Abstract
This is a randomized, double blind, placebo-controlled clinical trial that evaluated the efficacy of a higher than typical daily dose of naltrexone (150mg/day), taken for 12 weeks, in 164 patients (n=116 men and n=48 women) with co-occurring cocaine and alcohol dependence. Patients were stratified by gender, and then randomly assigned to naltrexone or placebo, and to either cognitive behavioral therapy, or a type of medical management. The two primary outcomes were cocaine and alcohol use. Significant gender by medication interactions were found for cocaine use via urine drug screens [3-way with time] and self-reports [2-way], for drug severity [2-way], and for alcohol use [2-way]. Type of psychosocial treatment did not affect outcomes. Thus, 150mg/day of naltrexone added to a psychosocial treatment resulted in reductions in cocaine and alcohol use, and drug severity in men, compared to higher rates of cocaine and alcohol use and drug severity in women.
Keywords: naltrexone, cocaine dependence, alcohol dependence, CBT, BRENDA, adverse-events
1. Introduction
Studies indicate that up to 60% of patients seeking treatment for cocaine dependence are also dependent on alcohol (Higgins, Budney, Bickel, Foerg, & Badger, 1994; McCance-Katz et al., 1993; Miller, Gold, Belkin, & Klahr, 1989), and combined addiction to these two agents presents a formidable challenge to treatment providers. Cocaine-alcohol dependent patients suffer more adverse addiction-related consequences, greater psychosocial problems, and higher rates of recidivism than patients addicted only to cocaine (Brady, Sonne, Randall, Adinoff, & Malcolm, 1995; Carroll, Rounsaville, & Bryant, 1993; Heil, Badger, & Higgins, 2001; Mengis, Maude-Griffin, Delucchi, & Hall, 2002). Furthermore, these patients are particularly difficult to treat and almost always require special treatment interventions.
Cocaine-alcohol dependent patients are inherently prone to relapse, due to their vulnerability to environmental cues associated with cocaine and alcohol. Commonly, the use of one substance leads to the use of the other (Dackis & O'Brien, 2001; Heil et al., 2001; Mengis et al., 2002). Additionally, concurrent use of cocaine and alcohol notably yields cocaethylene (McCance-Katz et al., 1993), an active transesterified metabolite associated with more lethality (Andrews, 1997; Jatlow et al., 1991; Katz, Terry, & Witkin, 1992) and toxicity (Cami, Farre, Gonzalez, Segura, & de la Torre, 1998; McCance-Katz, Kosten, & Jatlow, 1998; Pennings, Leccese, & Wolff, 2002; Wilson, Jeromin, Garvey, & Dorbandt, 2001) than cocaine alone. Postmortem studies link lethal overdose with cocaethylene (Jatlow et al., 1991), which has been estimated to increase the risk of sudden death by 18–25 fold when compared to cocaine alone (Andrews, 1997). Cocaethylene has pharmacological properties that are similar to those of cocaine (Jatlow et al., 1991; McCance-Katz et al., 1998; McCance-Katz et al., 1993), and controlled studies report that the combined use of cocaine and alcohol produces more euphoria than cocaine alone (Farre et al., 1993; McCance-Katz et al., 1998; Perez-Reyes & Jeffcoat, 1992). Aside from using alcohol to enhance cocaine euphoria, these patients also drink to counter irritability and insomnia produced by cocaine binges. Therefore, addiction to both cocaine and alcohol from a neurobiological perspective may be more tenacious because these agents have different and additive effects on reward-related glutamate and GABA neurons (Dackis & O'Brien, 2002).
Thus, developing an effective pharmacological treatment for this treatment-refractory population is a logical goal to improve clinical outcomes. Currently, there are no medications approved by the Food and Drug Administration (FDA) for treating cocaine dependence, or cocaine-alcohol dependence. On the other hand, there are four medications approved for treating alcohol dependence. Naltrexone, one of the FDA-approved medications for the treatment of alcohol dependence, is an opioid receptor antagonist that has been found in a number of trials to prevent heavy drinking in alcoholics (Anton et al., 1999; Krystal, Cramer, Krol, Kirk, & Rosenheck, 2001; O'Brien, Volpicelli, & Volpicelli, 1996; O'Malley et al., 1992; Volpicelli, Alterman, Hayashida, Muentz, & O'Brien, 1990; Volpicelli, Alterman, Hayashida, & O'Brien, 1992). The beneficial effect of naltrexone in alcoholism appears to stem from its ability to attenuate alcohol-induced euphoria (King, Volpicelli, Frazer, & O'Brien, 1997), a phenomenon that is linked to the release of beta-endorphins in a large number of animal and human studies (Dackis & O'Brien, 2003). Therefore, because drinking alcohol in cocaine-alcohol dependent patients can lead to cocaine use, it is reasonable to propose that naltrexone’s ability to reduce alcohol consumption may indirectly reduce cocaine use.
In addition, naltrexone may have direct beneficial effects on the compulsive abuse of cocaine, without respect to alcohol. Animal studies report that cocaine releases beta-endorphins (Olive, Koenig, Nannini, & Hodge, 2001), and that cocaine self-administration is reduced by naltrexone and other opioid antagonists (Corrigall & Coen, 1991). Dynorphin upregulation is produced by chronic exposure to either alcohol or cocaine (Dackis & O’ Brien, 2003), and probably contributes significantly to dopamine hypoactivity in both conditions (Wise, 1996). Dynorphin inhibits dopamine neurons via kappa-opioid receptors, which are antagonized by naltrexone, suggesting that this agent might reverse a common neuroadaptation produced by chronic exposure to alcohol and cocaine. Furthermore, even though naltrexone has not been found to block cocaine euphoria in humans (Walsh, Sullivan, Preston, Garner, & Bigelow, 1996), naltrexone (50 mg/day) given with relapse-prevention therapy reduced cocaine use in a placebo-controlled study (n = 85) of cocaine-addicted patients (Schmitz, Stotts, Rhoades, & Grabowski, 2001).
While at first, treating co-occurring cocaine and alcohol dependence with naltrexone appears to be a reasonable pharmacological strategy, two double-blinded, placebo-controlled naltrexone studies failed to find an advantage for treating cocaine-alcohol dependent patients with naltrexone (Hersh, Van Kirk, & Kranzler, 1998; Schmitz, Stotts, Sayre, DeLaune, & Grabowski, 2004). A published, open-label report (Oslin et al., 1999) questioned these findings by showing an effect with a higher daily dose of naltrexone (150 mg/day) than what has been the typical 50mg/day dose used in the former two studies. Naltrexone dosage of 50 mg/day may be inadequate for this treatment-resistant, cocaine-alcohol patient population. Higher naltrexone daily doses may positively influence blockade of kappa opioid receptors, as well as medication adherence (higher doses last longer) (Oslin et al., 1999).
Accumulating data have suggested that men and women with alcohol or drug dependence can respond differently to the same pharmacological treatment, typically with men having better treatment outcomes than women (Garbutt et al., 2005; Hernandez-Avila et al., 2006; Nich et al., 2004; Pettinati, Dundon, & Lipkin, 2004). However, at least one naltrexone study has suggested that alcohol-dependent women treated with 50mg/day of naltrexone abstained from alcohol for a longer duration than men (Kiefer, Jahn, & Wiedemann, 2005). In addition, the COMBINE study, which is the largest double-blind naltrexone trial completed to date in alcohol dependent patients, found no gender differences in attrition or drinking outcomes with 100mg/day dose (Anton et al., 2006). Nonetheless, in reference to the present naltrexone study and the dose under investigation, there is literature which has suggested that women may be more vulnerable than men to the medication’s common side effects, nausea and vomiting (O'Malley, Krishnan-Sarin, Farren, & O'Connor, 2000). A potential rationale suggested by O’Malley and colleagues was the possibility that there are gender differences in their sensitivity of the endogenous opioid system, which is targeted by naltrexone. This hypothesis was generated once the investigators ruled out body weight, smoking status, concomitant medications, and a number of other factors that potentially account for the gender differences in medication responses they found in their study (O'Malley, Krishnan-Sarin, Farren, & O'Connor, 2000). In summary, the literature has been inconsistent with regard to predicting differential gender response to naltrexone treatment. In order to address the possibility of gender differences in treatment response in the present study, the design dictated that randomization to naltrexone or placebo occur within gender subgroups so that treatment response could be evaluated separately for males and females.
Another potentially important factor may be the type of psychosocial intervention provided with pharmacotherapy, because the type of psychosocial treatment may influence outcomes in cocaine dependent patients (Anton et al., 2005; Schmitz et al., 2001). While the traditional “platform” treatments that are used in cocaine treatment studies have been based on the principles of cognitive-behavioral relapse prevention therapy (Marlatt & Gordon, 1985), medical management --a supportive treatment that can be provided when prescribing pharmacotherapy, has been successfully provided in treatment studies of alcohol dependent patients (Garbutt et al., 2005; O'Malley et al., 2003; Pettinati, Volpicelli, Pierce, & O’Brien, 2000; Pettinati et al., 2005; Volpicelli, Pettinati, McLellan, & O'Brien C, 2001). Desirable features of a medical management-supportive intervention is that it typically includes an emphasis on patient strategies to maintain good medication adherence, and it is likely to be more easily transportable into nonspecialty settings. Gender differences in response to type of psychosocial treatment have not been established, although differences in treatment adherence found in this or any study between types of psychosocial treatment could possibly have implications for gender differences in treatment response.
Thus, the present study was designed to assess in a controlled clinical trial, stratified on gender, the effect of treating cocaine-alcohol dependence with a daily dose of naltrexone that is higher than is typically prescribed for alcohol dependence, with one of two types of psychosocial interventions — a traditional cognitive behavioral relapse prevention therapy, or a type of medical management, supportive intervention.
2. Materials and Methods
2.1 Participants
Participants were 164 males and females between the ages of 18 and 65 with current DSM-IV cocaine and alcohol dependence diagnoses, as determined by the Structured Clinical Interview for DSM–IV (First, Spitzer, Gibbon, & Williams, 1996). These patients were seeking treatment at the Treatment Research Center, a university-affiliated outpatient substance abuse treatment-research facility in Philadelphia, PA. This study was approved by the University of Pennsylvania Institutional Review Board and all participants provided written informed consent.
To be eligible for this treatment study, individuals had to be abstinent from alcohol for at least 3 consecutive days, and have a negative urine drug screen prior to randomization. These requirements for short-term abstinence prior to randomization promoted evaluating naltrexone as a relapse-prevention medication, rather than an abstinence-initiating one. Individuals were excluded if they met any of the following conditions: a) current DSM-IV diagnosis of any substance dependence other than cocaine and alcohol; b) opiate use in the past 30 days; c) current severe psychiatric symptoms, including psychosis, suicidal or homicidal ideation or mania; d) current treatment with psychiatric medications, including antipsychotic, antidepressants and antianxiety medications; e) history of unstable or serious medical illness, including epilepsy, seizure disorder, AIDS, active hepatitis, or severe hepatocellular injury, evidenced by elevated bilirubin levels, or elevated levels over 4x normal of aspartate aminotransferase (AST) or alanine aminotransferase (ALT); and f) pregnancy, nursing or use of unreliable method of contraception.
2.2 Treatments
This was a randomized, placebo-controlled, double-blind factorial (2×2) 12-week trial of 150mg/day of naltrexone or placebo, and one of two psychosocial treatments for cocaine and alcohol dependence – either traditional cognitive behavioral therapy (CBT), or a low-intensity type of medical management treatment known as BRENDA (explained below). Participants were stratified by gender and then randomly assigned to 1 of the following 4 treatment conditions: naltrexone-CBT; naltrexone-BRENDA; placebo-CBT; and placebo-BRENDA.
2.2.1 Naltrexone and Placebo
At randomization, participants were prescribed 50mg naltrexone or placebo (matching capsules) for the first 3 days and incremented by 50mg every 3 days to the target dose of 150mg naltrexone or placebo. Participants were then maintained on 150mg naltrexone, as tolerated, until the last week of the trial, when the naltrexone dosage was tapered to 100mg for 3 days and then 50 mg for the last 3 days. Medication adherence was monitored for every patient by the combination of systematic weekly pill counts based on the return of well-marked blister packs and patient self-reports.
2.2.2 Cognitive Behavioral Therapy (CBT)
Manually-guided CBT, an individual psychotherapy treatment based on the relapse prevention model of Marlatt and Gordon (Marlatt & Gordon, 1985) and adapted by Carroll (Carroll, 1998) for treatment of cocaine and alcohol dependence, was conducted by CBT-trained, experienced, masters- or doctoral-level therapists in 45-minute weekly sessions for the duration of the 12-week treatment trial. Treatment goals were to reduce the likelihood of relapse and abstain from alcohol and cocaine use by identifying high-risk situations and utilizing coping strategies. In CBT treatment, therapists focused on promoting motivation for abstinence, teaching skills to cope with drug cravings and high-risk situations, facilitating life-enhancement and affect-management skills, and improving interpersonal functioning. CBT sessions were audiotaped and reviewed for clinician manual adherence by the supervising therapist. Individual supervision was provided weekly by the clinical supervisor to correct clinician drift.
2.2.3 BRENDA
BRENDA is a low-intensity, manualized psychosocial intervention to enhance treatment adherence and motivation to achieve health (Volpicelli et al., 2001). Participants in the BRENDA condition attended 30-minute individual, weekly meetings with a BRENDA-trained and experienced nurse practitioner during the 12-week treatment period. BRENDA techniques include providing empathic support and structured feedback about the nature of the addictive illness, the motivation toward achieving health, and the behaviors necessary to derive maximum benefit from the treatment. BRENDA is an acronym for the following 6 steps that guide its delivery: 1) use of a Biopsychosocial evaluation to comprehensively review the biological, psychological and family-sociodemographic status as related to substance use; 2) Report and provide feedback to the participant about his /her biopsychosocial status, focusing on any negative drug- and alcohol-related consequences; 3) Provision of Empathy in order to manage patient resistance to change; 4) Identification of a patient’s Needs or problems in treatment; 5) Provision of Direct advice to enhance treatment adherence; and 6) Assessment of the patient’s ability to follow advice or get involved in new problems. All sessions were audiotaped and random selections of tapes were rated for clinician adherence to the BRENDA manual guidelines and the treatment’s unique principles.
2.3 Procedures
2.3.1 Baseline/Screening Assessments
During the screening phase of the study, medical and psychosocial evaluations, laboratory tests (including a pregnancy test for women), and diagnostic, problem severity, and substance use interviews were administered. All participants who provided written consent and met eligibility criteria were randomized, separately by gender, to one of four treatment conditions.
2.3.2 Trial Assessments
During the 12-week trial, all participants met once a week with the following: 1) a physician for dispensing medications and checking for side effects (M.D. visit was kept to 15 min). Adverse events were measured at each M.D. visit using the Systematic Assessment for Treatment Emergent Effects (Rabkin, Markowitz, Ocepek-Welikson, & Wager, 1992). There was a small list of potential adverse events that patients were specifically asked about, which might be most associated with naltrexone. However, time was also allotted for patients to report on other, more general side effects. Liver enzymes were collected monthly, and female patients received urinary pregnancy tests prior to starting medications, and at monthly intervals throughout the study; 2) a CBT or BRENDA clinician for psychosocial treatment, and 3) a research technician who obtained various study-related measures. Participants were not financially compensated for treatment visits, but were provided with compensation for transportation, completing research assessments, and returning the medication blister cards.
2.4 Collecting Primary and Secondary Outcome Measures
2.4.1 Self-reported Substance Use
The Timeline Follow-Back (TLFB) method (Sobell, Maisto, Sobell, & Cooper, 1979), adapted to collect both alcohol and cocaine self-reports, was used to obtain subject reports of alcohol and cocaine use at the screening visit (the previous 30-days), and at the weekly treatment visits (daily use during treatment). In studies with alcohol dependent subjects, there has been good agreement between TLFB data and collateral and biological data (Ehrman & Robbins, 1994; Fals-Stewart, O'Farrell, Freitas, McFarlin, & Rutigliano, 2000; Maisto, Sobell, & Sobell, 1979). The primary outcome measures derived from TLFB data were the amount and frequency of both alcohol and cocaine use. TLFB was administered by a trained Research Technician at screening and at each weekly visit throughout the trial.
2.4.2 Cocaine Urine Toxicological Examination
Urine benzoylecgonine (BE) tests were obtained at screening and, thereafter, patients were required to provide weekly urine specimens, collected directly before each treatment visit with the physician. Urine collection was not observed but urine sample temperature was monitored. Samples less than 90 degrees, or greater than 100 degrees Fahrenheit were not accepted (fewer than 1%). Samples were analyzed for benzoylecgonine by fluorescent polarization assay. Samples were analyzed qualitatively [greater than or equal to 300 ng/ml of BE was considered positive].
2.4.3 Problem Severity
The Addiction Severity Index (ASI) (McLellan et al., 1992) was used to obtain sociodemographics and information on the severity of medical, employment, drug use, alcohol use, legal, family/social, and psychiatric problems. The ASI has demonstrated moderate to excellent internal consistency, test-retest, and interrater reliabilities in different groups of substance abusers (Alterman, Brown, Zaballero, & McKay, 1994; McLellan et al., 1985). The ASI was administered by a trained Research Technician at pre-treatment, and then every 4 weeks during the 12 weeks of treatment.
2.5 Statistical Analyses
The analysis was by intention-to-treat. The two main outcome measures were: 1) abstinence from cocaine; and 2) abstinence from alcohol. The patients were compared on a variety of baseline characteristics, using logistic regression for categorical characteristics, and linear regression (ANOVA) for continuous characteristics, to assess how well the randomization had balanced patient characteristics across the four treatment groups, both across and within gender.
The primary analyses did not include additional covariates, but characteristics that showed significant imbalance across the groups were considered for inclusion as covariates in supplementary analyses, together with characteristics known to be of importance such as a BE positive urine at baseline.
With respect to abstinence from cocaine, the repeated binary outcomes obtained from the BE assays were analyzed using generalized estimating equation (GEE) models (Diggle, Heagerty, Liang, & Zeger, 2002). In these analyses, missing urine screens were imputed as drug-positive, which is a standard practice in clinical trials for which cocaine abstinence is a primary outcome (Shoptaw, Kintaudi, Charuvastra, & Ling, 2002). The other primary outcome, i.e., abstinence from alcohol, and secondary repeated outcomes were also analyzed using GEE models for continuous or count responses from the TLFB. Secondary outcomes of self-reported cocaine use (TLFB) and of alcohol and drug severity (composite scores for alcohol and for drug use on the ASI) were analyzed in the same manner. In all the repeated measures analyses, the models included terms for medication group (placebo versus naltrexone), therapy group (BRENDA versus CBT) together with linear and quadratic time effects, and some group by time interactions. Quadratic time effects were included in the models to allow for the possibility that rates of cocaine or alcohol use might decrease (or increase) early in treatment, and remain at a lower (or higher) level through the rest of the trial. Group by quadratic time interactions allow these patterns to be different for the different groups. In fitting these models to the data, terms were included in the GEE models if they were significant at the 5% level, and lower order effects contained in a significant interaction effect were also included. Empirical standard errors were used to assess significance.
3. Results
3.1 Demographics and Baseline Data
For the total study group of 164 cocaine and alcohol dependent patients, the average age of the participants was 39.1 years (sd=7.0). Most were African American (76.2%), of lower socioeconomic status (61.2%), never married (52.5%), employed (70.9%) with 12.5 years (sd=1.9) of education. Most smoked crack cocaine (71.1%). On average, participants had used cocaine for 12.2 years (sd=6.5), about 12.5 days (sd=7.3) in the 30 days prior to treatment, and spent on average $1,122 per month (sd=1138) on cocaine. On average, participants reported problem use of alcohol for 19.7 years (sd=8.2), drinking about 12.0 drinks (sd=7.2) per drinking day, on 17.1 days (sd=7.9) in the 30 days prior to treatment.
At pre-treatment there were no differences in pre-treatment variables between groups assigned to different psychosocial treatments on age, race, gender, route of cocaine administration, days of cocaine or alcohol use in the 30 days prior to treatment, years in their lifetime of cocaine use, or the alcohol composite score from the ASI at the time of treatment entry.
There was some evidence of gender, medication group, and gender by medication group interactions for days of cocaine and alcohol use in the 30 days before treatment, years of alcohol use in lifetime, alcohol and drug composite scores at treatment entry from the ASI. Generally, at the start of treatment females had higher cocaine severity scores than males (ASI drug composite index at treatment entry of 0.26 vs. 0.24, p < 0.05, respectively), but males reported more years of alcohol use (20.7 yrs vs 17.3 yrs, p < 0.05). Within gender, the males in the naltrexone-treated group had higher drug composite scores-- higher cocaine severity—at treatment entry than placebo-treated males, but no such differences were observed in the females.
The data for all pre-treatment characteristics are provided for placebo-treated and naltrexone-treated patients, separately for men and women, in Table 1.
Table 1.
Pre-Treatment Characteristics for Cocaine-Alcohol Dependent Males and Females Assigned to Naltrexone or Placebo Conditions
| Total Sample | Overall | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Male | (A) | Female | PLAC | (B) | NTX | PLAC | (C) | NTX | |
| N (%) | 164 | 116 (70.7) | 48 (29.3) | 58 | 58 | 24 | 24 | |||
| Age, M (sd) | 39.1 (7.0) | 39.0 (7.5) | 39.2 (5.8) | 39.8 (8.0) | 38.2 (6.8) | 38.3 (5.8) | 40.3 (5.8) | |||
| Caucasian, n (%) | 39 (23.8) | 31 (26.7) | 8 (16.7) | 14 (24.1) | 17 (29.3) | 3 (12.5) | 5 (20.8) | |||
| Married, n (%) (1) | 28 (17.3) | 22 (19.3) | 6 (12.5) | 10 (17.2) | 12 (20.7) | 4 (16.7) | 2 (8.3) | |||
| Years of Education, M (sd) | 12.5 (1.9) | 12.7 (2.0) | 12.2 (1.8) | 12.6 (2.0) | 12.8 (2.0) | 11.7 (1.6) | * | 12.8 (1.8) | ||
| Mid/Upper SES, n (%) (2) | 59 (38.8) | 43 (40.2) | 16 (35.6) | 21 (37.5) | 22 (43.1) | 6 (28.6) | 10 (41.7) | |||
| Days Employed, past 30 days, M (sd) | 10.6 (10.3) | 11.6 (10.2) | * | 8.1 (10.0) | 11.4 (10.3) | 11.8 (10.3) | 7.6 (9.6) | 8.6 (10.6) | ||
| Cocaine/Alcohol Use Pre-Treatment | ||||||||||
| Lifetime | ||||||||||
| Prior Alcohol Treatment, M (sd) | 2.0 (2.5) | 2.1 (2.5) | 1.8 (2.6) | 2.3 (2.3) | 2.0 (2.7) | 2.1 (2.5) | 1.5 (2.7) | |||
| Prior Drug Treatment, M (sd) | 2.2 (2.5) | 2.3 (2.5) | 1.9 (2.5) | 2.5 (2.3) | 2.0 (2.6) | 2.2 (2.4) | 1.7 (2.6) | |||
| Years of Alcohol Use, M (sd) | 19.7 (8.2) | 20.7 (8.1) | * | 17.3 (8.0) | 21.7 (7.6) | 19.7 (8.5) | 16.6 (7.2) | 18.0 (8.7) | ||
| Cocaine Use, M yrs (sd) | 12.2 (6.5) | 12.5 (6.6) | 11.5 (6.2) | 13.1 (6.8) | 11.8 (6.3) | 10.8 (5.4) | 12.2 (7.0) | |||
| Crack-cocaine Preference, n (%) (3) | 113 (71.1) | 78 (69.6) | 35 (74.5) | 42 (73.7) | 36 (65.5) | 17 (70.8) | 18 (78.3) | |||
| Past 30 Days | ||||||||||
| ASI Alcohol composite score, M (sd) | 0.61 (0.2) | 0.59 (0.2) | 0.65 (0.2) | 0.62 (0.2) | * | 0.55 (0.2) | 0.63 (0.2) | 0.67 (0.2) | ||
| ASI Drugs composite score, M (sd) | 0.25 (0.1) | 0.24 (0.1) | * | 0.27 (0.1) | 0.25 (0.1) | 0.23 (0.1) | 0.28 (0.1) | 0.26 (0.1) | ||
| Days of Alcohol Use, M (sd) | 17.1 (7.9) | 16.4 (7.8) | 18.5 (8.2) | 17.6 (7.4) | 15.3 (8.0) | 17.0 (8.1) | 20.1 (8.2) | |||
| Number of drinks per drinking day, M (sd) | 12.0 (7.2) | 12.2 (6.7) | 11.5 (8.3) | 13.3 (7.1) | 11.1 (6.2) | 13.0 (10.0) | 10.0 (6.0) | |||
| Percentage of days drinking, M% (sd) | 57.1 (26.6) | 56.0 (26.2) | 68.0 (27.3) | 59.0 (25.1) | 51.1 (26.8) | 56.5 (26.9) | 67.4 (27.1) | |||
| Percentage of days heavy drinking, M% (sd) | 53.7 (27.7) | 52.0 (26.9) | 57.8 (29.5) | 56.4 (25.7) | 47.6 (27.5) | 55.7 (27.5) | 59.8 (31.8) | |||
| Days of Cocaine Use, M (sd) | 12.5 (7.3) | 12.1 (7.0) | 13.6 (8.0) | 13.1 (7.0) | 11.1 (6.8) | 14.5 (7.9) | 12.7 (8.2) | |||
| Money spent on cocaine, M$ (sd) | 1122 (1138) | 1178 (1146) | 988 (1118) | 1285 (1160) | 1071 (1131) | 1322 (1397) | * | 652 (608) | ||
N = 162
N = 152
N = 159
p < 0.05
Test of Male vs. Female
Test of Naltrexone vs. Placebo in Males only
Test of Naltrexone vs. Placebo in Females only
Note: M= Mean; M%= Mean percentage; sd=standard deviation; PLAC= placebo; NTX=high dose naltrexone
3.2 Treatment Attrition
Treatment attrition was defined as discontinuing medication treatment, either high dose naltrexone or placebo, for three or more consecutive weeks over the planned treatment course. Sixty-four percent (n=105) of the total study sample completed the 12-week trial. Men and women dropped out at similar rates (35.3% and 37.5%, respectively) (log-rank = 0.09, p = 0.76). Survival analyses showed no significant difference in treatment retention among the 4 treatment groups (placebo/Brenda 42.2%; placebo/CBT 35.1%; naltrexone/Brenda 34.2%; naltrexone/CBT 31.8%) (log-rank = 0.87, p = 0.83). A higher proportion of the patients in the placebo condition (39.0%) did not complete the treatment when compared to the high-dosage condition (32.9%), but this difference was not statistically significant (chi-square = 0.66, df =1, p = 0.42). Among men, 43.1% in the placebo condition and 27.6% in the naltrexone condition did not complete the treatment, but this difference did not reach statistical significance (chi-square = 3.06, df=1, p < 0.10). The treatment attrition rate in women, however, showed an inverse relationship; 29.2% of women in the placebo condition and 45.8% of women in the naltrexone condition dropped out from the treatment (chi-square = 1.42, df=1, p = 0.23), although this difference was not statistically significant.
The number of CBT or BRENDA sessions attended between the medication and placebo groups and between men and women did not differ significantly. Among women, the medication group (M = 4.7, sd = 2.4) attended significantly fewer BRENDA sessions than the placebo group (M = 7.6, sd = 3.4; p < 0.05), but no such difference was found among men.
3.3 Primary and Secondary Outcomes
3.3.1 Cocaine Abstinence (Urine drug screens)
A full GEE model of the log-odds of missing and drug-positive urines vs. drug-negative urines showed a significant quadratic time effect (β = −0.03, Z = −6.34, p < 0.0001) and a significant medication by gender by time interaction (Z = 2.02, p = 0.04), and no effects of therapy (main effect: Z = −0.09, p = 0.93; no interactions significant). Thus, there were significant medication by gender differences in how patterns of cocaine use changed over the course of the trial. Table 2 shows the coefficients, standard errors, Z-statistics, and p-values from the GEE model, rounded to two places of decimals. The coefficients represent log-odds of missing and drug-positive urines (“use”) versus drug-negative urines. The factors Male, Naltrexone, and CBT represent binary variables taking the value 1 for male, naltrexone, and CBT groups, respectively.
Table 2.
The coefficients, standard errors, Z-statistics, and p-values from The GEE model analysis on cocaine abstinence outcomes over 12 weeks
| Model Term | Coefficient (SE) | Z-statistic | p-value |
|---|---|---|---|
| Intercept | −2.07 (0.40) | −5.12 | < 0.0001 |
| Male | −0.04 (0.45) | −0.08 | 0.93 |
| NTX | −0.04 (0.56) | −0.07 | 0.95 |
| CBT | 0.00 (0.22) | −0.02 | 0.98 |
| Male*NTX | −0.01 (0.66) | −0.09 | 0.93 |
| Slope (Week) | 0.56 (0.07) | 7.51 | < 0.0001 |
| Male*Slope | 0.02 (0.05) | 0.45 | 0.66 |
| NTX*Slope | 0.10 (0.07) | 1.42 | 0.16 |
| Male*NTX*Slope | −0.16 (0.08) | −2.02 | 0.04 |
| Quadratic | −0.03 (0.01) | −6.34 | < 0.0001 |
Note: NTX=high dose naltrexone; CBT=cognitive behavior therapy
The choice of binary factors means that the Slope coefficient (0.56) represents the increase in log-odds of use per week for the female-placebo group, while the three coefficients for group by slope interactions give the adjustments necessary to obtain the corresponding rates of increase for the male-placebo, female-naltrexone, and male-naltrexone groups, respectively. These rates are 0.65 for female-naltrexone, 0.58 for male-placebo, and 0.52 for male-naltrexone. These rates are all significantly different from zero (p < 0.0001 for all), and positive, so all four groups showed significant increases in use over the course of the trial. This finding is typically expected across groups in protocols where abstinence is a requirement for randomization and initiation of medication. The significant negative coefficient for the quadratic effect (−0.03), and lack of significant quadratic by group interactions, showed that these rates of increase in use gradually slowed down at about the same rate for each of the four groups. Between group comparisons on the rates of increase showed that the rate for female-naltrexone group was significantly higher than that for the male-placebo group (Z = 2.21, p = 0.03). The other pairwise differences were not significantly different from each other, reflecting the low power available because of the overall sample size, and the small number of women.
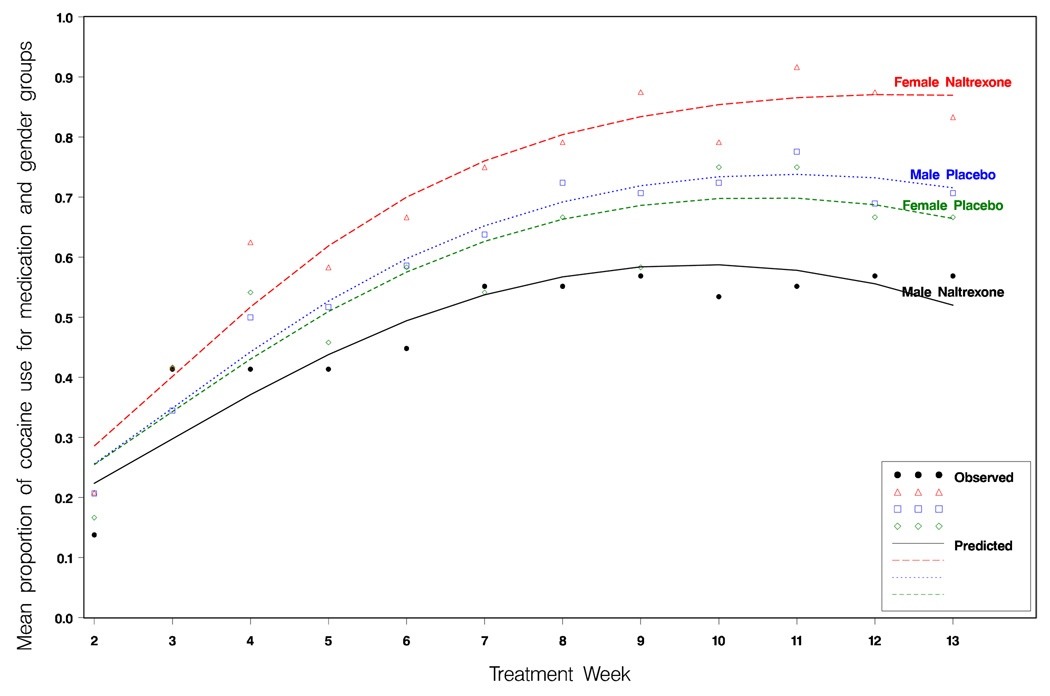
Figure 1 shows the observed and model-estimated proportions of cocaine use (based on identifying positive or missing urine drug screen results each week) for the naltrexone and placebo groups for men and women across the 12 weeks of the study. We see that the estimated lines provide a good fit to the data. We note that the inclusion of the gender by naltrexone by time interaction allow the model to capture the observed pattern of use at the start of the study. In a model where those interactions were omitted, the model incorrectly suggested that there were differences between the groups at the start of the treatment phase.
Figure 1.
The observed and model estimate proportion of male and female patients in the naltrexone and placebo groups using cocaine each week based on urine drug screen results.
Table 3 gives some further insight into the nature of the groups by time interaction. Columns 2 through 4 give the proportions of patients who are using cocaine according to urine drug screen results for the four groups at the beginning, middle, and end of the treatment phase. The proportion per group illustrates that the patients increase their use until the middle of the study, and then remain at that level through to the end of the trial. We also see the greater increase in use for the female-naltrexone group, relative to the other three groups. The remaining columns of Table 3 show the gender by naltrexone interactions at each of the three chosen weeks. We see that there are no significant differences in use at the start of treatment, but that differences between groups emerged by the middle or treatment, and persist through to the end of treatment.
Table 3.
The proportion of subjects with cocaine use according to urine drug screens across treatment, with the medication by gender interaction: Z-statistics, and p-values from The GEE model analysis on cocaine
| Female | Male | Gender × NTX Interaction | NTX Main Effect | Gender Main Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WEEK | PLAC | NTX | PLAC | NTX | Z | p | Z | p | Z | p |
| 1 | 0.25 | 0.29 | 0.26 | 0.22 | 0.58 | 0.56 | −0.80 | 0.42 | −0.32 | 0.75 |
| 7 | 0.66 | 0.80 | 0.69 | 0.57 | 2.46 | 0.01 | −3.16 | 0.002 | −1.65 | 0.10 |
| 13 | 0.66 | 0.87 | 0.72 | 0.52 | 2.76 | 0.006 | −3.27 | 0.001 | −1.87 | 0.06 |
Note: PLAC= placebo; NTX=high dose naltrexone
Thus, within males, 150mg/day of naltrexone was associated with lower rates of increase in use, while among females this dosage was associated with higher rates of increase in use. While the effect of naltrexone was significantly different between men and women, the within-gender differences were not significantly different. Adjustments for baseline covariates (including presence of a drug-positive urine at baseline; previous 30-day measures of days of alcohol use, days of alcohol use to intoxication, days of cocaine use, lifetime years of alcohol use, lifetime years of cocaine use, and the ASI composites for drugs, alcohol, and psychiatric domains) yielded the same conclusions, with very similar estimates and tests of significance. Among these baseline covariates, only the number of days of cocaine use in the 30 days prior to the study, and the ASI alcohol composite, were significant (p-values of 0.03 and 0.02, respectively, each indicating higher baseline levels being associated with higher in-treatment use), but had no influence on the group and time effects.
3.3.2 Cocaine Abstinence (TLFB Self-report)
Of 1189 urine specimens collected during the treatment period, 959 (80.7%) were consistent with self-report, 149 (12.5%) were found positive for cocaine but patients denied cocaine use, and 81 (6.8%) were found negative for cocaine despite patients’ admission of cocaine use. These figures are comparable to previous studies using self-report data in cocaine dependent samples (Carroll et al., 2004; Hersh, Mulgrew, Van Kirk, & Kranzler, 1999; Zanis, McLellan, & Randall, 1994). This indicated that in this sample, self-reported cocaine use is relatively reliable. Therefore, no data corrections were made to accommodate the relatively few discrepant results between UDS results and self-reported cocaine use. Also, some of these discrepancies may only appear to be discrepancies because qualitative drug-positive urine results within a particular week can reflect the same cocaine use, and not a new use. Nonetheless, the primary cocaine outcome analysis relied on the UDS results and not on the self-reported use of cocaine
A similar GEE model for self-reported cocaine use, with missing weeks regarded as use, showed similar patterns to those observed with the UDS analysis for determining cocaine use. There were similar time trends (linear week coefficient = 0.21, Z = 3.26, p = 0.001; quadratic week coefficient = −0.01, Z = −2.33, p = 0.02), with the CBT group showing slightly slower rates of increased use (β = −0.08, Z = −2.47, p = 0.01). There were no significant interactions with time. However, the pattern of use across the four groups was about the same as in the UDS analyses: there was a significant medication group by gender interaction (β = 1.43, Z = 2.71, p = 0.01), with the naltrexone group in males being 1.74 (Z = 2.00, p = 0.05) times less likely to self-report cocaine use than placebo-treated males, but the naltrexone-treated group in females being 2.39 (Z = 1.97, p = 0.05) times more likely to self-report cocaine use than placebo-treated females. Thus, within males, 150mg/day naltrexone was associated with significantly lower self-reported rates of cocaine use, while among females this treatment was associated with significantly higher rates.
3.3.3 Alcohol Abstinence (TLFB Self-report)
A similar GEE model for presence of any drinking showed patterns comparable to those found for the self-reported cocaine outcomes. There were similar time trends (linear week coefficient = 0.18, Z = 2.85, p = 0.004; quadratic week coefficient = −0.01, Z = −2.19, p = 0.03), with no significant differences in these trends across medication, therapy, or gender groups. Again, there was a significant medication group by gender interaction (β = 1.12, Z = 2.29, p = 0.02), with the naltrexone group in males being 1.57 (Z = 1.61, p = 0.09) times less likely to report drinking alcohol than placebo males, but the naltrexone group in females being 1.87 (Z = 1.35, p = 0.18) times more likely to drink alcohol than placebo females. Thus, within males, 150mg/day of naltrexone was associated with lower rates of drinking, while among females this treatment was associated with higher rates, but neither within-gender differences were significant. There were no significant effects of therapy (p = 0.23 for the therapy main effect). Adjustments for baseline covariates yielded the same conclusions, with very similar estimates and tests of significance.
A similar GEE model for presence of heavy drinking showed no significant time effects (β=0.10, Z = 1.15, p = 0.25 for linear; β= −0.00, Z = −0.80, p = 0.42 for quadratic), no medication by therapy interaction (β= −0.18, Z = −0.33, p = 0.74), and nonsignificant main effects for medication (β=0.46, Z=1.22, p = 0.22), and therapy (β=−0.19, Z = −0.48, p = 0.63).
Outcome and related variables reflecting primary and secondary outcomes are provided in Table 4.
Table 4.
Treatment Variables for Cocaine-Alcohol Dependent Males and Females Assigned to Naltrexone or Placebo Conditions during the 12-Week Trial
| Total Sample | Overall | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male Total | (A) | Female Total | PLAC | (B) | NTX | PLAC | (C) | NTX | ||
| Treatment Adherence | ||||||||||
| # CBT completers, n (%) | 54 (66.7) | 40 (67.8) | 14 (63.6) | 16 (61.5) | 24 (72.7) | 8 (72.7) | 6 (54.6) | |||
| # CBT sessions attended, M (sd) | 6.2 (3.9) | 6.6 (3.8) | 5.4 (4.0) | 6.1 (4.0) | 7.0 (3.6) | 4.1 (3.7) | 6.4 (4.1) | |||
| BRENDA completers, n (%) | 51 (61.5) | 35 (61.4) | 16 (61.5) | 17 (53.1) | 18 (72.0) | 9 (69.2) | 7 (53.9) | |||
| BRENDA sessions attended, M (sd) | 7.0 (3.8) | 7.5 (4.0) | 5.9 (3.1) | 7.0 (3.9) | 8.0 (4.0) | 7.6 (3.4) | * | 4.7 (2.4) | ||
| Percentage of days taking medication,M% (sd) | 61.0 (36.0) | 62.4 (36.0) | 57.6 (36.3) | 58.0 (35.0) | 66.9 (36.7) | 60.9 (36.3) | 54.4 (36.9) | |||
| Med. Compliance > 80%, n (%) | 75 (45.7) | 54 (46.6) | 21 (43.8) | 24 (41.4) | 30 (51.7) | 11 (45.8) | 10 (41.7) | |||
| Cocaine/Alcohol Use in Treatment | ||||||||||
| Cocaine use days, M (sd) | 7.4 (11.6) | 7.2 (11.1) | 7.7 (13.0) | 9.0 (12.7) | 5.3 (8.8) | 6.2 (10.1) | 9.4 (15.6) | |||
| Money spent on cocaine, M$ (sd) | 465 (1045) | 432 (795) | 543 (1,488) | 478 (761) | 384 (833) | 495 (1,505) | 593 (1,502) | |||
| Alcohol use days, M (sd) | 9.7 (12.9) | 9.7 (13.1) | 9.7 (12.6) | 11.3 (13.6) | 8.1 (12.5) | 8.8 (11.0) | 10.6 (14.2) | |||
| Percentage of days drinking, M% (sd) | 11.9 (15.0) | 11.8 (15.2) | 12.3 (14.5) | 14.4 (16.2) | 9.1 (13.7) | 9.8 (12.0) | 14.8 (16.6) | |||
| Percentage of days heavy drinking, M% (sd) | 7.6 (11.4) | 7.6 (11.4) | 7.4 (11.3) | 9.6 (12.7) | 5.6 (9.6) | 7.8 (12.2) | 6.9 (10.6) | |||
| Number of drinks per drinking, M (sd) | 5.2 (5.2) | 5.2 (5.2) | 5.0 (5.4) | 5.6 (6.0) | 4.9 (4.2) | 6.4 (6.7) | 3.7 (3.1) | |||
| Percentage of clean UDS - cocaine (1) M % (sd) | 40.0 (32.2) | 43.3 (32.2) | * | 32.3 (31.3) | 37.1 (31.6) | * | 49.4 (31.8) | 39.2 (36.0) | 25.4 (24.6) | |
p < 0.05
Missing UDS assumed drug positive (Reviewer #1)
Test of Male vs. Female
Test of Naltrexone vs. Placebo in Males only
Test of Naltrexone vs. Placebo in Females only
Note: M= Mean; M%= Mean percentage; sd=standard deviation; CBT=cognitive behavior therapy; PLAC= placebo; NTX=high dose naltrexone
3.3.4 ASI composite scores
We also examined GEE models for the ASI composite scores for the alcohol, drugs, and psychiatric domains, considering three time-points across the 12 weeks as our repeated outcomes, and used the baseline value for a given composite score as a covariate in the model for that score. Since we have only three time points in the model, we modeled time as a categorical factor. As might be expected, each baseline covariate was positively correlated with the later values (p<0.0001 for each outcome). For the alcohol and psychiatric domains, there were no other significant group, time, or group by time effects. For the drug composite score, there was a significant medication group by gender interaction (β = 0.09, Z = 2.82, p < 0.005), with the naltrexone group in males having scores 0.04 (Z = 2.86, p = 0.004) lower than placebo males, but the naltrexone group in females having scores 0.05 (Z = 1.69, p = 0.09) higher than placebo females. Thus, within males, naltrexone was associated with significantly lower drug composite scores (lower drug severity), while among females there was a trend for naltrexone to be associated with higher drug composite scores (higher drug severity), compared to their respective placebo-treated cohorts.
3.5 Adverse Events (AEs)
There were no participant deaths or serious medical conditions during the clinical trial. Adverse events or AEs ranged from mild to severe. Two subjects (1 placebo and 1 naltrexone) left treatment early due to enrolling in inpatient substance abuse treatment. The most frequently reported AEs were headache (61.6%), anxiety/irritability (61.0%) and nausea (40.2%). Table 5 provides the prevalence of the most frequent AEs reported (i.e., reported by 10% or more subjects) by men and women treated with naltrexone or placebo. The top half of the table represents AEs that we specifically ask each patient; the bottom half represent AEs that are spontaneously reported.
Table 5.
Reported Adverse Events (10% or more subjects reporting) for Males and Females in Naltrexone or Placebo Conditions
| Total Sample | Overall | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male Total | (A) | Female Total | PLAC | (B) | NTX | PLAC | (C) | NTX | ||
| Study Specific Adverse Events | ||||||||||
| Headache, n (%) | 101 (61.6) | 69 (59.5) | 32 (66.7) | 36 (62.1) | 33 (56.9) | 16 (66.7) | 16 (66.7) | |||
| Nausea, n (%) | 66 (40.2) | 41 (35.3) | ** | 25 (52.1) | 10 (17.2) | *** | 31 (53.4) | 12 (50.0) | 13 (54.2) | |
| Vomiting, n (%) | 34 (20.7) | 21 (18.1) | 13 (27.1) | 7 (12.1) | * | 14 (24.1) | 6 (25.0) | 7 (29.2) | ||
| Increased Sexual Desire, n (%) | 45 (27.4) | 34 (29.3) | 11 (22.9) | 14 (24.1) | 20 (34.5) | 8 (33.3) | * | 3 (12.5) | ||
| Decreased Sexual Desire, n (%) | 46 (28.0) | 32 (27.6) | 14 (29.2) | 13 (22.4) | 19 (32.8) | 8 (33.3) | 6 (25.0) | |||
| Anxiety/Irritability, n (%) | 100 (61.0) | 69 (59.5) | 31 (64.6) | 34 (58.6) | 35 (60.3) | 15 (62.5) | 16 (66.7) | |||
| General Adverse Events | ||||||||||
| Insomnia, n (%) | 19 (11.6) | 12 (10.3) | 7 (14.6) | 5 (8.6) | 7 (12.1) | 4 (16.7) | 3 (12.5) | |||
| Aches/Pains, n (%) | 28 (17.1) | 17 (14.7) | 11 (22.9) | 7 (12.1) | 10 (17.2) | 6 (25.0) | 5 (20.8) | |||
| Upper Respiratory Problems, n (%) | 59 (36.0) | 43 (37.1) | 16 (33.3) | 22 (37.9) | 21 (36.2) | 11 (45.8) | 5 (20.8) | |||
| Other GI Problems, n (%) | 39 (23.8) | 23 (19.8) | * | 16 (33.3) | 9 (15.5) | 14 (24.1) | 9 (37.5) | 7 (29.2) | ||
| Musculoskeletal, n (%) | 32 (19.5) | 25 (21.6) | 7 (14.6) | 8 (13.8) | * | 17 (29.3) | 3 (12.5) | 4 (16.7) | ||
p < 0.10
p < 0.05
p < 0.001; all reported p-values are for 2-sided Chi Square Test
Test of Male vs. Female
Test of Naltrexone vs. Placebo in Males only
Test of Naltrexone vs. Placebo in Females only
Note: PLAC= placebo; NTX=high dose naltrexone; GI: gastro-intestinal
Nausea was associated not only with the naltrexone condition (vs. placebo) (53.7% vs. 26.8%, chi square = 12.3, df=1, p < 0.05), but also women were more likely to report nausea than men (52.1% vs. 35.3%, chi square = 3.96, df=1, p < 0.05). Naltrexone-treated men were more likely to report nausea than placebo-treated men (53.4% vs. 17.2%, chi square = 16.6, df=1, p < .001).
4. Discussion
Our present findings suggest that a higher than typical daily dose of naltrexone (i.e., 150mg/day) is associated with gender differences in response to treatment in patients suffering from both cocaine and alcohol dependence. That is, for men, this medication regimen may reduce the amount of cocaine use and reduce drug severity in treatment of men dependent on both cocaine and alcohol. However, as indicated by a medication group by gender interaction, women dependent on both cocaine and alcohol did not appear to benefit from the 150mg/day naltrexone daily dose, and in fact, the women in the 150mg/day naltrexone subgroup, compared to placebo-treated females, used more cocaine during the trial period. Overall, there was a similar pattern of results for alcohol abstinence and a significant correlation between cocaine and alcohol abstinence/use in this trial. There also were no significant differences on any outcome or treatment mediator variable with respect to the type of psychosocial support (CBT or a type of medical management called BRENDA). While no conclusions can be drawn about the role psychosocial treatment in this study, the results suggest that medical management treatment might be a useful alternative when specialty treatment is not available. Hopefully, these results will stimulate more studies that investigate what conditions support medical versus specialty psychosocial treatments like the ones that were used in this study.
While there are a number of possible reasons for differences in outcomes between men and women in our study, the most likely explanation is the 150mg/daily dose of naltrexone was more difficult for women to take than men. For example, pill adherence in the naltrexone-only subgroups appeared different in the men than women, although the difference did not reach significance. That is, while overall men and women took their medications daily at similar rates, and stayed in treatment at similar rates, there were greater disparities in adherence rates when only the two patient subgroups that were actually taking naltrexone were considered: e.g., alcohol dependent men who actually took naltrexone in this study had a higher rate of naltrexone adherence compared to a similar cohort of women taking naltrexone (67% vs. 54%, respectively). In addition, naltrexone-treated men had a lower rate of attrition than naltrexone-treated women (28% vs. 46%; respectively). However, neither of these differences were statistically significant. In our original pilot study of 150mg/day, we (Oslin et al., 1999) reported good adherence rates and outcomes with this higher daily dose of naltrexone. However, on hindsight, 85% of the pilot study sample were men. In summary, while we found no significant difference in the attrition rates between men and women in this study, there was a strong trend within the subjects who received active naltrexone for the women to attend/receive less treatment than the men.
Previous studies indicate that female patients are likely to be more sensitive to medications due to unique sensory processes and/or cognitive hyper-vigilance than men (Berkley, K. J., 1997; Lee, Mayer, Schmulson, Chang, & Naliboff, 2001). In addition, it is known that there are gender-based pharmacokinetic differences in bioavailability, distribution, metabolism and elimination of medications (Gandhi, Aweeka, Greenblatt, & Blaschke, 2004). Thus, it has been hypothesized that perhaps one or more of those gender differences have elicited more naltrexone side effects in women than men at a 150mg/day dose, namely, nausea and vomiting (O'Malley, Krishnan-Sarin, Farren, & O'Connor, 2000). Nausea and vomiting associated with medication have been reported as predictors of patients stopping treatment (Rosenhow et al, 2000; Oncken et al, 2001), thus, resulting in an increase of their alcohol or drug use during the trial evaluation period. Thus, it is possible that our observation of distinct differences in clinical responses for men and women could be due to the consequences of naltrexone side effects at the 150mg/day dose. However, in contrast to the present study’s findings with an 150mg/day dose in this alcohol-dependent population, a small but well-controlled pre-clinical study of brief exposure to naltrexone at 100mg daily dose was reportedly safe and well-tolerated in both men and women (Johnson, O’Malley, Ciraulo, Roache, Chambers, Sarid-Segal & Couper, 2003). Although we cannot posit here that cocaine-alcohol dependent women are likely to have poorer outcomes than their male counterparts with 150mg/day of naltrexone compared to placebo only because of medication side effects, the data from this study are suggestive that females taking this high of a daily dose of naltrexone will not benefit via associated side effects, potentially leaving treatment, and/or possibly increasing substance use – all of which are likely due to the patient’s discomfort and need to seek remediation. Differential increased sensitivity of the opioid pathways between men and women is a viable explanation for the more dramatic gender differences in response to medication. This rationale was previously suggested by other investigators, after they empirically ruled out body weight, concomitant medications and other variables that typically differ between men and women patients (O'Malley, Krishnan-Sarin, Farren, & O'Connor, 2000).
Of note, differential gender response to 100mg/day of naltrexone between male and females has not been reported to date, and it is likely that this dose might be a more feasible targeted daily dose of naltrexone for females. Also, at the very least, these results suggest that females likely require a slower titration up to a target dose of naltrexone in order to avoid common naltrexone side effects, and potential attrition and negative outcomes. Further study of this issue is warranted (Suh, Pettinati, Kampman, Lynch, & O'Brien C, 2005).
The results of this study should be considered in light of several methodological limitations. First, our findings are specific to outpatient treatment-seeking patients with co-occurring cocaine and alcohol dependence, and they may not be representative of the general cocaine-alcohol dependent population. Second, the presence of a gender interaction prevented us from pooling data across males and females in comparing naltrexone to placebo. Thus, our naltrexone comparisons have less power than was originally planned. However, while the number of female subjects was small in this study compared to the number of male patients, the male to female ratio in this study is within the range reported in other published addiction treatment studies (O'Malley et al., 2000; Wong, Badger, Sigmon, & Higgins, 2002). Additionally, although few patients were completely lost to follow-up, a number dropped out during the trial and did not complete a full course of treatment.
Naltrexone, an opioid antagonist, has been shown to be effective in treating alcohol dependence at 50mg/day, unless the alcoholic patients were also cocaine dependent. In the present study, a higher daily dose of 150mg/day for 12 weeks was associated with less self-reported cocaine use and lower drug severity in men than women with co-occurring cocaine and alcohol dependence. Abstinence from alcohol also appeared to increase in the men, compared to women. Higher naltrexone daily doses may positively influence blockade of kappa opioid receptors, which may directly impact cocaine intake, unrelated to naltrexone’s purported mechanisms of action on reducing drinking. Treatment providers using a higher dose of naltrexone, however, need to ensure good medication adherence in their patients in order to obtain a good treatment response, and this study’s findings suggest that 150mg/day naltrexone is not beneficial in women dependent on both cocaine and alcohol. Further studies are needed in developing an efficacious treatment for females with co-occurring cocaine and alcohol dependence.
Acknowledgments
The work was supported by grants from the National Institute on Drug Abuse (P60 DA05186 to Dr. O’Brien; P50 DA12756 to Dr. Pettinati), and from the VISN 4 Mental Health Research Education and Clinical Center (MIRECC) at the Philadelphia Veterans Affairs Medical Center. Dupont Pharmaceuticals generously donated naltrexone and matching placebo.
We thank Thea Gallis, Hu Xie, William Dundon, Donna Giles, Maya McAllister, Michelle Teti and Thomas Whittingham for technical assistance. Address reprint requests to: Dr. Helen Pettinati, University of Pennsylvania, Treatment Research Center, 3900 Chestnut Street, Philadelphia PA 19104-6178.
A portion of this paper was presented in an oral communication session at the 65th Annual Scientific Meeting of The College on Problems of Drug Dependence, June 14–19, 2003, Bal Harbour, FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterman AI, Brown LS, Zaballero A, McKay JR. Interviewer severity ratings and composite scores of the ASI: a further look. Drug and Alcohol Dependence. 1994;34(3):201–209. doi: 10.1016/0376-8716(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Andrews P. Cocaethylene toxicity. Journal of Addictive Diseases. 1997;16(3):75–84. doi: 10.1300/J069v16n03_08. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. Journal of Clinical Psychopharmacology. 2005;25(4):349–357. doi: 10.1097/01.jcp.0000172071.81258.04. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. American Journal of Psychiatry. 1999;156(11):1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Journal of American Medical Association. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bain GT, Kornetsky C. Naloxone attenuation of the effect of cocaine on rewarding brain stimulation. Life Sciences. 1987;40(11):1119–1125. doi: 10.1016/0024-3205(87)90575-3. [DOI] [PubMed] [Google Scholar]
- Brady K, Sonne S, Randall C, Adinoff B, Malcolm R. Features of cocaine dependence with concurrent alcohol abuse. Drug and Alcohol Dependence. 1995;39:69–71. doi: 10.1016/0376-8716(95)01128-l. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Gonzalez ML, Segura J, de la Torre R. Cocaine metabolism in humans after use of alcohol. Clinical and research implications. Recent Developments in Alcoholism. 1998;14:437–455. doi: 10.1007/0-306-47148-5_22. [DOI] [PubMed] [Google Scholar]
- Carroll K, Rounsaville B, Bryant K. Alcoholism in treatment seeking cocaine abusers: clinical and prognostic significance. Journal of Studies on Alcoholism. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Archives of General Psychiatry. 2004;61(3):264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology (Berl) 1991;104(2):167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’ Brien CP. Glutamatergic agents for cocaine dependence. Annals of New York Academy of Sciences. 2003;103:1–18. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. Journal of Substance Abuse Treatment. 2001;21(3):111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. The neurobiology of addiction. In: Asbury A, Goadsby P, McArthur J, editors. Diseases of the Nervous System. Third ed. Cambridge: Cambridge University Press; 2002. pp. 431–444. [Google Scholar]
- Dackis CA, O'Brien CP. The neurobiology of alcoholism. In: Soires JC, Gershon S, editors. Handbook of Medical Psychiatry. New York: Marcel Dekker; 2003. pp. 563–580. [Google Scholar]
- Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data. Oxford: Oxford University Press; 2002. [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62(4):843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, et al. Alcohol and cocaine interactions in humans. Journal of Pharmacology and Experimental Therapeutics. 1993;266(3):1364–1373. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Subject Edition (SCID-I/P, Version 2.0) New York: New York: Biometrics Research Department New York State Psychiatric Institute; 1996. [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annual Review of Pharmacology and Toxicology. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. Journal of American Medical Association. 2005;293(13):1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. Journal of Studies on Alcohol. 2001;62(1):14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcoholism: Clinical and Experimental Research. 2006;30(5):860–865. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Hersh D, Mulgrew CL, Van Kirk J, Kranzler HR. The validity of self-reported cocaine use in two groups of cocaine abusers. Journal of Consulting and Clinical Psychology. 1999;67(1):37–42. doi: 10.1037//0022-006x.67.1.37. [DOI] [PubMed] [Google Scholar]
- Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology (Berl) 1998;139(1–2):44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Badger GJ. Alcohol dependence and simultaneous cocaine and alcohol use in cocaine-dependent patients. Journal of Addictive Diseases. 1994;13(4):177–189. doi: 10.1300/j069v13n04_06. [DOI] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, et al. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sciences. 1991;48(18):1787–1794. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- Johnson BA, O’Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, Couper D. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. Journal of Clinical Psychopharmacology. 2003;23(3):281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- Katz JL, Terry P, Witkin JM. Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene) Life Sciences. 1992;50(18):1351–1361. doi: 10.1016/0024-3205(92)90286-x. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Wiedemann K. A neuroendocrinological hypothesis on gender effects of naltrexone in relapse prevention treatment. Pharmacopsychiatry. 2005;38(4):184–186. doi: 10.1055/s-2005-871244. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129(1):15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Naloxone depresses cocaine self-administration and delays its initiation on the following day. Neuroreport. 2003;14(2):251–255. doi: 10.1097/00001756-200302100-00019. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine. 2001;345(24):1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. American Journal of Gastroenterology. 2001;96(7):2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics' self-reports of drinking behavior with reports of collateral informants. Journal of Consulting and Clinical Psychology. 1979;47(1):106–112. [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention. New York: New York: Guilford Press; 1985. [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone--a multiple-dose study. Biological Psychiatry. 1998;44(4):250–259. doi: 10.1016/s0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 1993;111(1):39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mengis MM, Maude-Griffin PM, Delucchi K, Hall SM. Alcohol use affects the outcome of treatment for cocaine abuse. American Journal on Addictions. 2002;11(3):219–227. doi: 10.1080/10550490290087992. [DOI] [PubMed] [Google Scholar]
- Miller NS, Gold MS, Belkin BM, Klahr AL. The diagnosis of alcohol and cannabis dependence in cocaine dependents and alcohol dependence in their families. British Journal of Addiction. 1989;84(12):1491–1498. doi: 10.1111/j.1360-0443.1989.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Nich C, McCance-Katz EF, Petrakis IL, Cubells JF, Rounsaville BJ, Carroll KM. Sex differences in cocaine-dependent individuals' response to disulfiram treatment. Addictive Behaviors. 2004;29(6):1123–1128. doi: 10.1016/j.addbeh.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13(1):35–39. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. Journal of Neuroscience. 2001;21(23):RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Archives of General Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, O'Connor PG. Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. Journal of Clinical Psychopharmacology. 2000;20(1):69–76. doi: 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, et al. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Archives of Internal Medicine. 2003;163(14):1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- Oncken C, Van Kirk J, Kranzler HR. Adverse effects of oral naltrexone: analysis of data from two clinical trials. Psychopharmacology. 2001;154(4):397–402. doi: 10.1007/s002130000666. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O'Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. Journal of Substance Abuse Treatment. 1999;16(2):163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Pennings EJ, Leccese AP, Wolff FA. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97(7):773–783. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sciences. 1992;51(8):553–563. doi: 10.1016/0024-3205(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Volpicelli J, Pierce J, O'Brien C. Improving naltrexone response: An intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. Journal of Addictive Diseases. 2000;19(1):71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in Type A alcohol dependence. American Journal on Addictions. 2004;13(3):236–247. doi: 10.1080/10550490490459906. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, et al. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of Studies on Alcohol. 2005 Suppl (15):170–178. doi: 10.15288/jsas.2005.s15.170. discussion 168–179. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS, Ocepek-Welikson K, Wager SS. General versus systematic inquiry about emergent clinical events with SAFTEE: implications for clinical research. Journal of Clinical Psychopharmacology. 1992;12(1):3–10. doi: 10.1097/00001573-199202000-00002. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Colby SM, Monti PM, Swift RM, Martin RA, Mueller TI, Gordon A, Eaton CA. Predictors of compliance with naltrexone among alcoholics. Alcoholism Clinical and Experimental Research. 2000;24(10):1542–1549. [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addictive Behaviors. 2001;26(2):167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J. Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. American Journal on Addictions. 2004;13(4):333–341. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Kintaudi PC, Charuvastra C, Ling W. A screening trial of amantadine as a medication for cocaine dependence. Drug and Alcohol Dependence. 2002;66:217–224. doi: 10.1016/s0376-8716(01)00205-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, Lynch KG, O'Brien CP. Gender differences in predicting treatment retention with high-dose naltrexone; Paper presented at the 67th Annual Scientific Meeting of The College on Problems of Drug Dependence; Orlando, FL. 2005. [Google Scholar]
- Volpicelli J, Alterman A, Hayashida M, Muentz L, O'Brien C. Naltrexone and the treatment of alcohol dependence. In: Reid LD, editor. Opioids, Bulimia and Alcoholism. New York: Springer-Verlag; 1990. pp. 195–214. [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, O'Brien CP. Combining medication and psychosocial treatments for addictions: The BRENDA approach. New York, NY: Guilford Press; 2001. [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. Journal of Pharmacology and Experimental Therapeutics. 1996;279(2):524–538. [PubMed] [Google Scholar]
- Wilson LD, Jeromin J, Garvey L, Dorbandt A. Cocaine, ethanol, and cocaethylene cardiotoxity in an animal model of cocaine and ethanol abuse. Academic Emergency Medicine. 2001;8(3):211–222. doi: 10.1111/j.1553-2712.2001.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wong CJ, Badger GJ, Sigmon SC, Higgins ST. Examining possible gender differences among cocaine-dependent outpatients. Expermental and Clinical Psychopharmacology. 2002;10(3):316–323. doi: 10.1037//1064-1297.10.3.316. [DOI] [PubMed] [Google Scholar]
- Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug and Alcohol Dependence. 1994;35(2):127–132. doi: 10.1016/0376-8716(94)90119-8. [DOI] [PubMed] [Google Scholar]