Abstract
As only a handful of ligands have been identified, the general nature of the ligands recognized by γδ T cells remains unresolved. In this study, soluble multimerized γδ T cell receptors (smTCRs) representing the TCRs of two γδ T cell subsets common in the mouse were used to detect and track their own ligands. Ligands for both subsets were found on resident peritoneal macrophages taken from untreated mice, and the expression of both was further induced by Listeria monocytogenes infection. Nevertheless, the two types of ligand differ from one another in abundance, in the kinetics of their induction following Listeria infection, and in their ability to be induced by in vitro culture with LPS. Surprisingly, because both are detectable on normal macrophages, these host-derived ligands are likely expressed constitutively, but are induced to higher levels of expression by stress or inflammation. In contrast to T22 and other known cell-surface ligands for γδ T cells in mice and humans, expression of these smTCR-defined ligands does not depend on β2-microglobulin, suggesting that they are not MHC class I or class I-like molecules.
Keywords: Rodent, T cells, Cell Surface Molecules, T Cell Receptors
Introduction
γδ T cells have attracted much interest because of their protective potential in malignancies and their ability to regulate immune responses and tissue repair, but little is known about how their functions are triggered. Presumably, these functions are induced via the T cell receptor (TCR), but the nature of ligands for the γδ TCR and a coherent theory of ligand recognition remain elusive.
Despite this, some ideas about ligand recognition by γδ T cells and the nature of γδ TCR ligands have gained broad acceptance. Because of structural similarities between the putative ligand-binding-sites of the γδ TCRs and the BCR (Rock et al., 1994), and because several cell-surface-expressed ligands for γδ T cells are apparently recognized as intact molecules without the need for antigen processing or presentation (Crowley et al., 2000; Groh et al., 1998; Sciammas et al., 1994; Spada et al., 2000), ligand recognition by γδ TCRs has been postulated to have features in common with that of immunoglobulin molecules. Conversely, this concept is difficult to reconcile with the observed stimulation of certain human γδ T cells by small pyrophosphate-containing compounds, such as isopentenyl pyrophosphate (IPP), because they are too small by themselves to provide TCR crosslinking. IPP has been found to accumulate in cells when the mevalonate biosynthetic pathway has been blocked by drugs, infection, or mutation, providing an explanation as to why these particular γδ T cells can be stimulated in many different ways (Bonneville and Scotet, 2006). However, recognition of IPP may more resemble antigen recognition by the αβ TCR than by immunoglobulin, because IPP is thought to be “presented” in some fashion by a larger cell surface molecule, including as a possible candidate an F1-ATPase-apolipoprotein complex which has been found on certain tumor cells and has also been implicated as a ligand (Scotet et al., 2005).
The idea that ligands for γδ TCRs are stress-inducible is also supported by much of the available evidence. Most of the currently acknowledged ligands are induced to higher levels of expression under conditions of cellular stress, including in mice the non-classical MHC class I molecules T22 and T10 (Crowley et al., 2000), and in humans the class I-like molecules MIC-A, MIC-B (Groh et al., 1998), and CD1c (Spada et al., 2000), as well as IPP (Bonneville and Scotet, 2006).
Progress has also been made in defining the γδ TCR components needed for ligand recognition. At least one ligand, the murine T22 molecule, turned out to be recognized almost exclusively by the CDR3 component of the TCR-δ chain (Adams et al., 2005; Shin et al., 2005). In fact, nearly all of the required motif could be supplied by the Dδ2 element when expressed in one of 3 possible reading frames. In contrast, no contributions from the γ chain were seen, and only one amino acid was supplied by Vδ, a residue that could alternatively be encoded by Dδ1, or by appropriately-placed N or P nucleotides in the rearranged δ gene. The interaction of this γδ TCR with its ligand contrasts markedly to that typical of αβ TCRs with MHC-peptide ligands, both in the involvement of only the CDR3 of one TCR chain, rather than a combination of variable region and CDR3 contributions from both chains, and in the tilted angle of binding that was seen (Adams et al., 2005), as opposed to the nearly parallel alignment generally observed for the αβ TCR bound to peptide/MHC complex. Further examples are needed in order to determine whether or not this is typical of γδ TCR ligand recognition. The problem remains that no method has been available that would allow for the detection and characterization of ligands for any given γδ TCR.
Towards this end, we have taken the approach of generating soluble multimeric γδ TCRs (smTCRs), which can be used as reagents to detect their own specific ligands (Aydintug et al., 2004). We demonstrated that an smTCR derived from the T22-specific γδ T cell hybridoma KN6 readily detects the cell-surface-expressed transfected T22 molecule, and showed, by staining with several other smTCRs representative of common subsets of γδ T cells in mice, that many immortalized cell lines express unknown ligands for γδ TCRs. Whether this would also be true for normal non-stressed, non-transformed cells was not clear.
We now show that normal resident peritoneal macrophages also stain with two smTCRs representative of common subsets of γδ T cells in mice, suggesting that they express at least two different ligands for γδ T cells, even in untreated mice. Because of changes in the smTCR staining patterns noted during an infection of mice with the bacterium L. monocytogenes, or following in vitro stimulation with lipopolysaccharide (LPS), our data further suggest that these ligands are induced to higher levels of expression under conditions of cellular stress. In contrast to T22 and other previously described cell-surface-expressed ligands in mice, these newly detected ligands for γδ T cells do not require expression of β2-microglobulin, which is typically associated with class I and class I-like MHC molecules.
Materials and Methods
Mice
Fully backcrossed C57BL/6 background mice homozygous for a genetically inactive TCR-δ constant region gene [B6.TCRδ−/− mice (Mombaerts et al., 1993)] were used for most of the experiments and were bred in our facility from commercially available stock (Jackson Labs, Bar Harbor, ME). Additional strains examined include B6.FcRγ−/−, B6.β2-microglobulin−/−, and C57BL/6 wildtype mice, also bred in-house from commercially available stock (Jackson Labs, Bar Harbor, ME). C57BL/6 mice for some experiments were obtained from Harlan Sprague Dawley, Inc., (Indianapolis, IN). These studies were reviewed and approved by the National Jewish Institutional Animal Care and Use Committee.
Unstimulated peritoneal macrophages
Cells from naive or Listeria-infected mice (see below) were isolated from the peritoneal cavity of untreated mice by peritoneal lavage. Briefly, ~7 ml of Hank’s BSS containing 5% heat-inactivated FBS (Atlanta Biologicals) were injected into the peritoneal cavity, and the abdomen massaged before withdrawing the BSS. The cell preparation was treated with Gey’s solution to remove any RBCs, and the cells enumerated using a Coulter counter. The cells were then placed in culture medium (O'Brien et al., 1992) containing 10% heat-inactivated FBS. To purify/enrich macrophages, the cells were next cultured at 37°C for 1–2 hours, to allow the macrophages to adhere to tissue culture flasks or plates. Any non-adherent cells were then poured off. Next, the bound macrophage-enriched population was removed from the plate by culturing them for 30 minutes at 37°C in calcium/magnesium-free PBS containing 10 mM EDTA, followed by vigorous pipetting. The cells were then placed in round-bottom 96-well tissue culture plates, stained and analyzed by flow cytometry, taking care to keep the cells on ice or refrigerated at all times during the staining to reduce adherence to the culture plate.
In vivo stimulation of macrophages
Mice were infected with Listeria monocytogenes, strain 10403S (Portnoy et al., 1988) (kind gift of Dr. Laurel Lenz, National Jewish Medical and Research Center). Fresh overnight cultures in tryptose phosphate broth were grown from frozen stock, then diluted in pyrogen-free PBS on ice, just prior to injection. An approximate dose of 2 × 104 colony-forming units was given per mouse by i.p. injection; the number of Listeria injected was verified by plating a small aliquot of the inoculum on Tryptic Soy agar plates.
In vitro stimulation of macrophages
Fresh peritoneal resident macrophages were isolated and maintained at 37°C in a 10% CO2 atmosphere in culture medium (O'Brien et al., 1992) containing 10% heat-inactivated FBS, as described above, along with LPS from Escherichia coli O55:B5 (Sigma-Aldrich Co., St. Louis, MO) at a concentration of 3 µg/ml. Tripalmitoyl-S-glycerylcysteine (Pam3Cys), a TLR2 agonist, was the kind gift of Dr. Ross Kedl, Univ. of Colorado at Denver and Health Sciences Center, and was used at a concentration of 200 ng/ml. Cells were cultured for 2–4 days in T-75 culture flasks, and removed from the flasks for staining as described above.
Preparation of spleen macrophages
Spleens were dispersed in cold BSS + 5% heat-inactivated FBS by passing the cells through a mesh screen. After letting the debris settle, this single cell suspension was spun down at 1000 rpm for 8 minutes in the cold, and the RBCs lysed with Gey’s solution. The cells were then resuspended in culture medium and transferred to T-75 culture flasks (Corning Corp., Corning, NY) using 2 flasks per spleen, and incubated for 3 hours at 37°C in a 10% CO2 atmosphere. The nonadherent cells were at this time poured off, fresh medium was added, and the cells were then again cultured overnight. Adherent cells were removed from the flasks for staining as described above.
Flow Cytometry
Cells were stained in round-bottom 96-well plates, containing 5 × 105 or fewer cells/well. Cells were pre-blocked to prevent Fc-receptor background binding by treatment with 40 µg/ml of a purified rat monoclonal antibody against mouse CD16/CD32 [clone 2.4G2 (Unkeless, 1979)] prepared in our laboratory, plus 20–40 µg/ml mouse IgG (Jackson ImmunoResearch, West Grove, PA), for 45 minutes at 4°C. Affinity-purified γδ smTCRs were generated in our laboratory after the method of Crawford et al. (Crawford et al., 2004), and used to stain cells as previously described (Aydintug et al., 2004), using a TCR concentration of 30 µg/ml. Unless otherwise indicated in the figure legends, a low-level biotinylated anti-Cδ “core” monoclonal antibody [clone 403A.10 (Itohara et al., 1989)], first tetramerized using Alexa fluor-647 streptavidin (InVitrogen Corp., Carlsbad, CA), was used to generate individual smTCRs; anti-histidine-tag monoclonal antibody (anti-HisTag, clone AD1.1.10, Serotec, Oxford, UK), and an anti-FLAG-tag monoclonal antibody (clone M2, Sigma-Aldrich Co., St. Louis, MO) were also used as core monoclonal antibodies in particular experiments as noted in the figure legends. In all experiments, core monoclonal antibodies used to generate the smTCRs were matched, except in one experiment in which the use of different core monoclonal antibodies was necessary to avoid cross reactions (shown in Fig. 5D). In experiments in which monomeric soluble TCRs were used, the preparations were spun at 16,000 g for 15–20 minutes before use, to remove any aggregated material. In most experiments, dead cells were excluded from the analyses based on staining with 7-AAD or propidium iodide. In some experiments, cells were also co-stained with rat anti-mouse F4/80-FITC [a pan-macrophage marker (Austyn and Gordon, 1981; Schaller et al., 2002); clone BM8, eBioscience, San Diego, CA], and hamster anti-mouse Cδ FITC [prepared in our own laboratory, clone GL3 (Goodman and Lefrancois, 1989)]. Unlabeled antibodies used to inhibit staining, or as a negative control, include a rat anti-mouse Vδ 6.3 [17C (Belles et al., 1996); prepared in our laboratory], and polyclonal rat IgG (Sigma-Aldrich Co., St. Louis, MO). All flow cytometry experiments shown were carried out at least twice to ensure that the results were reproducible, allowing for some differences in the intensity of staining when using different smTCR preparations. Nomenclature used for expressed Vγ genes is as in (Heilig and Tonegawa, 1986) and is in accordance with the IMGT numbering system; however, Vδ 1 is termed TRDV4 in the IMGT system and Vδ 6.3 is termed DV6D-1.
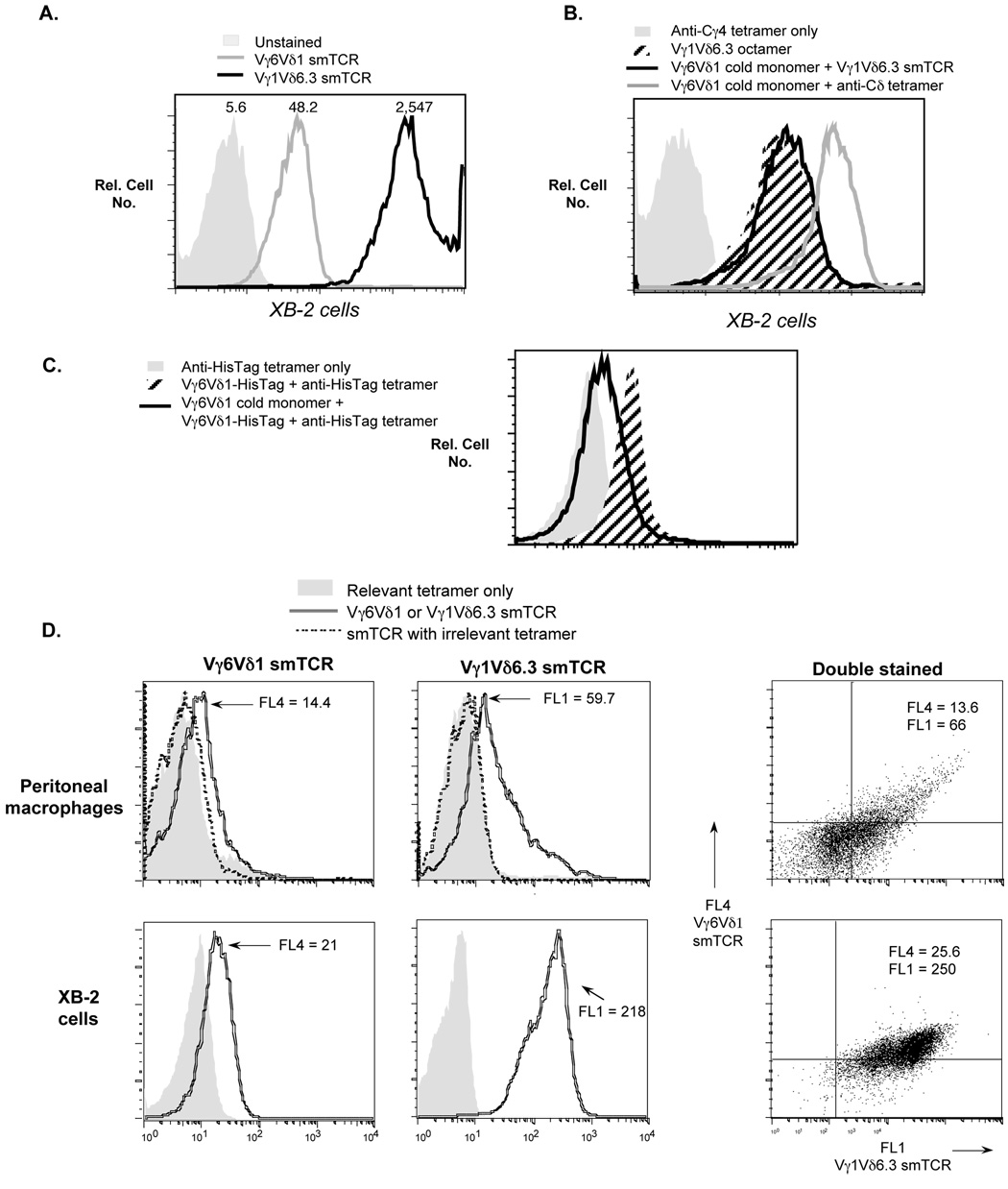
Fig. 5.
A. Differential staining of XB-2 cells with two different γδ smTCRs. The smTCRs in this experiment were prepared using histidine-tagged soluble TCRs together with an anti-histidine tag monoclonal antibody tetramer core. Mean fluorescence values obtained with each smTCR are indicated above the relevant peak. B. Pretreatment of XB-2 cells with an excess of unlabeled (cold) Vγ6Vδ1 monomer fails to block subsequent staining with labeled Vγ1Vδ6.3 smTCR. For cells pre-blocked with Vγ6Vδ1 monomer, staining with V©1Vδ6.3 smTCR is undiminished compared to that of untreated cells. Note that in this experiment, to prevent cross-recognition, the labeled Vγ1Vδ6.3 smTCR octamer was prepared using an anti-Cγ4 core monoclonal antibody [clone 2.11 (Pereira and Boucontet, 2004)], which cannot recognize the Vγ6Vδ1 TCR. The ability of the “cold” Vγ6Vδ1 monomer to bind to the XB-2 cells was verified by showing that cells incubated with the Vγ6Vδ1 monomer only can be detected with a labeled anti-Cδ tetramer secondary reagent [clone GL3 (Goodman and Lefrancois, 1988)]. C. Control experiment verifying that a soluble TCR can block itself. Compared to untreated cells, pre-treatment of XB-2 cells with cold Vγ6Vδ1 monomer substantially reduces their subsequent ability to stain with Vγ6Vδ1 HisTag monomer, whose binding is detected using anti-HisTag tetramer as a secondary reagent. D. Double staining of macrophages (top row) and XB-2 cells (bottom row) with differentially labeled Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs. The smTCRs for this experiment were prepared with core monoclonal antibodies that recognize only the bound TCR: an anti-Cγ4 monoclonal antibody for the Vγ1Vδ6.3 smTCR (Pereira and Boucontet, 2004), and an anti-histidine tag monoclonal antibody for the Vγ6Vδ1 smTCR (which carries a Cδ-terminal histidine tag not present on this Vγ1Vδ6.3 TCR). Mean fluorescence values obtained for each peak are indicated (shown as FL4 for the Vγ6Vδ1 smTCR and FL1 for the Vγ1Vδ6.3 smTCR). The smTCRs when used to double-stain either cell type (right panel) are as bright in mean fluorescence as when used to single-stain (left two panels), indicating that they do not cross-block one another during double-staining.
Proliferation Assays
Vγ6/Vδ1 [line 134, (Sim et al., 1995)] transgenic spleen cells were prepared and passed over nylon wool to enrich for T cells, then were treated with a biotinylated anti-Cβ monoclonal antibody (Kubo et al., 1989), washed, incubated with streptavidin-conjugated magnetic beads, and then passed over a MACS-streptavidin bead column (Miltenyi Biotec, Gladbach, Germany) to remove most of the αβ TCR-bearing cells. The nonadherent cells were collected and placed on a 96-well plate at 2 × 104 T cells/well. Irradiated XB-2 cells were added at various effector:target (E:T) ratios, using triplicate wells for each condition, and the plate incubated for 3 days, after which 1 µCi/well of 3H-thymidine was added to each well. After an additional 18-hour incubation, wells were harvested and counts per well determined. The stimulation index was calculated by dividing the cpm obtained per well by the average obtained from triplicate wells containing the T cells only. As a control, the same experiment was carried out using αβ T cells together with XB-2 cells. The αβ T cells were enriched from the spleen of a B6.TCRδ−/− mouse, using nylon wool purification.
Competitive soluble TCR binding
XB-2 keratinocytes, 3 × 105, were first pre-incubated for 45 minutes at 4°C with unlabeled Vγ6/Vδ1 monomeric soluble TCR at a concentration of 1.2 mg/ml. Following a single wash in staining buffer, the cells were then stained with labeled Vγ1/Vδ6.3 smTCR having an anti-Cγ4 monoclonal antibody core [clone 2.11 (Pereira and Boucontet, 2004; Pereira et al., 1995)] at a concentration of 16 µg/ml for the TCR. Staining with smTCR only was carried out as previously described (Aydintug et al., 2004). To verify binding of the unlabeled Vγ6/Vδ1 monomer, XB-2 cells were incubated with unlabeled Vγ6/Vδ1 soluble TCR monomer at 200 µg/ml, which was subsequently detected by using labeled tetrameric anti-Cδ monoclonal antibody [clone 403A.10 (Itohara et al., 1989)] as a secondary reagent. As a control experiment to demonstrate that preincubation with Vγ6Vδ1 monomer can block itself, pretreatment of XB-2 cells with unlabeled Vγ6Vδ1 soluble TCR monomer was carried for 45 minutes at 4°C using a concentration of 800 µg/ml, followed by a single wash, and then by staining with soluble Vγ6Vδ1-HisTag monomer at a concentration of 125 µg/ml. Binding of Vγ6Vδ1-HisTag was detected with labeled tetrameric anti-HisTag monoclonal antibody as a secondary reagent.
Results
Normal peritoneal macrophages express ligands for at least two common γδTCRs
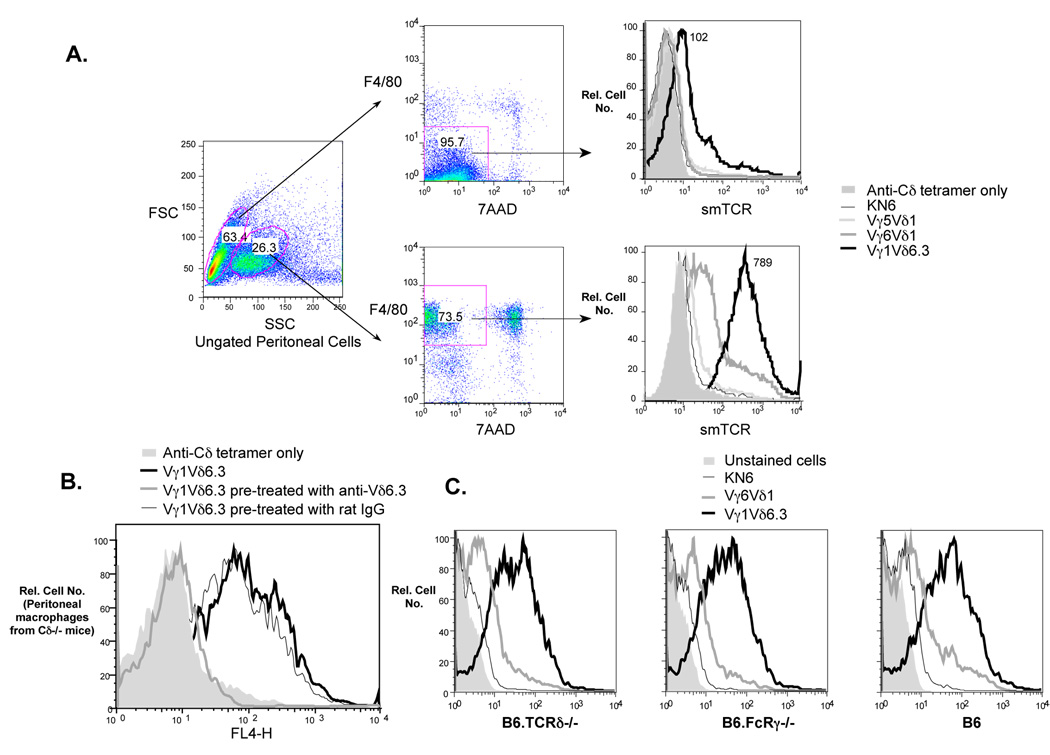
Previously, we reported that two unrelated γδ TCRs of mice, a Vγ1Vδ6.3 TCR and the invariant Vγ6Vδ1 TCR, stained many of the same mouse cell lines, most of which were derived from epithelial cells, and that multimeric versions of these TCRs (smTCRs) gave superior staining compared to monomeric soluble TCRs (Aydintug et al., 2004). We have found that staining of these cell lines with the Vγ1Vδ6.3 smTCR is consistently brighter than that obtained with the Vγ6Vδ1 smTCR, although in a previous study (Aydintug et al., 2004), we underestimated the degree of staining with the Vγ1Vδ6.3 TCR due to use of either monomeric TCR, which stains poorly for this TCR, or of a different anti-Cδ core monoclonal antibody to generate the smTCR, which gave higher backgrounds than the anti-Cδ core monoclonal antibody used in this study. We speculated that the expression of multiple γδ TCR ligands might be a consequence of the transformed nature of immortal cell lines, and we thus went on to see whether ligands for the same TCRs could be detected on normal mouse cells as well. Although no substantial staining was detected on suspensions of spleen or liver cells (data not shown), a large portion of normal resident peritoneal cells was found to stain well with both the Vγ1Vδ6.3 and Vγ6Vδ1 smTCRs. Resident peritoneal cells include both macrophages, which stain with the monoclonal antibody F4/80 that detects a pan-macrophage marker (Austyn and Gordon, 1981), and B and T lymphocytes, which do not, and the two populations are also largely distinguishable by differences in their forward/side scatter properties (Fig. 1A). The F4/80 positive population, much like the epithelial cell lines we previously studied, stained brightly with the Vγ1Vδ6.3 smTCR, and weakly but clearly with the Vγ6Vδ1 smTCR. In contrast, a γδ smTCR derived from the hybridoma KN6 (Ito et al., 1990), which recognizes the nonclassical MHC class I molecule T22 (Crowley et al., 2000), showed no detectable staining for the F4/80 positive population, and with the invariant Vγ5Vδ1 smTCR, which is closely related to the Vγ6Vδ1 TCR and carries an identical δ chain, it was also essentially negative (Fig. 1A). In contrast, the F4/80 negative population showed no detectable staining with the Vγ6Vδ1, the Vγ5Vδ1, or the KN6-derived smTCRs, although these cells showed a small but reproducible shift when stained with the Vγ1Vδ6.3 smTCR (Fig. 1A), suggesting low level expression of a ligand for this TCR on lymphocytes.
Fig. 1.
A. Freshly isolated peritoneal cells from B6.TCRδ−/− mice: two main peritoneal cell types can be distinguished by forward/side scatter properties (left panel), which also stain differentially with F4/80 (middle panel), a pan macrophage marker. All F4/80-positive cells stain brightly with the Vγ1/Vδ6.3 smTCR, and whereas most stain weakly with the Vγ6/Vδ1 smTCR, a small percentage of the macrophages stain brightly with this smTCR as well. The F4/80-negative peritoneal cells (largely lymphocytes) did not stain with the Vγ6/Vδ1 smTCR, but stained weakly with Vγ1/Vδ6.3 smTCR (mean fluorescence values obtained with this TCR are indicated, right panel). Neither the F4/80-positive or negative population stained appreciably with either the KN6-derived or Vγ5Vδ1 canonical smTCR. Note that staining here was carried out with double the concentration of smTCR used in the other experiments in this study, in an attempt to reveal any weak staining. B. Pretreatment of the Vγ1Vδ6.3 smTCR with a monoclonal antibody specific for Vδ6.3, but not with rat IgG, inhibits its ability to stain peritoneal macrophages. C. Peritoneal macrophages from both B6.FcRγ−/− and C57BL/6 (B6) wildtype mice show staining profiles with the Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs similar to those of B6.TCRδ−/− peritoneal macrophages.
Specificity of the soluble TCR staining
We previously found that staining of cell lines with soluble γδ TCRs could be inhibited by pre-treatment of the soluble TCRs with relevant, but not irrelevant, anti-V region specific monoclonal antibodies, a result that is consistent with binding of the soluble TCR via specific recognition of a ligand through the variable portion of the molecule. We therefore attempted to verify the specificity of binding of the Vγ1Vδ6.3 smTCR to peritoneal macrophages in a similar way. As shown in Fig. 1B, pretreatment of the Vγ1Vδ6.3 smTCR with an anti-Vδ6.3 monoclonal antibody greatly reduced its ability to bind macrophages, whereas pretreatment with nonspecific rat IgG had no effect. This result implies that the Vγ1Vδ6.3 smTCR binds specifically to peritoneal macrophages. Since peritoneal macrophages stain only weakly with the Vγ6Vδ1 smTCR, and because the one available monoclonal antibody which specifically recognizes the Vγ6Vδ1 TCR is a low affinity IgM antibody (Roark et al., 2004), we were not able to carry out a similar experiment for the Vγ6Vδ1 smTCR. Because these γδ smTCRs contain monoclonal antibody “cores,” there was still a possibility that the Vγ1Vδ6.3 and Vγ6Vδ1 smTCRs might be binding nonspecifically via Fc receptors on the macrophages, even though the KN6-derived smTCR and Vγ5Vδ1 smTCR which contain the same core failed to bind. Although we attempted to block any potential Fc receptor binding by pretreatment with unlabeled monoclonal antibody 2.4G2 and mouse IgG in all staining experiments shown in this study, potential background from FcγRI, which is not specifically recognized by monoclonal antibody 2.4G2, was still possible. We therefore attempted to stain, using the same γδ smTCRs, peritoneal macrophages from B6.FcRγ−/− mice, which lack all activating FcγRs, including FcγRI (Takai et al., 1994). Although these mice can potentially express the inhibitory receptor FcγRII, monoclonal antibody 2.4G2 was again used in this experiment to preblock the cells, which specifically recognizes FcγRII as well as activating receptor FcγRIII (Araujo-Jorge et al., 1993; Takai et al., 1994). As shown in Fig. 1C, the staining profile of FcRγ−/− peritoneal macrophages was similar to that of B6.TCRδ−/− peritoneal macrophages, indicating that the binding of the Vγ1Vδ6.3 and Vγ6Vδ1 smTCRs to peritoneal macrophages is not the result of nonspecific binding via FcRs.
In addition to other lymphocytes, the F4/80 negative population potentially contains γδ T cells, which might be able to bind soluble γδ TCR multimers via the core anti-Cδ monoclonal antibody used to multimerize them. Because they are a rare T cell type (about 3% of the lymphoid T cells in C57BL/6 mice) compared to the large peritoneal macrophage populations that stained with both Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs, the presence of γδ T cells cannot explain the staining seen among F4/80 positive cells. Many experiments shown in this study (including Fig. 1A and B) were carried out using cells from B6.TCRδ−/− mice, to prevent this possibility. However, peritoneal macrophages derived from wildtype C57BL/6 mice showed a very similar staining pattern to those from B6.TCRδ−/− mice (right panel, Fig. 1C).
Levels of ligand for both the Vγ6Vδ1 and Vγ1Vδ6.3 TCRs are higher on macrophages from Listeria-infected mice
Responses by both Vγ1Vδ6.3+ and Vγ6Vδ1+ γδ T cells have been previously reported in mice infected with Listeria monocytogenes (Belles et al., 1996; Dalton et al., 2003; O'Brien et al., 2000; Roark et al., 1996). Therefore, we tested whether the ligands on macrophages for either of these two γδ TCRs were affected by infection of the mice with Listeria. We found that staining with the Vγ1Vδ6.3 smTCR was stronger overall on macrophages of mice infected intraperitoneally with Listeria. As well, a small population that stained brightly with the Vγ6Vδ1 smTCR was induced on Listeria-infected macrophages. We next examined the expression of these ligands for the γδ TCRs over a timecourse during Listeria infection (Fig. 2). Staining of macrophages with the Vγ1Vδ6.3 smTCR rose gradually over time, increasing from about 1.4 times above the uninfected level at day 4 to more than 2.5 times above the uninfected level by day 8 of the infection, based on mean fluorescence values. The level of Vγ1Vδ6.3 smTCR staining was still comparably high on days 8 and 10 (not shown) following infection, although we have not followed the expression further than this. In contrast, staining with the Vγ6Vδ1 smTCR decreased over time, such that the mean fluorescence of the peak of bright cells was clearly reduced by day 8. By day 10, staining of a Vγ6Vδ1-bright peak was even lower, nearly down to the background level in uninfected macrophages (not shown). In contrast, the KN6-derived smTCR, which generally does not stain uninfected macrophages, remained essentially unchanged in macrophages from Listeria-infected mice, as was staining with the Vγ5Vδ1 smTCR (not shown). Thus, it appears that ligands for both Vγ1Vδ6.3+ and Vγ6Vδ1+ γδ T cells are indeed induced on macrophages following Listeria infection, but to different degrees and with different kinetics, when compared with one another. In contrast, Listeria infection under the conditions used does not appear to induce ligands for the KN6-derived or the Vγ5Vδ1 canonical TCR.
Fig. 2.
Time course of ligand expression on peritoneal macrophages. B6.TCRδ−/− mice were injected intraperitoneally with Listeria monocytogenes (2 × 104 CFU). The mice were sacrificed postinfection after the number of days indicated under each profile, for analysis. Freshly isolated peritoneal macrophages were identified by F4/80 staining, and any dead/dying cells in this population (propidium iodide-positive) were excluded. Mean fluorescence values for the entire peak stained with Vγ1Vδ6.3 smTCR are indicated above the peak, and above the indicated gate for cells stained with the Vγ6Vδ1 smTCR. Note that unstained and KN6 smTCR stained cells are shown as the negative control, rather than anti-Cδ tetramer, because the peritoneal cells from infected mice often show high background staining with anti-Cδ tetramer.
Stimulation of macrophages in vitro can also induce γδ TCR ligands
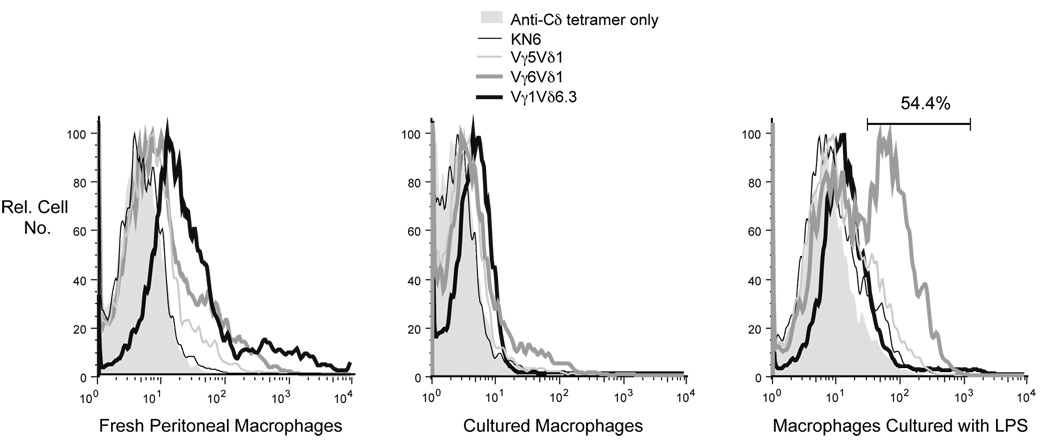
During the course of a peritoneal Listeria infection, neutrophils and macrophages are recruited to the site of infection, whereas many of the resident macrophages disappear (Barth et al., 1995; Skeen et al., 2004). Therefore, the changes we saw in expression of ligands for the Vγ6Vδ1 and Vγ1Vδ6.3 TCRs during Listeria infection could reflect differential expression of ligands on newly recruited macrophages, rather than a change in ligand expression following activation of resident macrophages present before infection. We therefore attempted to stimulate resident peritoneal macrophages in vitro, by culture in medium containing LPS, to see whether levels of ligands for either of these two TCRs increase. As shown in Fig. 3, after 4 days of culture in LPS, staining of the cells with the Vγ6Vδ1 smTCR was greatly enhanced, such that over 50% of the F4/80-positive cells now stained with this reagent. This change was not yet evident after 2 days of culture with LPS (data not shown). In contrast, staining with the Vγ1Vδ6.3 smTCR was not induced by this treatment at either timepoint. Culture of peritoneal macrophages with the synthetic lipopeptide TLR2 agonist Pam3Cys also induced a ligand for the Vγ6Vδ1 but not the Vγ1Vδ6.3 smTCR, although fewer cells were induced (data not shown), implying that stimulation through particular Toll-like receptors, including TLR4 and TLR2, is sufficient for induction of this ligand. Macrophages cultured alone, without LPS, showed a decrease in the expression of ligands for all smTCRs tested, compared to freshly isolated peritoneal macrophages (except for the KN6-derived smTCR, which stained neither fresh nor cultured macrophages), and perhaps expression of other cell surface proteins as well, since nonspecific background staining with the core monoclonal antibody was also evidently reduced.
Fig. 3.
Macrophages were isolated from the peritoneum of B6.TCRδ−/− mice. Staining results with these cells obtained on the day of isolation are shown (left panel), and compared to results obtained following in vitro culture. Macrophages cultured for 4 days without LPS express no or only very low levels of ligands for any of the smTCRs tested (middle panel). However, following 4 days of in vitro activation with LPS (right panel), the ligand for the Vγ6Vδ1 smTCR is strongly induced on over half of the cells. Ligands for the KN6-derived, the Vγ5Vδ1, and the Vγ1Vδ6.3 smTCRs in contrast show no induction.
Evidence that the molecule detected by staining with an smTCR is actually a ligand
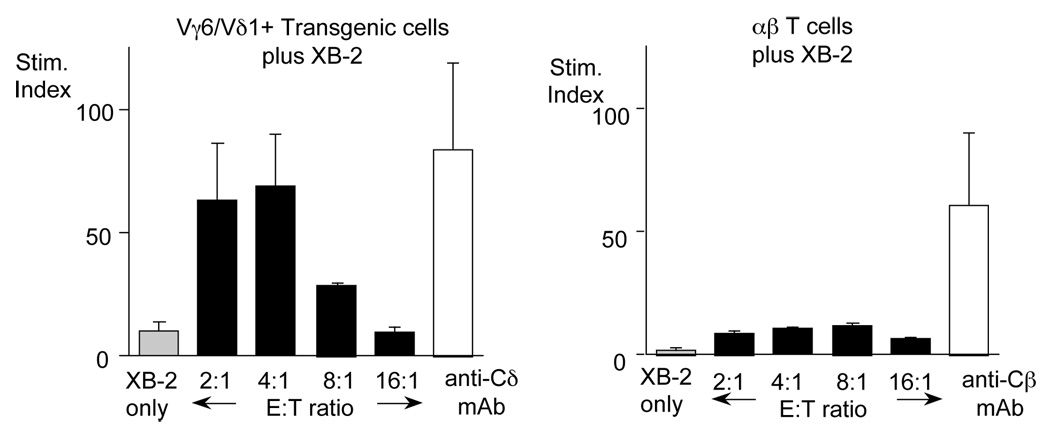
In a previous study (Aydintug et al., 2004), we showed that as for peritoneal macrophages, XB-2 cells, a keratinocyte-derived cell line, also stain with both the Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs. This was somewhat surprising, because Vγ5Vδ1+ γδ T cells, but not Vγ6Vδ1+ or Vγ1Vδ6.3+ γδ T cells, are normally found in the skin in close association with keratinocytes (Jameson et al., 2004). If the molecules these smTCR detect on XB-2 are actually ligands, they should be capable of stimulating the relevant γδ T cells. To test whether XB-2 cells can stimulate Vγ6Vδ1+ T cells, we cultured XB-2 together with Vγ6Vδ1-purified cells from a TCR-transgenic mouse strain. As shown in Fig. 4, a response to XB-2 by Vγ6Vδ1 γδ T cells was evident, whereas αβ T cells did not respond. This supports the interpretation that the molecule detected on XB-2 cells by the Vγ6Vδ1 soluble TCR is in fact a ligand. We were unable to similarly verify the presence of a ligand for the Vγ1Vδ6.3 soluble TCR due to a lack of appropriate Vγ1Vδ6.3 transgenic mice to provide a clonally identical T cell population.
Fig. 4.
Irradiated XB-2 cells were cultured with either Vγ6Vδ1 transgenic T cells (left) or αβ T cells (right), in the presence of tritiated thymidine, at the effector:target ratios indicated. The white bar shows as a positive control the response of the T cells when incubated in a well pre-coated with the appropriate anti-TCR monoclonal antibody.
Evidence that the ligands detected by different soluble γδ TCRs are distinct molecules
Because they are affected differentially following a Listeria infection or after culture with LPS, the molecules that macrophages express which bind to the Vγ6Vδ1 and the Vγ1Vδ6.3 smTCR appear to be distinct moieties. To test whether the molecules detected on cells by these two soluble TCRs are in fact different from one another, we carried out experiments with the XB-2 cell line rather than macrophages, due to the variable background staining characteristics of macrophages, which fluctuate considerably among different preparations; the XB-2 line in contrast has reproducibly low background staining. We previously found that the Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs stain XB-2 cells differentially, and as shown in Fig. 5A, although variations are seen among different smTCR preparations, the Vγ1Vδ6.3 smTCR consistently stains them more brightly even when the smTCRs are generated with a different core monoclonal antibody, suggesting that the ligands for these TCRs are indeed distinct. To more rigorously assess whether these ligands are different molecules, we tested whether it was possible to block the staining of a soluble TCR of one type by pre-treating the cells to be stained with unlabeled soluble TCR of another type. Specifically, we pretreated XB-2 cells with an excess of unlabeled Vγ6Vδ1 monomer, and then compared them with untreated cells for their ability to bind a labeled Vγ1Vδ6.3 smTCR. Importantly, in this experiment shown in Fig. 5B, the monoclonal antibody core used to generate the Vγ1Vδ6.3 smTCR is specific for Vγ1Jγ4Cγ4, and is thought to recognize Cγ4, the Cγ uniquely associated with Vγ1 but not other Vγs (Pereira and Boucontet, 2004). Thus, the core monoclonal antibody cannot cross-react with pre-bound Vγ6Vδ1 monomer (although the need to use a different core monoclonal antibody for the smTCR in this experiment resulted in somewhat weaker staining) The staining of the XB-2 cells with the labeled Vγ1Vδ6.3 smTCR was undiminished by pretreatment with “cold” Vγ6Vδ1 monomer, even when using as much as a 75-fold excess of the monomer. The ability of the Vγ6Vδ1 monomer to bind to XB-2 cells was confirmed by the staining monomer-treated cells subsequently with a labeled secondary reagent. This result implies that these two γδ TCRs are binding to distinct ligands on XB-2. We were unable to carry out a reciprocal experiment using monomeric Vγ1Vδ6.3 soluble TCR to block staining of the Vγ6Vδ1 smTCR, because the Vγ1Vδ6.3 TCR stains very poorly in monomeric form. However, to validate the experimental method used here, we verified that pretreatment of XB-2 cells with unlabeled Vγ6Vδ1 soluble TCR monomer does effectively block subsequent staining of the cells by a histidine-tagged version of the same TCR (Fig. 5C).
As an additional test, we also compared the binding of these two different smTCRs at the same time. As shown in Fig. 5D, this was accomplished by double-staining macrophages and XB-2 cells with differentially labeled smTCRs prepared with core monoclonal antibodies that cannot cross-react with the other TCR. The two left panels show single staining of XB-2 cells with either the Vγ6Vδ1 smTCR or Vγ1Vδ6.3 smTCR. For the peritoneal macrophages (top row), the single staining is shown next to a specificity control verifying that the core monoclonal antibody used to generate each multimer does not detect the irrelevant TCR. XB-2 cells double-stained with both smTCRs, added at the same time, are shown in the right panel. Clearly, as shown by the mean fluorescence values, each smTCR stains with equivalent brightness whether used to double-stain or single-stain the cells. If the smTCRs recognized the same molecule, when added together for the double staining, they would be expected to stain with about half of the intensity obtained when each was used alone, but no decrease was seen. Therefore, several lines of evidence indicate that the ligands detected by the Vγ1Vδ6.3 TCR and the Vγ6Vδ1 TCR are distinct molecules.
The Vγ1Vδ6.3 and Vγ6Vδ1 ligands do not appear to be MHC class I or class I-like molecules
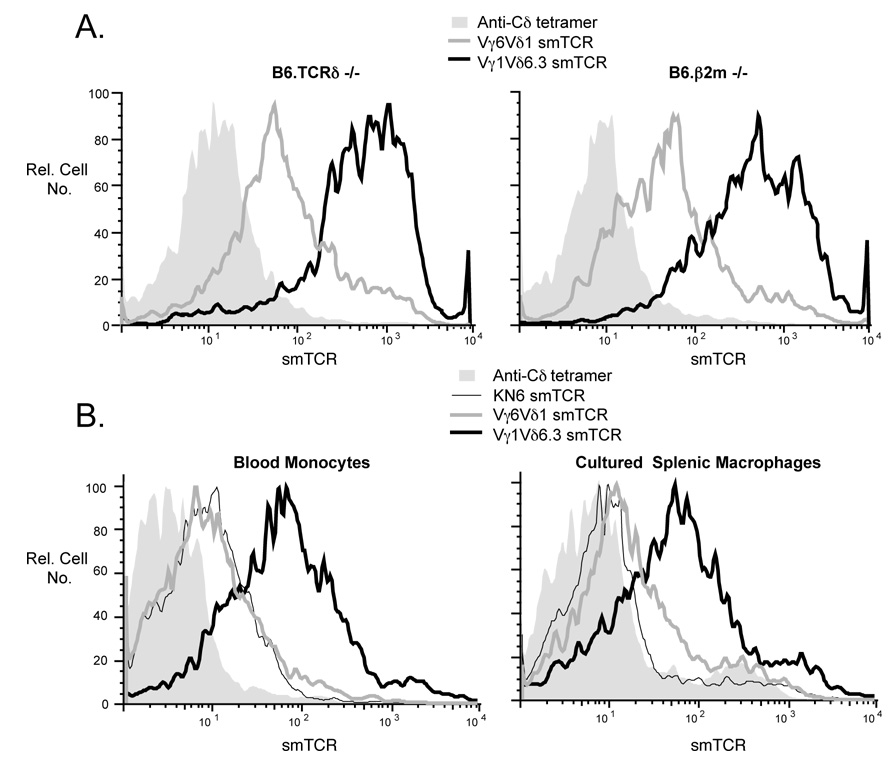
In both mice and humans, among the few molecules that have been identified thus far as ligands for known γδ TCRs, several are MHC class I or class I-like molecules associated with β2-microglobulin (Crowley et al., 2000; Groh et al., 1998; Spada et al., 2000). Therefore, the possibility that the molecules being detected by the Vγ1Vδ6.3 and Vγ6Vδ1 smTCRs are class I or class I-like was examined by testing whether macrophages from β2-microglobulin−/− mice also stained with these reagents. As can be seen in Fig. 6A, peritoneal macrophages from these mice gave staining patterns very similar to those from B6.TCRδ−/− mice, which are wildtype for β2-microglobulin. Consistently, in other experiments, we found that macrophages from B6.TAP1−/− mice produce staining patterns with these two smTCRs that are very similar to those of macrophages from B6.TCRδ−/− or C57BL/6 wildtype mice (data not shown). Thus, the ligands recognized by the Vγ1Vδ6.3 and Vγ6Vδ1 soluble TCRs are not likely to represent class I or class I-like molecules.
Fig. 6.
A. Freshly isolated peritoneal macrophages from B6.TCRδ−/− vs. B6.β2m−/− mice were stained with either the Vγ1Vδ6.3 or Vγ6Vδ1 smTCR. Macrophages, first identified by staining with F4/80, were then analyzed for staining with each smTCR, as shown. B. Freshly isolated blood monocytes from B6.TCRδ−/− mice were identified by F4/80 staining and any dead cells were excluded by propidium iodide staining; this population was then examined for co-staining with the indicated smTCR. For splenic macrophages, plastic-adherent spleen cells from C57BL/6 mice were cultured overnight before staining. F4/80 positive, propidium iodide negative cells were then analyzed for co-staining with smTCRs as indicated.
Macrophages in other sites also stain with the Vγ1Vδ6.3 and Vγ6Vδ1 smTCRs
To test whether the expression of ligands for the Vγ1Vδ6.3 and V©6Vδ1 TCRs is an unusual property of peritoneal macrophages, or instead is common among macrophages in general, we tested both blood monocytes and macrophages derived from spleen for their ability to stain with these smTCRs. To enrich the splenic macrophages enough for analysis, we first cultured splenic plastic-adherent cells overnight before staining, because fresh ex vivo splenic macrophages express low levels of F4/80 and also require enrichment. We found that the Vγ1Vδ6.3 smTCR also stained both blood monocytes and cultured splenic macrophages. Staining with the Vγ6Vδ1 smTCR was weak but also detectable on cultured splenic macrophages and blood monocytes. With the KN6-derived smTCR, staining varied, and was as strong as that of the Vγ6Vδ1 smTCR on blood monocytes, but lower than Vγ6Vδ1 on cultured splenic macrophages (Fig. 5B). It therefore appears that strong expression of a Vγ1Vδ6.3 ligand, and weak expression of a Vγ6Vδ1 ligand, are common properties of macrophages in general, even before they exit the blood for the tissues.
Discussion
In a previous study, we showed that soluble forms of γδ TCRs can be used as reagents to detect their own ligands, using as a control the γδ TCR from the hybridoma KN6, whose ligand, T22, had already been identified. Although the monomeric form of this soluble TCR was sufficient for ligand-detection, a multimerized form provided brighter staining with less reagent. Using soluble multimerized forms of other γδ TCRs (smTCRs) representative of several of the major γδ T cell subsets in mice, we then went on to show that a variety of transformed cell lines express ligands for γδ T cells as defined by smTCR-staining, and often several different ones simultaneously. Because these ligands were protease-sensitive, they appear to be protein or protein-associated molecules expressed on the cell surface (Aydintug et al., 2004). These first experiments established cell-staining with smTCRs as a viable approach for the systematic detection and discovery of new γδ T cell-ligands. They also raised the question of whether such endogenous ligands can be expressed in unstimulated normal cells or are only induced as a consequence of transformation or stress.
In this study, we have addressed this question, and have extended our findings to show that normal cells, in this case peritoneal macrophages identified by their scatter-profile and expression of F4/80, also express ligands for at least two common γδ TCRs in mice: the invariant Vγ6Vδ1 TCR and a representative Vγ1Vδ6.3 TCR. This staining revealed co-expression of these two smTCR-detected ligands, indicating that the expression of multiple ligands for certain γδ TCRs in cells which have not been stressed or triggered by inflammation is likely a common property rather than a peculiarity of immortal or transformed cell lines. We also showed that the levels of ligands for these two TCRs are increased on macrophages from Listeria-infected mice, and that expression of the Vγ6Vδ1 ligand is increased on peritoneal macrophages following in vitro culture with LPS. These data therefore suggest that host-derived endogenous ligands for γδ T cells, already expressed under steady state conditions, can be induced to higher levels of expression during the course of an infection. However, it must be kept in mind that, because their molecular structure remains unknown, the smTCR-detected ligands on the cell populations that express them may instead vary in form under different conditions. That the same ligands are expressed at different levels seems more likely, especially in the case of the ligand detected by the Vγ6Vδ1 smTCR, because it is naturally invariant and probably recognizes an equally invariant ligand.
This study also provides evidence that the ligands detected by two smTCRs are different molecules, because they are distinct in abundance and expression kinetics in normal cells, and because of the absence of crossblocking between the smTCRs. Finally, neither smTCR-defined ligand appears to be an MHC class I or a class I-like molecule, because nearly all such molecules require β2-microglobulin to form a stable structure on the cell surface (Rodgers and Cook, 2005). This was a somewhat surprising finding, since most of the known ligands for both mouse and human γδ TCRs are nonclassical class I or class I-like molecules. Because the TCRs we focused on in these experiments represent common γδ TCR subsets, this finding may indicate that many γδ TCR ligands, perhaps even the majority, are not MHC class I-like molecules.
We and others have previously noted in vivo responses of γδ T cells bearing both Vγ6Vδ1 and Vγ1, particularly Vγ1Vδ6.3+ cells, during Listeria infection (Belles et al., 1996; Dalton et al., 2003; O'Brien et al., 2000; Roark et al., 1996). Our discovery here that ligands for both Vγ6Vδ1 and Vγ1Vδ6.3 TCRs are induced on peritoneal macrophages during Listeria infection fits well with these earlier findings. Additionally, in vivo evidence has been published that Vγ6Vδ1+ γδ T cells, expressing the canonical TCR, respond during a peritoneal E. coli infection (Mokuno et al., 2000), and that the response is LPS-dependent, whereas Vγ1+ γδ T cells do not appear to respond in this system. Our finding in this study that, despite strong induction of the Vγ6Vδ1 ligand, the Vγ1Vδ6.3 ligand does not appear to be induced by in vitro culture in LPS, further suggests that this differential subset-specific response is in fact driven by the peripherally induced expression of the Vγ6Vδ1 ligand. We were, however, surprised to find that ligands for both the Vγ6Vδ1 and Vγ1Vδ6.3 TCRs are generally detectable on peritoneal macrophages freshly isolated from untreated mice. Although the cell preparation procedure could potentially induce ligands for γδ T cells, it seems more likely that the peritoneal macrophages express these ligands due to some degree of continuous “background” stimulation. Indeed, culturing macrophages in medium only, without any deliberate inflammatory stimuli, leads to decreased staining with both the Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs, perhaps due to the lack of normal background stimulation (Fig. 3). Interestingly, established macrophage cell lines differ in their ability to stain with these soluble γδ TCRs. Specifically, J774 cells stained weakly with the Vγ1Vδ6.3 smTCR but not with the Vγ6Vδ1, Vγ5Vγ1, or KN6-derived TCR smTCRs, whereas RAW-264 cells stained with both the Vγ6Vδ1 and the Vγ1Vδ6.3 smTCR, but only very weakly. In contrast, the monocyte line WEHI-3.5 showed no staining with any of these smTCRs (data not shown). Although the lack of staining of these cells lines might be taken to suggest that macrophages require some sort of stimulation to express ligands for the Vγ6Vδ1 and Vγ1Vδ6.3 TCRs, this does not appear to be so at least for the Vγ1Vδ6.3 TCR, because F4/80 monocytes derived from peripheral blood stained well with this smTCR (Fig. 5B).
Although induction of both smTCR-defined ligands was evident by day 4 of Listeria infection, a difference was seen in the duration of the induced expression levels, with faster kinetics for the ligand for the Vγ6Vδ1 TCR. The kinetics of the response of Vγ6Vδ1+ cells following Listeria infection depends to some degree on the dose given, but was at its highest in liver and spleen at day 5 for a fairly heavy systemic infection (Roark et al., 1996). In contrast, an early and a late biphasic response of Vγ1Vδ6.3 cells during Listeria infection has been previously noted (Belles et al., 1996). Therefore, there appears to be a positive correlation between the expression of the smTCR-defined ligands and the cellular responses of the corresponding γδ T cell subsets, with ligand-expression preceding the cellular responses. Although we have yet to show that the molecules detected on macrophages by these γδ smTCRs are functional ligands, this correlation suggests that they are indeed ligands which act to stimulate γδ T reactivity in vivo.
Our finding that macrophages deficient in β2-microglobulin stain with both the Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs equivalently to macrophages from wildtype mice strongly suggests that the ligands these TCRs recognize are not MHC class I or class I-like molecules. This was unexpected because several class I-type molecules have previously been shown to act as ligands for both mouse and human γδ TCRs, including MICA (Groh et al., 1998), T10 and/or T22 (Shin et al., 2005), and CD1c (Spada et al., 2000). This may indicate that class I molecules are not typically γδ TCR ligands, and indeed, one potential ligand described recently for human Vγ9Vδ2+ cells is neither class I nor class II-like (Scotet et al., 2005). Because γδ TCRs do not generally recognize MHC class II molecules either, this would represent a major difference between αβ and γδ T cells. However, nearly all of the ligands thus far described for γδ TCRs are inducible by stress or inflammation, including natural “phosphoantigens” for human Vγ9Vδ2+ cells (Bonneville and Scotet, 2006), a characteristic shared by the ligands we detected in this study using the mouse Vγ6Vδ1 and Vγ1Vδ6.3 smTCRs. A better understanding of these ligands awaits their structural characterization, but this study shows that even without knowing their structure, a considerable amount of information can be gained by detecting and tracking the ligands with soluble γδ TCR multimers.
Acknowledgments
We thank Fran Crawford and John Kappler for advice in growing and preparing smTCRs, and Molly Taylor for technical assistance. This work was supported by NIH grants R01 AI044920 and R21 AI063400 to R.L.O., and NIH grant R01 HL65410 to W.K.B.
Abbreviations used in this paper
- smTCR
soluble multimeric T cell receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no financial conflict of interest.
References
- Adams EJ, Chien YH, Garcia KC. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:209–210. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- Araujo-Jorge T, Rivera MT, el Bouhdidi A, Daeron M, Carlier Y. An FcγRII-, FcγRIII-specific monoclonal antibody (2.4G2) decreases acute Trypanosoma cruzi infection in mice. Infect. & Immun. 1993;61:4925–4928. doi: 10.1128/iai.61.11.4925-4928.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Aydintug MK, Roark CL, Yin X, Wands JM, Born WK, O'Brien RL. Detection of cell surface ligands for the γδ TCR using soluble TCRs. J. Immunol. 2004;172:4167–4175. doi: 10.4049/jimmunol.172.7.4167. [DOI] [PubMed] [Google Scholar]
- Barth MW, Hendrzak JA, Meinicoff MJ, Morahan PS. Review of the macrophage disappearance reaction. J. Leuk. Biol. 1995;57:361–367. doi: 10.1002/jlb.57.3.361. [DOI] [PubMed] [Google Scholar]
- Belles C, Kuhl AL, Donoghue AJ, Sano Y, O'Brien RL, Born W, Bottomly K, Carding SR. Bias in the γδ T cell response to Listeria monocytogenes. Vδ6.3+ cells are a major component of the γδ T cell response to Listeria monocytogenes. J. Immunol. 1996;156:4280–4289. [PubMed] [Google Scholar]
- Bonneville M, Scotet E. Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006;18:1–18. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Crawford F, Huseby E, White J, Marrack P, Kappler JW. Mimotopes for alloreactive and conventional T cells in a peptide/MHC display library. Pub. Libr. Sci. Biol. 2004;2:523–533. doi: 10.1371/journal.pbio.0020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MP, Fahrer MA, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y-H. A population of murine γδ T cells that recognizes an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- Dalton JE, Pearson J, Scott P, Carding SR. The interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J. Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Intraepithelial lymphocytes: Anatomical site, not T cell receptor form, dictates phenotype and function. J. Exp. Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy DB, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse γδ T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor γδ: Analysis of γδ T cells during thymic ontogeny and in peripheral lymphoid organs. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J. Immunol. 1989;142:2736–2742. [Google Scholar]
- Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of toll-like receptor 2 on γδ T cells bearing invariant Vγ6/Vδ1 induced by Escherichia coli infection in mice. J. Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- O'Brien RL, Fu Y-X, Cranfill R, Dallas A, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp-60 reactive γδ cells: A large, diversified T lymphocyte subset with highly focused specificity. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J. Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- Pereira P, Boucontet L. Rates of recombination and chain pair biases greatly influence the primary γδ TCR repertoire in the thymus of adult mice. J. Immunol. 2004;173:3261–3270. doi: 10.4049/jimmunol.173.5.3261. [DOI] [PubMed] [Google Scholar]
- Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J. Exp. Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark CE, Vollmer M, Campbell P, Born WK, O'Brien RL. Response of a γδ TCR monomorphic subset during bacterial infection. J. Immunol. 1996;156:2214–2220. [PubMed] [Google Scholar]
- Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O'Brien RL. Subset-specific, uniform activation of Vγ6/Vδ1+ γδ T cells elicited by inflammation. J. Leuk. Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien Y-H. CDR3 length in antigen-specific immune receptors. J. Exp. Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- Schaller E, Macfarlane AJ, Rupec RA, Gordon S, McKnight AJ, Pfeffer K. Inactivation of the F4/80 glycoprotein in the mouse germline. Mol. Cell. Biol. 2002;22:8035–8043. doi: 10.1128/MCB.22.22.8035-8043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R, Johnson RM, Sperling AI, Brady W, Linsley PS, Spear PG, Fitch FW, Bluestone JA. Unique antigen recognition by a herpesvirus-specific TCR-γδ cell. J. Immunol. 1994;152:5392–5397. [PubMed] [Google Scholar]
- Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- Sim G-K, Olsson C, Augustin A. Commitment and maintenance of the αβ and γδ T cell lineages. J. Immunol. 1995;154:5821–5831. [PubMed] [Google Scholar]
- Skeen MJ, Freeman MM, Ziegler HK. Changes in peritoneal myeloid populations and their proinflammatory cytokine expression during infection with Listeria monocytogenes are altered in the absence of γδ T cells. J. Leuk. Biol. 2004;76:104–115. doi: 10.1189/jlb.1103574. [DOI] [PubMed] [Google Scholar]
- Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by γδ T cells: implications for innate immunity. J. Exp. Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcRγ chain deletion results in pleiotropic effector cell defects. Cell. 1994;78:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 1979;150:580–588. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]