G proteins revealed that progesterone activated an inhibitory G protein in the immortalized T cells, consistent with previous studies showing that mPRα is coupled to and activates an inhibitory G protein. Taken together these results strongly suggest that progesterone acts on T cells through mPRs resulting in activation of an inhibitory G protein pathway. The functional consequences of activation of this pathway, however, remain unclear.
5. Progestin down-regulation of GnRH secretion in fish and rodents
Only circumstantial evidence has been obtained to date for a role of mPRα in progestin negative feedback of gonadotropin releasing hormone (GnRH) secretion in vertebrates. Both the mPRα mRNA and protein are expressed in the brains of spotted seatrout and Atlantic croaker [150, 81]. Treatment of preoptic anterior hypothalamic (POAH) tissue slices excised from adult Atlantic croaker at the beginning and at a midpoint of the reproductive cycle in vitro with the fish progestin, 20β-S (290 nM) caused a rapid decrease in the secretion of seabream GnRH, the hypophysiotropic GnRH in this species 124]. The release of GnRH into the incubation medium declined to less than 20% that of controls within the initial 10-minute incubation period and was also significantly decreased during longer incubation periods. The physiological significance of this rapid negative feedback effect of 20β-S on GnRH secretion is unclear because experiments have not been conducted with POAH tissues collected from croaker at the end of the reproductive cycle during oocyte maturation and spermiation when plasma 20β-S levels are elevated. However, the previous finding that 20β-S exerts a similar negative feedback effects on GnRH content in the POAH and LH secretion at the midpoint of the reproductive cycle as at the end of the cycle [60], suggests that the these rapid effects are also manifest at the end of the cycle. The feedback mechanism may switch off LH secretion once production of 20β-S and maturation of the largest most responsive cohort of oocytes are underway, to prevent over stimulation and precocious maturation of the remaining smaller, less developed oocytes. Additional studies using specific mPRα agonists will be required, however, to clearly establish a role for mPRα in mediating these rapid feedback effects of progestins on GnRH secretion in teleosts.
Similarly, only indirect evidence has been obtained so far implicating mPRα in progesterone feedback effects on GnRH secretion in mammals. The existence of rapid progesterone actions on reproductive neuroendocrine functions in rodents has been known for two decades [43] and specific progesterone binding sites have been detected on brain membrane preparations [131]. Both mPRα and mPRβ subtypes are present in the preoptic anterior hypothalamic region of the rodent brain and in immortalized GnRH-secreting neurons, GT1-7 cells. The finding that rapid down regulation of luteinizing hormone secretion by progesterone also occurs in PRKO mice indicates it is mediated by a novel progesterone receptor [Sleiter, Levine, pers. com.].
Progesterone membrane receptor component 1-PGMRC1
1. Progesterone binding sites on mammalian liver membranes
Progestin binding moieties have been detected in microsomal membranes prepared from rat and porcine livers [64, 145]. Partial purification of the microsomal membranes by anion-exchange chromatography produced two protein fractions with apparent molecular weights of 28 and 56 kDa with progesterone binding activity. Subsequent amino acid sequencing of the amino terminus of the two protein fractions revealed that they are identical, suggesting that they represented monomers and dimers of the same protein [64]. Receptor binding assays were conducted with both microsomal and solubilized membrane preparations. Both high affinity (Kd 11 nM) and low affinity (286 nM) binding sites for progesterone were identified in the microsomal protein fractions. The kinetics of association and dissociation of [3 H]-progesterone binding to the both the microsomal and solubilized membrane fractions are rapid, with t1/2s of 3-8minutes [64]. In single point competition assays progesterone displays the highest affinity of the compounds tested, with an IC 50 of 0.5μM followed by corticosterone which has an approximate relative binding affinity (RBA) 25 % that of progesterone and an IC50 of 2μM. Testosterone and cortisol also are effective competitors for [3 H]-progesterone binding, with IC50s of (RBAs 16% and 4%) respectively, whereas estradiol-17β has no affinity for the progesterone binding site. Such high relative binding affinities of corticosteroids for a progesterone binding protein are unique and may indicate that the putative receptor is activated by corticosteroids rather than progesterone, because plasma corticosteroid levels are higher than progesterone levels under many physiological conditions [64]. It was suggested, on the basis of a subsequent study showing that sigma (σ) ligands such as haloperidol, carbetapentane citrate and Rimeazole compete with [3 H]-progesterone binding to the porcine liver membrane preparations, that the progesterone binding sites are related to the σ receptor binding site superfamily [65]. However, no follow up studies have been conducted to test the binding of σ ligands to PGMRC1 so the significance of these findings remains unclear.
2. Cloning, identification and characterization of porcine PGMRC1
A cDNA was cloned from porcine smooth muscle cells with a deduced amino acid terminal sequence identical to that of the progesterone/corticosterone binding moiety previously purified from porcine liver tissue [27, 64]. The cDNA encodes a protein with 194 amino acids and is now known as progesterone membrane receptor component one (PGMRC1) [12]. The binding characteristics of recombinant PGMRC1 fused to glutathione S transferase (GST) produced in a prokaryotic expression system (E.coli) were investigated. Recombinant PGMRC1 (named IZA1) was incubated with [3H]-P4 for two hours, GSH-sepharose was added, and a GST pull-down assay performed. No specific [3H]-progesterone binding was detected, whereas the recombinant protein was able to bind heme, suggesting a functional protein was produced by this recombinant expression system [67]. Moreover, addition of IZA1 antibody to a mixture of [3H]-P4 and recom IZA1 did not show any specific isotope binding [67]. In contrast, stable transfection of PGMRC1 into CHO cells [26] and over expression in spontaneously immortalized granulosa cells (SIGCs) caused an increase in specific [3H]-progesterone binding to the microsomal fraction [91]. Single point competition assays showed that whereas progesterone showed highest affinity for the receptor (IC50s 0.5μM), testosterone and corticosterone also competed for progesterone binding, with IC50s of approximately 1 μM and 10 μM, respectively. There are several plausible explanations for the differences in the ability of recombinant PGMRC1 produced in the prokaryotic and eukaryotic expression systems to bind progesterone, including deficiencies in protein expression or the lack of another protein(s) in the E. coli system which are present in eukaryotic cells and forms a complex with PGMRC1 that is required for progesterone binding.
In contrast to the extensive studies on PGMRC1, there are very few reports on the closely human gene PGMRC2 which was cloned by Wehling and coworkers [33]. PGMRC2, together with PGMRC1, is expressed on human sperm and has been suggested to be involved in progesterone-induced acrosome reaction [51].
2. Characteristics of PGMRC1
Progesterone membrane receptor component 1 is a vertebrate 26-28 kDa protein with a single transmembrane domain and a potential internal cytochrome b5 binding sequence. PGMRC1 and the closely-related PGMRC2 have homologues belonging to the gene family named membrane associated progestin receptor (MAPR) in other eukaryotes, including nematodes, insects, yeast and Aripidopsis [12]. The gene has been cloned independently in vertebrates by several research groups and has been given several other names related to the diverse biological processes or tissues it has been associated with, such as its induction by dioxin (25-Dx0 [110], homology of a human gene to the porcine progesterone receptor (Hpr6) [33], a rat 195 amino acid protein (ratp28) [77], and 75 amino acid truncated form of ratp28 (HC5) [48], embryogenesis (VemaA) [107],and inner zone antigen in the adrenal cortex (IZA) [100]. A bewildering variety of ligands have been shown or suggested to bind to PGMRC1, including heme [68, 112], cholesterol [62] and steroids with 21 carbons, glucocorticoids and progestins [12]. A corresponding wide range of physiological roles have been suggested for this protein such as steroid synthesis and metabolism [100], cholesterol regulation, axonal guidance [107], endocytosis, alteration of reproductive behaviors [46], a component of a progesterone receptor and as and adapter protein. Recently evidence has been obtained in several laboratories that PGMRC1 forms complexes with other proteins. Complexes have been identified with proteins believed to be involved in the cholesterol system, SCAP and Insig1, in cells co-transfected with PGMRC1 and either of these proteins [113], consistent with a possible role for PGMRC1 in cholesterol regulation [12]. Only the proposed progesterone receptor functions of PGMRC1 will be discussed here.
Functions of PGMRC1
Antiapoptotic progesterone actions on ovarian follicle cells
Progesterone has an important physiological role in the ovarian follicle to inhibit apoptosis of granulosa and thecal cells, but the mechanism of progesterone action remains unclear because the nuclear progesterone receptor is not expressed in rat thecal cells and only transiently expressed in granulosa cells [86,116]. Progesterone has been shown to act at the cell surface to inhibit apoptosis in these cells, suggesting it is acting through a membrane progesterone receptor [87, 88.97,98]. PGMRC1 has been detected in rodent luteal and granulosa cells [13, 61] and its potential role in mediating these anti-apoptotic actions of progesterone in (SIGCs) have been investigated in detail by Peluso and collaborators [91]. These investigators showed that over expression of PGMRC1 in SIGCs is associated with increases in [3 H]-progesterone binding with cell survival and that the anti-apoptotic action of progesterone was abrogated by pretreatment with an antibody to the N-terminal of PGMRC1 [91]. Earlier it had been demonstrated that PGMRC1 interacts (immunoprecipitates) with plasminogen activator inhibitor RNA binding protein -1 (PAIRBP-1) which is present in rat granulosa and thecal cells [90]. Previously PAIRBP-1, which had been named RDA288, had been implicated in mediating progesterone's apoptotic actions in these cells [89]. Peluso and coworkers demonstrated that both PGMRC1 and PAIRBP-1 are localized on plasma membrane of SIGCs [91]. Information on the signal transduction pathways activated by progesterone through this PGMRC1/PAIRBP-1 complex should provide clues of how the antiapoptotic actions of the steroid are mediated.
Progesterone initiation of the acrosome reaction in mammalian sperm
The large number of putative progesterone receptors that have been identified on mammalian sperm using radioreceptor, immunological and biochemical approaches has complicated investigations of their roles in progesterone actions such as induction of the acrosome reaction and hyperactivation [6, 51,52,53, 58,102,103]. Wehling and coworkers identified The nPR and also PGMRC1 and PGMRC2 were identified on human sperm by researchers in the Wehling laboratory [51]. These researchers also showed that the rapid increase in intrasperm calcium concentration in response to progesterone was significantly reduced after incubation of sperm with an antibody to porcine PGMRC1 (formerly named mPR) compared to that observed after incubation with pre-immune serum [26]. Previously, immunostaining had been detected on the head of human sperm using this antibody [11]. Moreover, the progesterone-initiated acrosome reaction was inhibited 60% by the antibody, suggesting that PGMRC1 is involved in the acrosome reaction [11].
c. Summary of progestin signaling pathways through mPRα and PGMRC1
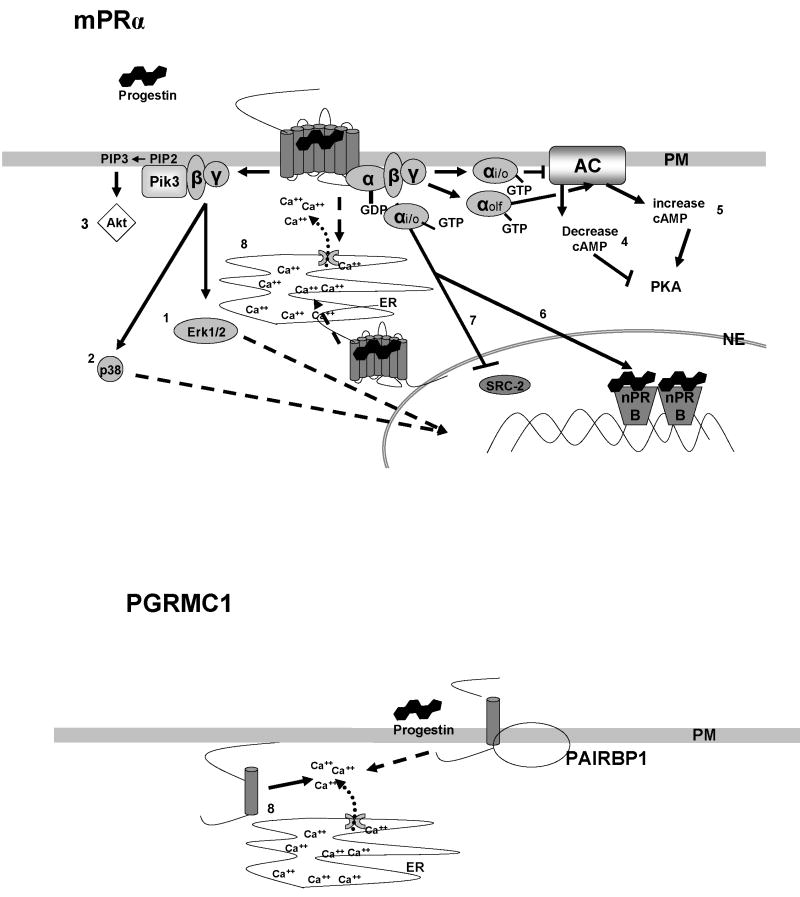
Progestins have been shown to activate a variety of signaling pathways through mPRα and PGMRC1 (Fig.5). In the majority of cell models investigated to date, progestin binding to mPRα alters second messenger pathways through activation of pertussis toxin-sensitive inhibitory G-proteins. Progestins activate mitogen activated protein kinases (MAPK) causing Erk 1/2 phosphorylation in a dose-dependent fashion in nPR negative cells transfected with seatrout mPRα [150] and with zebrafish mPRα and mPRβ [37], presumably through the βγ subunits of the G protein (Fig. 5, pathway 1). Another MAPK, p38, is activated in human myometrial cells through mPRα, resulting in phosphorylation of myosin light chain protein in these cells [41] (Fig. 5, pathway 2). A third signaling pathway likely initiated by the G protein βγ subunits through mPRα in Atlantic croaker oocytes is the Akt/PI3 kinase pathway 82][ (Fig.5, pathway 3). Progestin stimulation of the mPRα causes a decrease in cAMP levels which probably mediated by the inhibitory G protein α subunit, resulting in inhibition of adenylyl cyclase activity [21, 41, 83, 150] (Fig. 5, pathway 4). However, a stimulatory olfactory G protein appears to be activated upon progestin binding to mPRα in croaker sperm causing an increase in cAMP production [135] (Fig.5, pathway 5). Activation of a PTX-sensitive pathway through mPRα and mPRβ in human myometrial cells alters nPR transactivation activity [41] (Fig.5, pathway 6). Activation of this pathway in human myometrial cells also altered expression of the nuclear receptor coactivator, SRC-2 [6] (Fig. 5, pathway 7). There is evidence that progestins initiate calcium mobilization from intracellular stores through both mPRα and PGMRC1 [1, 26] (Fig.5, pathway 8). It is likely that additional signaling pathways mediated by these receptors will be identified when other target cells are investigated.
Figure 5.
Summary of the signal transduction pathways activated by progestins through mPRα (1-8) and PGMRC1 (8). mPRα pathways 1-4, 6,7 are mediated through activation of inhibitory G proteins (Gi), mPRα pathway 5 is mediated though an olfactory G protein (G olf). Pathway 1. Activation of Erk (Erk1, Erk2): Atlantic croaker oocytes during final maturation, probably through mPRα [82] and in MDA-MB-231 cells transfected with seatrout mPRα [150] and zebrafish mPRα and mPRβ [37]. Pathway 2: Activation of mitogen activated kinase p38: PTX-sensitive, via the mPRs in human myocytes resulting in phosphorylation of myosin light chain [41]. Pathway 3: Akt activation: Atlantic croaker oocytes during final maturation, probably through mPRα, resulting in upregulation of phosphodiesterase activity [82]. Pathway 4: Decrease cAMP levels: PTX-sensitive, in spotted seatrout oocytes during final maturation through mPRα [83, Fig.2], in MDA-MB-231 cells transfected with seatrout mPRα or human mPRα [122, 150], and in human myocytes via mPRα and mPRβ [41], in Jurkat cells via mPRα [21]. Pathway 5: Increase cAMP levels: in Atlantic croaker sperm through mPRα [135]. Pathway 6: Potentiation of PR-B transactivation: through mPRα- and mPRβ-mediated pathways in human myocytes [41]. Pathway 7: Down-regulation SRC2 coactivator expression: through mPRα in human myocytes [41]. Pathway 8: Increase intracellular free calcium concentrations: from internal calcium stores in CHO cells transfected with ovine mPRα [1] and in CHO cells transfected with PGMRC1 [26]. PM: plasma membrane, NE: nuclear envelope, ER: endoplasmic reticulum.
Methodological problems in expressing and characterizing membrane progestin receptors
a. Membrane localization of receptors
Most detailed studies of the characteristics of hormone membrane receptors have been conducted on GPCRs. The level and dynamics of GPCR expression on the cell surface are highly regulated and strongly influence the magnitude and duration of the physiological response upon ligand activation. After the receptor proteins are synthesized in the endoplasmic reticulum (ER), they undergo post-translational modifications such as folding with the aid of chaperone proteins. Defective proteins are detected by the cellular quality control system and selected for retention in the ER for recycling or destruction. Some GPCRs, such as the human GnRH receptor, rat LH receptor and the δ opioid receptor display weak expression at the plasma membrane and a large proportion of the synthesized protein is retained in the cytoplasm or in ER for degradation [18, 92, 95]. Conn and coworkers provide evidence that post-translational folding of the human GnRH receptor protein and its dimerization are important mechanisms controlling expression of the receptor on the plasma membrane [18]. Another major process regulating the cell–surface expression of GPCRs is their endocytic trafficking following activation [69]. As a consequence high levels of GPCR expression are rarely observed in the plasma membrane and instead the receptors show highest expression in the cytoplasm [129]. The receptors are phosphorylated by GPCRkinases enabling them to bind to arrestin which results in their desensitization. The GPCRs are subsequently targeted to endocytic sites on the plasma membrane such clatherin-coated pits where they are internalized for subsequent degradation or recycling by lysosomes.
It is not known what features of GPCR trafficking are shared by mPRα and PGMRC1. Although mPRs function as G protein coupled receptors, they have a different bacterial origin to GPCRs. However, both mPRs and PGMRC1 appear to function as oligomers, like GPCRs [80]. Moreover, a recent study by Karteris et al. shows human mPRα is rapidly internalized upon P4 stimulation and that the receptor is present in clatherin-coated pits [unpubl.obs].
b. Expression of recombinant putative progestin membrane receptors
The detection of recombinant progestin receptors on plasma membranes of eukaryotic cells by radioreceptor assay is frequently complicated by the presence of high background progestin binding. Greatest background binding to membrane preparations is frequently observed with progesterone, which is more lipophilic than the teleost progestins, 17,20βDHP and 20β-S, and other major sex steroids, estradiol-17β and testosterone. This problem can be minimized when expressing recombinant progestin membrane receptors in eukaryotic cells by selecting cell lines with low background binding and by maximizing expression of the receptor. Plasma membranes prepared from CHO and HEK293 cells were found to have higher background progesterone binding than those prepared from COS-7 and MDA-MB-231 breast cancer cells using a filtration assay [unpubl. obs.]. MDA-MB-231 cells do not express nuclear progesterone receptors and therefore have been used for expressing recombinant mPRα in order to determine its steroid binding and signaling characteristics [122,150]. However, CHO cells have been used successfully for the stable expression of Xenopus mPRβ and PGMRC1 [4, 26]. Both the putative membrane progestin receptors, mPRs and PGRMC-1, are widely expressed in mammalian tissues and immortal mammalian cell lines (Fig. 6), including MDA-MB-231 cells, and may partially account for the high background progestin binding in some of these cells. For example weak expression of mPRα protein and some specific progestin binding can be seen in MDA-MB-231 cells transfected with an empty vector (Fig. 7C). This endogenous expression of putative progestin membrane receptors in eukaryotic expression systems further complicates investigations of the characteristics of the recombinant receptor proteins. Fortunately, endogenous progestin receptor expression in MB-MDA-231 cells is insufficient to mediate activation of G proteins and alterations in the activities of adenylyl cyclase and MAPkinase by progestins and these responses are only observed when a membrane progestin receptor such as mPRα is overexpressed [122,150]. In the initial study expression of mPRα protein in MBA-MD-231 cells stably transfected with the seatrout mPRα cDNA was sufficient to transduce the progestin signal intracellularly to alter second messenger pathways, but not to demonstrate a significant increase in specific progestin binding [150]. Several strategies have been used to increase the expression of recombinant steroid membrane receptors. Stable transfection and continued selection over an extended period of up to 6 weeks has proven to be effective for increasing plasma membrane expression and progestin binding of goldfish and zebrafish mPRαs [37, 132]. However, for seatrout and human mPRα clones expressing high amounts of the receptor protein and progestin membrane binding were further selected by the limiting dilution method. Twelve aliquots of the transfected cells were serially diluted in 96-well plates and selection was continued for one week, after which selection was continued for a second week with plates containing only one or two healthy clones. The cells were then transferred to a cell culture flask and cultured for 10-14 days with several changes of medium. The cells were subcultured and after 1-2 passages there was a sufficient number of cells for expression and functional analyses. The expression of the human mPRα protein and [3 H]-progesterone binding in plasma membranes prepared from ten separate clones obtained by this procedure are shown in figure 7A and 7B. Binding of [3 H]-progesterone to the membrane preparations varied among the clones and in general was correlated with mPRα protein expression. Four clones with high receptor binding were selected for continued selection and subculture. High receptor binding was retained after several passages (fig.7 C) by which time there were enough cells for comprehensive characterization of the receptor. An alternative more rapid method of selecting cells with high cell surface expression of receptors is by cell sorting with a flow cytometer [14]. This method has been used successfully to enrich transfected cells expressing GPR30 on the cell surface [31]. Finally, transient transfection has been used successfully to express ovine mPRα and Xenopus mPRβ [1, 4] and may be adequate for limited characterization studies. The limiting dilution method has also been used successfully to obtain individual clones of CHO cells stably transfected (selection was maintained for two weeks) with PGMRC1 expressing sufficient amounts of the protein for [3 H]-progesterone binding studies [26].
Figure 6.

Detection of mPRα and PGMRC1 mRNAs in various cell lines after 35 cycles of RT-PCR. MDA: MDA-MB-231, SKBR: SKBR3, MYO: human myometrial cells after several passages, HEK: HEK293, RT-: lacking reverse transcriptase for actin controls, showing no DNA contamination.
Figure 7.

Selection of membrane progestin receptor-transfected cells for receptor characterization and signal transduction studies. Binding of [3 H]-P4 to plasma membranes of MDA-MB-231 cells stably transfected with human mPRα. A and B: Binding to ten subclones (a1-a10), obtained by limiting dilution, was assessed after three weeks selection. High [3 H]-P4 binding was associated with greater mPRα protein expression in the membrane fractions (insert) as assessed by Western blot analysis. C: [3 H]-P4 binding increased upon further selection for two weeks of the clones which had the highest binding in A&B (a1,a3,a5,a10). The receptor binding assay was conducted as described in Thomas et al., 2007. * P<0.05 compared to untransfected control cells (231-ve), Student's paired t test, N=4.
c. Measurement of recombinant putative progestin membrane receptors
Many hormone membrane receptors rapidly lose binding activity upon membrane disruption and homogenization unless precautions are taken to minimize degradation of the receptor protein. For the mPRα radioreceptor assay the entire procedure is conducted at 4C, a protease enzyme inhibitor cocktail is added in the homogenization and assay buffers, and the assay incubation period is shortened to 30 minutes which is sufficient for the binding to reach equilibrium. Progestin tracer bound to the receptor is rapidly separated from free steroid (< 20 sec.) by retaining the membrane fraction on glass fiber filters using a filtration manifold attached to a vacuum pump in the cold room (∼6C). Omission of the protease inhibitor cocktail from the buffers results in a 32 % loss of specific receptor binding (Fig.8). Progestin binding to recombinant mPRs was not detected in recent study by Brosens and coworkers [47] using an incubation period of 1hr at room temperature. Our results show that a 1hr at 4C does not cause a significant decline in receptor binding compared to the 30 minute incubation period, whereas conducting the incubation at room temperature for 1hr resulted in a 45-55 % decline in receptor binding even in the presence of protease enzyme inhibitors (Fig.7). Similar results were obtained for the progesterone membrane receptor on Xenopus oocytes; after one hr incubation at 20C, [3H]-progesterone binding had decreased to approximately 50% of that measured after incubation at 4C [50].
Figure 8.
Loss of [3 H]-P4 binding to plasma membranes prepared from MDA-MB-231 cells stably transfected with human mPRα during incubation at room temperature for 1hr (RT-1hr) or in the absence of protease inhibitors (-prot.inhib). The receptor binding assay was conducted as described in Thomas et al., 2007 [122] with modifications from the normal 30 min incubation procedure at 4C in the presence of protease inhibitors as indicated. Significant differences identified with a multiple range test, Fisher PLSD, are indicated with different letters (P<0.05), N=4.
Concluding Remarks
As expected for a new emerging field, many aspects of progestin signaling through novel putative progestin receptors remain unresolved and surrounded by controversy. However, considerable progress has been made in our understanding of the characteristics and physiological functions of mPRα in the short period since its discovery in seatrout ovaries was reported in 2003 [150]. The progestin binding characteristics of recombinant mPRαs has recently been confirmed in eukaryotic expressions systems for six vertebrate species (seatrout, goldfish, zebrafish, Xenopus, sheep, human) by four different research groups (1, 4, 122, 132] Activation of signal transduction pathways by progestins in mPRα-transfected cells has now been demonstrated by four research groups for mPRαs of five vertebrate species [1, 4, 37, 122]. Moreover, impairment of progestin actions (binding, induction of signal transduction pathways, or a biological response) by prior treatment with siRNA, antisense oligonucleotides, or specific antibodies has been independently confirmed in four laboratories in different vertebrate and cell models [1, 41, 133, 150]. In addition, the wildtype mPRα protein has been localized to the plasma membranes of a variety of cell types including seatrout, goldfish, zebrafish and Xenopus oocytes, fish sperm and human myocytes by investigators in five laboratories [4, 37,41,127,150], although the protein is also present in intracellular compartments [41]. Finally, clear evidence for hormonal regulation of mPRα has been obtained by three research groups in different vertebrate models [41, 133, 150]. Taken together, the results of these studies strongly support the original suggestion [150] that mPRα functions as a progestin membrane receptor that is expressed on the plasma membrane and acts as an intermediary in progestin activation of intracellular signaling pathways. In marked contrast to the results of these research groups, Brosens and coworkers reported that recombinant mPRαs of several vertebrate species were not present on the plasma membranes of transfected cells, but were localized in the endoplasmic reticulum [47]. In addition, they did not detect any specific progesterone binding to the microsomal fractions of these mPRα-transfected cells under the radioreceptor assay conditions they employed [47]. The reasons these investigators failed to detect progesterone binding to recombinant mPRα are unclear, but are probably at least partly attributable to methodological problems, such as inappropriate radioreceptor assay incubation conditions (Fig.8), receptor binding measurement only in microsomal fractions [122], and the expression of some of the recombinant proteins with apparent molecular weights (28 kD) much lower than that of mPRα (∼40kD) and the results obtained by other investigators. The findings obtained by other researchers were not directly evaluated by Brosens and coworkers because they did not examine [3 H]-progesterone binding or the presence of the mPRα protein by Western blotting in plasma membrane preparations obtained by subcellular fractionation of stably transfected cells [47], so their conclusions from this study are enigmatic. It should be noted, however, definitive evidence that mPRα is membrane progestin receptor has not been obtained to date. The demonstration of a plausible progestin binding site on mPRα by mutational analysis will be required for direct evidence that it functions as a progestin receptor. Moreover, an involvement of intracellular mPRα pools in activating signal transduction pathways cannot be excluded, since steroids can readily diffuse into the intracellular compartments. In addition, practically no information is currently available on the regulation of cell surface expression, trafficking, and endocytosis of mPRα, the role of dimerization in receptor function, its association with other proteins, and the mechanisms of cross-talk with nPRs. At the current rate of progress it is anticipated that new information on at least some of these processes will be obtained in the next few years.
Currently, even less is known about the progestin membrane receptor characteristics and functions of PGMRC1. The diverse range of ligands and proteins that can interact with the protein as well as the bewildering array of processes it is associated with, including physiological, developmental and toxicological ones, suggests PGMRC1 has widespread and important functions in the cell. However, in view of its widespread possible functions and interactions, it is unclear whether PGMRC1 has a distinct role as a specific membrane progestin receptor alone, or when coupled to another protein such as PAIRB1. A clear demonstration that progesterone activates signal transduction pathways via a plausible mechanism through PGMRC1 or this protein complex in several cell models, such as that obtained for mPRα through an inhibitory G protein, will be required to establish it as a functional progestin membrane receptor. Experiments demonstrating that down-regulation of PGMRC1 expression after treatment with siRNAs or antisense oligonucleotides is associated with decreases in [3 H]-progesterone binding and progesterone signaling are also necessary but have not been conducted. Moreover, the ability of the recombinant protein produced in a prokaryotic expression system to bind [3 H]-progesterone should be re-examined in view of the negative findings of a previous study [67]. In addition, mutation experiments, like those proposed for mPRα, will be required to identify the progesterone binding site. Finally, the potential role of PGMRC1 as an adaptor protein warrants further investigation.
A notable feature of both mPRα and PGMRC1 is their widespread tissue distribution. The finding that these putative progestin membrane receptors are co-expressed in all the eukaryotic expression systems examined to date (Fig.6), further complicates investigations of their individual characteristics. Therefore, future studies need to take into account the presence of the other putative progestin membrane receptor when examining the characteristics of mPRα or PGMRC1.
Acknowledgments
research on mPRα conducted by Thomas and coworkers was funded by Grant ESO ESO12961 from the National Institutes of Health Grant and on croaker sperm by National Research Initiative Competitive Grant no. 2006-35203-17130 from the USDA Cooperative State Research, Education, and Extension Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 2.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab. 1999;84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 3.Bayaa M, Booth RA, Sheng Y, Liu XJ. The classical progesterone receptor induces Xenopus oocyte maturation through a nongenomic mechanism. Proc Natl Acad Sci USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Yehoshua LF, Lewellyn AL, Thomas P, Maller JL. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol. 2007;21:664–673. doi: 10.1210/me.2006-0256. [DOI] [PubMed] [Google Scholar]
- 5.Berg AH, Thomas P, Olsson P-E. Characterization of a membrane progestin receptor in artic charr ovaries. Reprod Biol Endocrinol. 2005;3:64. doi: 10.1186/1477-7827-3-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface-binding sites for progesterone mediated calcium-uptake in human sperm. J Biol Chem. 1991;266(28):18655–18659. [PubMed] [Google Scholar]
- 7.Blois SM, Joachim R, Kandil J, Margni R, Tometten M, Klapp BF, Arck PC. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172:5893–5899. doi: 10.4049/jimmunol.172.10.5893. [DOI] [PubMed] [Google Scholar]
- 8.Blondeau JP, Baulieu E. Progesterone receptor characterized by photoaffinity labeling in the plasma membrane of Xenopus laevis oocytes. Biochem J. 1984;219:785–792. doi: 10.1042/bj2190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonyaratnakonkit V, McGowan E, Sherman L, Mancini D, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 10.Boonyaratnakonkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a praline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinase. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 11.Buddhikot M, Falkenstein E, Wehling M, Meizel S. Recognition of a human sperm surface protein involved in the progesterone-initiated acrosome reaction by antisera against an endomembrane progesterone binding protein from porcine liver. Mol Cell Endocrinol. 1999;158:187–193. doi: 10.1016/s0303-7207(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 12.Cahil MA. Progesterone receptor membrane component 1: An integrative review. J Steroid Biochem Molec Biol. 2007;150:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- 14.Carroll S, Al-Rubeau M. The selection of high-producing cell lines using flow cytometry and cell sorting. Expert Opin Biol Ther. 2004;4:1821–1829. doi: 10.1517/14712598.4.11.1821. [DOI] [PubMed] [Google Scholar]
- 15.Chapman NR, Kennelly MM, Harper KA, Europe-Finner GN, Robson SC. Examining the spatio-temporal expression of mRNA encoding the membrane-bound progesterone receptor-alpha isoform in human cervix and myometrium during pregnancy and labour. Mol Human Reprod. 2006;12:19–24. doi: 10.1093/molehr/gah248. [DOI] [PubMed] [Google Scholar]
- 16.Chidiac P. Rethinking receptor-G protein-effector interactions. Biochem Pharmacol. 1998;55:549–556. doi: 10.1016/s0006-2952(97)00361-4. [DOI] [PubMed] [Google Scholar]
- 17.Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PM, Knollman PE, Brothers SP, Janovick JA. Protein folding as post-translational regulation: Evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol. 2006;20:3035–3041. doi: 10.1210/me.2006-0066. [DOI] [PubMed] [Google Scholar]
- 19.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 20.DeManno DA, Goetz FW. Steroid-induced final maturation in brook trout (Salvelinus fontinalis) oocytes in vitro: the effects of forskolin and phosphodiesterase inhibitors. Biol Reprod. 1987;36:1321–1332. doi: 10.1095/biolreprod36.5.1321. [DOI] [PubMed] [Google Scholar]
- 21.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Guidice LC. Expression of membrane progesterone receptors (mPRs) on human T lymphocytes and Jurkat cells and activation of G proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 22.Dosiou C, Lathi RB, Tulac S, Huang ST, Giudice LC. Interferon-related and other immune genes are downregulated in peripheral blood leukocytes in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2501–2504. doi: 10.1210/jc.2003-031647. [DOI] [PubMed] [Google Scholar]
- 23.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, Negulescu PA, Cahalan MD. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- 26.Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- 27.Falkenstein E, Meyer C, Eisen C, Sciba PC, Wehling M. Full-length cDNA sequence of progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- 28.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones- a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 29.Fernandes MS, Pierron V, Michalovich D, et al. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J Endocrinol. 2005;187:89–101. doi: 10.1677/joe.1.06242. [DOI] [PubMed] [Google Scholar]
- 30.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 31.Filardo EJ, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Sequestration and redistribution of the novel estrogen receptor, GPR30, from the plasma membrane following estrogen stimulation. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. 2007. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA, Petralia SM. Progestins have actions through GABAA receptors. In: Watson CS, editor. The Identities of Steroid Membrane Receptors. Kluwer Academic Publishers; Boston: 2003. pp. 165–168. [Google Scholar]
- 33.Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- 34.Grazzini E, Guillon G, Mouilliac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 35.Haider S, Baqri SS. Cyclic AMP-mediated control of oocyte maturation in the catfish, Clarias batrachus (Bloch): effects of 17 alpha,20 beta-dihydroxy-4-pregnen-3-one and phosphodiesterase inhibitors. Indian J Exp Biol. 2000;38:967–973. [PubMed] [Google Scholar]
- 36.Haider S, Baqri SS. Role of cyclic AMP-dependent protein kinase in oocyte maturation of the catfish, Clarias batrachus. J Exp Zool. 2002;292:587–593. doi: 10.1002/jez.10102. [DOI] [PubMed] [Google Scholar]
- 37.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 38.Ho HC, Suarez SS. An inositol 1,4,5,-triphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 39.Jalabert B, Finet B. Regulation of oocyte maturation in rainbow trout, Salmo gairdneri: role of cyclic AMP in the mechanism of action of the maturation inducing steroid (MIS),17α-hydroxy, 20β-dihydro-progesterone. Fish Physiol Biochem. 1986;2:65–74. doi: 10.1007/BF02264074. [DOI] [PubMed] [Google Scholar]
- 40.Kalo-Klein A, Witkin SS. Candida albicans: cellular immune system interactions during different stages of the menstrual cycle. Am J Obstet Gynecol. 1989;161:1132–1136. doi: 10.1016/0002-9378(89)90649-2. [DOI] [PubMed] [Google Scholar]
- 41.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20(7):1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 42.Kazeto Y, Goto-Kazeto R, Thomas P, Trant JM. Molecular characterization of three forms of putative membrane-bound progestin receptors and their tissue-distribution in channel catfish, Ictalurus punctatus. J Mol Endocrinol. 2005;34:781–791. doi: 10.1677/jme.1.01721. 2005. [DOI] [PubMed] [Google Scholar]
- 43.Ke FC, Ramirez VD. Membrane mechanism mediates progesterone stimulatory effect on LHRH release from superfused rat hypothalami in vitro. Neuroendocrinology. 1987;45:514–517. doi: 10.1159/000124784. [DOI] [PubMed] [Google Scholar]
- 44.King W, Ghosh S, Thomas P, Sullivan CV. A receptor for the oocyte maturation-inducing hormone 17α,20β,21-trihydroxy-4-pregnen-3-one on ovarian membranes of striped bass. Biol Reprod. 1997;56:266–271. doi: 10.1095/biolreprod56.1.266. [DOI] [PubMed] [Google Scholar]
- 45.Kostellow AB, Weinstein SP, Morril GA. Specific binding of progesterone to the cell surface and its role in the meiotic divisions in Rana oocytes. Biochim Biophys Acta. 1982;720:356–363. doi: 10.1016/0167-4889(82)90112-4. [DOI] [PubMed] [Google Scholar]
- 46.Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW-F, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRα. β, and γ) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–64. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- 48.Leel V, Elrick LJ, Solares J, Ingram N, Charlton KA, Porter AJ, Wright MC. Identification of a truncated ratp28-related protein expressed in kidney. Biochem Biophys Res Commun. 2004;316:872–877. doi: 10.1016/j.bbrc.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Dillon JS. Dehydroepandrosterone activates endotherlial cell nitricoxide synthetase by a specific plasma membrane receptor coupled to Gα12,3. J Biol Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, Patiño R. High-affinity binding of progesterone to the plasma membrane of Xenopus oocytes: characteristics of binding and hormonal and developmental control. Biol Reprod. 1993;49:980–988. doi: 10.1095/biolreprod49.5.980. [DOI] [PubMed] [Google Scholar]
- 51.Losel R, Breiter S, Seyfert M, Wehling M, Falkenstein E. Classic and non-classic progesterone receptors are both expressed in human spermatozoa. Horm Metab Res. 2005;37:10–14. doi: 10.1055/s-2005-861023. [DOI] [PubMed] [Google Scholar]
- 52.Losel R, Dorn-Beineke A, Falkenstein E, Wehling M, Feuring M. Porcine spermatozoa contain more than one membrane progesterone receptor. Int J Biochem Cell Biol. 2004;36:1532–1541. doi: 10.1016/j.biocel.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Luconi M, Bonaccorsi L, Maggi M, Pecchioli P, Krausz C, Forti G, Baldi E. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab. 1998;83:877–885. doi: 10.1210/jcem.83.3.4672. [DOI] [PubMed] [Google Scholar]
- 54.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Nat Acad Sci USA. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maller JL. Recurring themes in oocyte maturation. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- 56.Maller JL. The elusive progesterone receptor in Xenopus oocytes. Proc Natl Acd Sci USA. 2001;98:8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mansour I, Reznikoff-Etievant MF, Netter A. No evidence for the expression of the progesterone receptor on peripheral blood lymphocytes during pregnancy. Hum Reprod. 1994;9:1546–1549. doi: 10.1093/oxfordjournals.humrep.a138746. [DOI] [PubMed] [Google Scholar]
- 58.Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod. 2004;70:1626–1633. doi: 10.1095/biolreprod.103.026476. [DOI] [PubMed] [Google Scholar]
- 59.Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- 60.Mathews S, Khan IA, Thomas P. Effects of maturation-inducing steroid on LH secretion and the GnRH system at different stages of the reproductive cycle in Atlantic croaker. Gen Comp Endocrinol. 2002;126:287–297. doi: 10.1016/s0016-6480(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 61.McRae RS, Johnson HM, Mihm M, O'Shaughnessy PJ. Changes in rat granulosa cell gene expression during early luteinization. Endocrinology. 2005;146:309–317. doi: 10.1210/en.2004-0999. [DOI] [PubMed] [Google Scholar]
- 62.Menzies GS, Howland K, Rae MT, Bramley TA. Stimulation of specific binding of [3H]-progesterone to bovine luteal cell-surface membranes: specificity of digitonin. Mol Cell Endocrinol. 1999;153:57–69. doi: 10.1016/s0303-7207(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 63.Meyer C, Christ M, Wehling M. Characterization and solubilization of novel aldosterone binding proteins in porcine liver microsomes. Eur J Biochem. 1995;229:736–740. doi: 10.1111/j.1432-1033.1995.tb20521.x. [DOI] [PubMed] [Google Scholar]
- 64.Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–731. doi: 10.1111/j.1432-1033.1996.0726u.x. [DOI] [PubMed] [Google Scholar]
- 65.Meyer C, Schmieding K, Falkenstein E, Wehling M. Are high-affinity progesterone binding site(s) from porcine liver microsomes members of the σ receptor family? Eur J Pharmacol. 1998;347:293–299. doi: 10.1016/s0014-2999(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 66.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Balancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras /Erk pathway iby progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:101–111. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Min L, Stushkevich V, Harnastai IN, Iwamoto H, Gilep AA, Takemori H, Usanov SA, Nanaka Y, Hori H, Vinson GP, Okamoto M. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS Journal. 2005;272:5832–5843. doi: 10.1111/j.1742-4658.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- 68.Min L, Takemori H, Nonaka Y, Katoh Y, Doi J, Horike N, Osamu H, Raza FS, Vinson GP, Okamato M. Characterization of the adrenal-specific antigen IZA (inner zone antigen) and its role in the steroidogenesis. Mol Cell Endocinol. 2004;215:143–146. doi: 10.1016/j.mce.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 69.Moore CAC, Milano SK, Benovic JE. Regulation of receptor trafficking by GRKs and arrestins. Ann Rev Physiol. 2007;69:19.1–19.32. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 70.Moore FL. Evidence that a membrane corticosteroid receptor is an opioid-like receptor. In: Watson CS, editor. The Identities of Steroid Membrane Receptors. Kluwer Academic Publishers; Boston: 2003. pp. 157–164. [Google Scholar]
- 71.Morrill GA, Ma G-Y, Kostellow AB. Progesterone binding to plasma membrane and cytosol receptors in the amphibian oocyte. Biochem Biophys Res Comm. 1997;232:213–217. doi: 10.1006/bbrc.1997.6190. [DOI] [PubMed] [Google Scholar]
- 72.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603. doi: 10.1073/pnas.97.21.11603. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagahama Y. 17 alpha, 20 beta-dihydroxy-4-pregnen-3-one, a teleost maturation-inducing hormone. Dev Growth Differ. 1987;29:1–12. doi: 10.1111/j.1440-169X.1987.00001.x. [DOI] [PubMed] [Google Scholar]
- 74.Nagahama Y, Yoshikuni M, Yamashita M, Tanaka M. Regulation of oocyte maturation in fish. In: Sherwood NM, Hew CL, editors. Fish Physiology XIII. Academic Press; 1994. pp. 393–439. 1994. [Google Scholar]
- 75.Nagahama Y, Yoshikuni M, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte growth and maturation in fish. Curr Top Dev Biol. 1995;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- 76.Nelson JL, Ostensen M. Pregnancy and rheumatoid arthritis. Rheum Dis Clin North Am. 1997;23:195–212. doi: 10.1016/s0889-857x(05)70323-9. [DOI] [PubMed] [Google Scholar]
- 77.Nolte I, Jeckel D, Wieland FT, Sohn K. Localization and topologu of rat28, a member of a novel familiy of putative steroid-binding proteins. Biochim Biophys Acta. 2000;1543:123–130. doi: 10.1016/s0167-4838(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 78.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 79.Nutu M, Weijdegård B, Thomas P, Bergh C, Thurin-Kjellberg A, Pang YF, Billig H, Larsson DG. Membrane progesterone receptor gamma: tissue distribution and expression in ciliated cells in the fallopian tube. Mol Reprod Devel. 2007;74:843–850. doi: 10.1002/mrd.20685. [DOI] [PubMed] [Google Scholar]
- 80.Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 81.Pace MC. Ph D Dissertation. University of Texas; Austin: 2005. Molecular mechanisms regulating oocyte maturation in sciaenid fishes. [Google Scholar]
- 82.Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–96. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- 83.Pace MC, Thomas P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol. 2005;285:70–79. doi: 10.1016/j.ydbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Patiño R, Thomas P. Characterization of a membrane receptor activity for 17α,20β,21-trihydroxy-4-pregenen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus) Gen Comp Endocrinol. 1990;78:204–217. doi: 10.1016/0016-6480(90)90007-9. [DOI] [PubMed] [Google Scholar]
- 85.Patiño R, Yoshizaki G, Thomas P, Kagawa H. Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanisms. Comp Biochem Physiol B. 2001;129:427–439. doi: 10.1016/s1096-4959(01)00344-x. [DOI] [PubMed] [Google Scholar]
- 86.Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5:967–978. doi: 10.1210/mend-5-7-967. [DOI] [PubMed] [Google Scholar]
- 87.Peluso JJ, Fernandez G, Pappalardo A, White BA. Characterization of a putative membrane receptor for progesterone in rat granulosa cells. Biol Reprod. 2001;65:94–101. doi: 10.1095/biolreprod65.1.94. [DOI] [PubMed] [Google Scholar]
- 88.Peluso JJ, Pappalardo A. Progesteone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-3σ. Biol Reprod. 2004;71:1870–1878. doi: 10.1095/biolreprod.104.031716. [DOI] [PubMed] [Google Scholar]
- 89.Peluso JJ, Pappalardo A, Fernandez G, Wu CA. Involvement of an unnamed protein, RDA288, in the mechanism through which progesterone mediates its antiapoptotic action in spontaneously immortalized granulosa cells. Endocrinology. 2004;145:3014–3022. doi: 10.1210/en.2004-0067. [DOI] [PubMed] [Google Scholar]
- 90.Peluso JJ, Pappalardo A, Losel R, Wehling M. Expression and function of PAIRBP1 within gonadotropin-primed rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability. Biol Reprod. 2005;73:261–270. doi: 10.1095/biolreprod.105.041061. [DOI] [PubMed] [Google Scholar]
- 91.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane component 1 expression in the immature rat ovary and its role in mediating progesterone's apoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- 92.Petäjä-Repo UE, Hogue M, Ghalla S, Laperrière A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of δ opiod receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 94.Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–879. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- 95.Pietila EM, Tuusa JT, Apaja PM, Aatsinki JT, Hakalahti AE, Rajaniemi HJ, Petäjä-Repo UE. Inefficient maturation of the rat luteinizing hormone receptor: a putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280:26622–26629. doi: 10.1074/jbc.M413815200. [DOI] [PubMed] [Google Scholar]
- 96.Pinter J, Thomas P. Characterization of a progestogen receptor in the ovary of the spotted seatrout Cynoscion nebulosus. Biol Reprod. 1995;52:667–675. doi: 10.1095/biolreprod52.3.667. [DOI] [PubMed] [Google Scholar]
- 97.Rae MT, Menzies GS, Bramley TA. Bovine ovarian non-genomic binding sites, presence in follicular and luteal cell membranes. J Endocrinol. 1988;159:413–427. doi: 10.1677/joe.0.1590413. [DOI] [PubMed] [Google Scholar]
- 98.Rae MT, Menzies GS, McNeilly AS, Woad K, Webb R, Bramley TA. Specific non-genomic, membrane-localized binding sites for progesterone in the bovine corpus luteum. Biol Reprod. 1988;58:1394–1406. doi: 10.1095/biolreprod58.6.1394. [DOI] [PubMed] [Google Scholar]
- 99.Rahman MA, Ohta K, Yoshikuni M, Nagahama Y, Chuda H, Matsuyama M. Characterization of ovarian membrane receptor for 17,20β-dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in yellowtail, Seriola quinqueradiata. Gen Comp Endocrinol. 2001;127:71–79. doi: 10.1016/s0016-6480(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 100.Raza FS, Takemori H, Tojo H, Okamato M, Vinson GP. Identification of the rat adrenal zona fasciculata/reticularis specifc protein, inner zone antigen (IZAg), as the putative membrane progesterone receptor. Eur J Biochem. 2001;268:2141–2147. doi: 10.1046/j.1432-1327.2001.02096.x. [DOI] [PubMed] [Google Scholar]
- 101.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 102.Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 103.Revelli A, Modotti M, Piffarettiyanez A, Massobrio M, Balerna M. Steroid-receptors in human spermatozoa. Hum Reprod. 1994;9:760–766. doi: 10.1093/oxfordjournals.humrep.a138592. [DOI] [PubMed] [Google Scholar]
- 104.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 105.Ropero AB, Soria B, Nadal A. A nonclasssical estrogen membrane receptor triggers differential actions in the endocrine pancreas. Mol Endocrinol. 2002;16:497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- 106.Rossato M, Nogara A, Merico M, Ferlin A, Foresta C. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane. Steroids. 1999;64:168–175. doi: 10.1016/s0039-128x(98)00104-4. [DOI] [PubMed] [Google Scholar]
- 107.Runko E, Kaprielian Z. Expression of the Vema in the developing mouse spinal cord and optic chiasm. J Comp Neurol. 2002;451:289–299. doi: 10.1002/cne.10356. [DOI] [PubMed] [Google Scholar]
- 108.Sabeur K, Edwards D, Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biol Reprod. 1996;54:993–1001. doi: 10.1095/biolreprod54.5.993. [DOI] [PubMed] [Google Scholar]
- 109.Sadler SE, Maller ML. Identification of a steroid receptor on the surface of Xenopus oocytes by photaffinity labeling. J Biol Chem. 1982;257:355–361. [PubMed] [Google Scholar]
- 110.Selmin O, Thorne PA, Blachere FM, Johnson PD, Romagnolo DF. Transcriptional activation of the membrane-bound progesterone receptor (mPR) by dioxin, in endocrine-responsive tissues. Mol Reprod Dev. 2005;70:166–174. doi: 10.1002/mrd.20090. [DOI] [PubMed] [Google Scholar]
- 111.Skildum A, Favre E, Lange CA. Progesterone receptors induce cell cycle progression via mitogen-activated protein kinases. Mol Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- 112.Song J, Vinarov D, Tyler EM, Shahan MN, Tyler RC, Marley JL. Hypothetical protein At2g24940.1 from Arabidopsis thaliana has a cytochrome b5 like fold. J Bioml NMR. 2004;30:215–218. doi: 10.1023/b:jnmr.0000048943.34504.29. [DOI] [PubMed] [Google Scholar]
- 113.Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- 114.Szekeres-Bartho J, Szekeres G, Debre P, Autran B, Chaouat G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol. 1990;125:273–283. doi: 10.1016/0008-8749(90)90083-4. [DOI] [PubMed] [Google Scholar]
- 115.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 116.Telleria CM, Stocco CO, Stati AO, Deis RP. Progesterone receptor is not required for progesterone action in the rat corpus luteum of pregnancy. Steroids. 1999;64:760–766. doi: 10.1016/s0039-128x(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 117.Thomas P. Hormonal control of final oocyte maturation in sciaenid fishes. In: Davey KG, Peter RE, Tobe SS, editors. Nat Res. Council of Canada; Ottawa: 1994. pp. 619–625. [Google Scholar]
- 118.Thomas P. Nongenomic steroid actions initiated at the cell surface: Lessons from studies in fish. Fish Biochem Physiol. 2004;28:3–12. [Google Scholar]
- 119.Thomas P. Discovery of a new family of membrane progesterone receptors in vertebrates and detection of the alpha and beta subtypes in mouse brain, testis and uterus. Med Chem Res. 2004;13:202–209. [Google Scholar]
- 120.Thomas P, Breckenridge-Miller D, Detweiler C. Binding characteristics and regulation of the 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor on testicular and sperm plasma membranes of spotted seatrout (Cynoscion nebulosus) Fish Physiol Biochem. 1997;17:109–11. [Google Scholar]
- 121.Thomas P, Das S. Correlation between binding affinities of C21 steroids for the maturation-inducing steroid membrane receptor in spotted seatrout ovaries and their agonist and antagonist activities in an oocyte maturation bioassay. Biol Reprod. 1997;57:999–1007. doi: 10.1095/biolreprod57.5.999. [DOI] [PubMed] [Google Scholar]
- 122.Thomas P, Pang Y, Dong J, Groenen J, Kelder J, van de Vlieg H, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 123.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G-protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 124.Thomas P, Pang Y, Zhu Y, Detweiler DC, Doughty K. Multiple rapid progestin actions and progestin membrane subtypes in fish. Steroids. 2004;69:567–574. doi: 10.1016/j.steroids.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Thomas P, Pinter J, Das S. Upregulation of the maturation-inducing steroid membrane receptor in spotted seatrout ovaries by gonadotropin during oocyte maturation and its physiological significance. Biol Reprod. 2001;64:21–29. doi: 10.1095/biolreprod64.1.21. [DOI] [PubMed] [Google Scholar]
- 126.Thomas P, Trant JM. Evidence that 17,20β,21-trihydroxy-4-pregnen-3-one is a maturation-inducing steroid in spotted seatrout. Fish Physiol Biochem. 1989;7:185–191. doi: 10.1007/BF00004706. [DOI] [PubMed] [Google Scholar]
- 127.Thomas P, Tubbs C, Detweiler D, Das S, Ford L, Breckenridge-Miller D. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids. 2005;70:427–433. doi: 10.1016/j.steroids.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 128.Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 129.Thompson MD, Burnham WM, Cole DE. The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci. 2005;42:311–392. doi: 10.1080/10408360591001895. [DOI] [PubMed] [Google Scholar]
- 130.Tian J, Kim S, Hellig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tischkau SA, Ramirez VD. A specific membrane protein for progesterone in rat brain: sex difernces and induction by estrogen. Proc Natl Acad Sci USA. 1993;90:1285–1289. doi: 10.1073/pnas.90.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tokumoto T, Tokumoto M, Thomas P. Interactions of diethylstilbestrol (DES) and DES analogues with membrane progestin receptor α (mPRα) and the correlation with their nongenomic progestin activities. Endocrinology. 2007;148:3459–3467. doi: 10.1210/en.2006-1694. [DOI] [PubMed] [Google Scholar]
- 133.Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 134.Trant JM, Thomas P. Isolation of a novel maturation-inducing steroid produced in vitro by ovaries of Atlantic croaker. Gen Comp Endocrinol. 1989;75:397–404. doi: 10.1016/0016-6480(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 135.Tubbs C. PhD Thesis. University of Texas; Austin: 2007. Mechanisms of progestin-stimulated sperm hypermotility in two teleosts: the Atlantic croaker (Micropogonias undulatus) and the southern flounder (Patatylichthys lethistomata) [Google Scholar]
- 136.Tubbs C, Thomas P. Functional characteristics of membrane progestin receptor alpha (mPRα) subtypes: A review with new data showing mPRα expression in seatrout sperm and its association with sperm motility. Steroids. doi: 10.1016/j.steroids.2007.12.022. in press. [DOI] [PubMed] [Google Scholar]
- 137.Tucker AL, Jia LG, Holeton D, Taylor AJ, Linden J. Dominance of G(s) in doubly G(s)/G(i)-coupled chimaeric A(1)/A(2A) adenosine receptors in HEK-293 cells. Biochem J. 2000;352:203–210. [PMC free article] [PubMed] [Google Scholar]
- 138.Uhler ML, Leung A, Chan SYN, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fert Steril. 1992;58:1191–1198. [PubMed] [Google Scholar]
- 139.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 140.Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab. 1984;58:629–639. doi: 10.1210/jcem-58-4-629. [DOI] [PubMed] [Google Scholar]
- 142.Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 143.Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 144.Weiler PJ, Wiebe JP. Plasma memebrane receptors for the cancer-regulating progesterone metabolites, 5α-pregnane-3,20-dione and 3α-hydroxy-4-pregnen-20-one in MCF7 breast cancer cells. Biochem Biophys Res Commun. 2000;272:731–737. doi: 10.1006/bbrc.2000.2847. [DOI] [PubMed] [Google Scholar]
- 145.Yamada M, Miyaji H. Binding of sex hormones by male rat liver microsomes. J Steroid Biochem. 1982;16:437–446. doi: 10.1016/0022-4731(82)90057-7. [DOI] [PubMed] [Google Scholar]
- 146.Yamashita M. Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin Cell Dev Biol. 1998;9:569–79. doi: 10.1006/scdb.1998.0251. [DOI] [PubMed] [Google Scholar]
- 147.Yen S. Endocrine-metabolic adaptations in pregnancy. In: Yen S, Jaffe R, editors. Reproductive endocrinology. Philadelphia: Saunders; pp. 936–981. [Google Scholar]
- 148.Yoshikuni M, Nagahama Y. Involvement of an inhibitory G-protein in the signal transduction pathway of maturation-inducing hormone (17α,20β-dihydroxy-4-pregnen-3-one) action in rainbow trout (Oncorhynchus mykiss) oocytes. Dev Biol. 1994;166:615–622. doi: 10.1006/dbio.1994.1341. [DOI] [PubMed] [Google Scholar]
- 149.Yoshikuni M, Shibata N, Nagahama Y. Specific binding of [3H]17α,20β-dihydroxy-4-pregnen-3-one to oocyte cortices of rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 1993;11:15–24. doi: 10.1007/BF00004546. [DOI] [PubMed] [Google Scholar]
- 150.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]