Abstract
Testosterone is assumed to be the key hormone related to resource-defence aggression. While this role has been confirmed mostly in the context of reproduction in male vertebrates, the effect of testosterone on the expression of resource-defence aggression in female vertebrates is not so well established. Furthermore, laboratory work suggests that progesterone inhibits aggressive behaviour in females. In this study, we investigated the hormonal changes underlying territorial aggression in free-living female African black coucals, Centropus grillii (Aves; Cuculidae). Females of this sex-role reversed polyandrous bird species should be particularly prone to be affected by testosterone because they aggressively defend territories similar to males of other species. We show, however, that territorial aggression in female black coucals is modulated by progesterone. After aggressive territorial challenges female black coucals expressed lower levels of progesterone than unchallenged territorial females and females without territories, suggesting that progesterone may suppress territorial aggression and is downregulated during aggressive encounters. Indeed, females treated with physiological concentrations of progesterone were less aggressive than females with placebo implants. This is one of the first demonstrations of a corresponding hormone–behaviour interaction under challenged and experimental conditions in free-living females. We anticipate that our observation in a sex-role reversed species may provide a more general mechanism, by which progesterone—in interaction with testosterone—may regulate resource-defence aggression in female vertebrates.
Keywords: territorial aggression, females, testosterone, progesterone, sex-role reversal, classical polyandry
1. Introduction
Testosterone represents a key hormonal player in the modulation of resource-defence aggression in males (Harding 1981; Balthazart 1983; Wingfield et al. 1990, 2006; Wingfield & Silverin 2002; Oliveira 2004; Hirschenhauser & Oliveira 2006; Hau 2007) and is often assumed to play a similar role in females. Testosterone levels of females sampled after experimental encounters with female decoys increased in some instances (Desjardins et al. 2006; Gill et al. 2007) but not in others (Elekonich & Wingfield 2000; Davis & Marler 2003; Rubenstein & Wikelski 2005; Jawor et al. 2006; see also review in Voigt & Goymann 2007). Thus, testosterone may be affected differently depending on the species or context (see also Gill et al. 2007), just as in males (see reviews in Goymann et al. 2007 and Landys et al. 2007). Similar to males, testosterone implants may increase song and aggression in female birds (reviewed in Staub & de Beer 1997; Fusani et al. 2003; Ketterson et al. 2005; but see Kriner & Schwabl 1991). Yet, it is important to note that many testosterone implantation studies aimed at investigating the pharmacological mechanisms of testosterone action (and often use females as a model for males to distinguish organizational from activational effects). Hence, the testosterone levels generated by such implants often by far exceed the levels present in the circulation of unmanipulated females. As a consequence, the results of such pharmacological studies—while being interesting as such—may be of limited relevance for questions related to effects of physiological levels of testosterone in ecologically relevant contexts. Recently, however, Sandell (2007) demonstrated that free-living female European starlings (Sturnus vulgaris) treated with presumably physiological levels of testosterone respond more aggressively towards female competitors than controls, indicating that testosterone mediates aggressive behaviour in female starlings.
While testosterone facilitates territorial aggression in males, the main effect of steroid hormones on female aggression has been instead described as suppressive (Simon 2002). For example, high-dosage injections of progesterone decrease territorial aggression in captive female rodents that have had their ovaries removed (Fraile et al. 1988; Meisel et al. 1988; Meisel & Sterner 1990; Kapusta 1998; Kohlert & Meisel 2001). Yet again, the focus of most of these studies was pharmacological, using implants that often exceeded progesterone levels present in the circulation of unmanipulated females. During experimental challenges progesterone levels have been described to decrease in some species (Davis & Marler 2003), whereas there was an increase (Rubenstein & Wikelski 2005) or no change at all in others (Elekonich & Wingfield 2000).
We are convinced that the conundrum of the hormonal control of female aggression will only be solved when we use physiological levels of hormones and apply them in an ecologically relevant context (e.g. Sandell 2007). This is why we decided to study and manipulate territorial aggression in intact free-living black coucals. Black coucals (Centropus grillii) are non-parasitic cuckoos (Cuculidae) and belong to the less than 1% of bird species with reversed sex roles in which females compete over males who raise the offspring. Female black coucals are 70% larger than males and display conspicuous ‘male-like’ behaviours, such as aggressive defence of breeding territories and singing. Moreover, they typically mate with more than one male in a breeding system termed classical polyandry (Vernon 1971; Goymann et al. 2004, 2005). Black coucals thus represent an excellent model system to study the hormonal modulation of resource-defence aggression in females. Analogous to males, testosterone represents an obvious candidate for the modulation of territorial aggression in females of sex-role reversed species. However, in all those species investigated so far—including black coucals—males have far higher levels of testosterone than females, thus resembling socially monogamous birds with conventional sex roles (reviewed in Eens & Pinxten 2000; Goymann & Wingfield 2004). Furthermore, GnRH injections in female black coucals did not elevate testosterone concentrations (Goymann & Wingfield 2004), indicating that testosterone may not increase during territorial challenges. The aim of this study was to elucidate the hormonal mechanisms that regulate female aggression. First, we elicited aggressive behaviour in free-living female black coucals using a standardized stimulus (a simulated territorial intrusion or STI)—induced by placing a stuffed female black coucal (the decoy) into a focal female's territory and playing back the two main vocalizations typically uttered by females during territorial displays. After the challenge, we caught the focal female to take a blood sample for steroid hormone measurements. Having identified progesterone as the main hormone influenced by such challenges (see §3), we then as a next step manipulated progesterone levels to test for a causal relationships between territorial aggression and this hormone.
2. Material and methods
(a) Study area
We studied black coucals in the Usangu plains in southwestern Tanzania (8°41′ S, 34°5′ E; for details see Goymann et al. 2004). The experiments were conducted between January and February 2004, February and April 2006 (hormonal response to STIs), and February and March 2007 (progesterone implant study).
(b) Hormonal response to STIs with female black coucals
STIs were conducted with one of two stuffed female black coucals. We played back territorial vocalizations of a female (‘whoot’ and ‘k'tuc’; see Goymann et al. 2004) with a Sony TCD-D100 DAT-recorder and a Pignose 7-100 speaker placed underneath the stuffed decoy using recordings. For each trial, we selected one playback out of seven recorded from seven different females. After onset of the playback, we collected for 15 min the following behavioural responses towards the decoy: latency to respond (time interval between onset of playback and first movement towards the decoy), closest approach (the estimated closest perching distance between territory owner and decoy), number of attacks and flights over the decoy, and the number of whoot and k'tuc vocalizations. After the 15 min observation period we briefly interrupted the playback, opened a mist net close to the decoy and then continued the playback to attempt to catch the focal female. We successfully caught 7 of the 11 tested females after a mean catch time (mean ± s.e.m.) of 26.7±3.1 min (range 22–45 min). A 10-min challenge is the minimum time required to measure changes in plasma sex steroids in response to STIs in male song sparrows (Wingfield & Wada 1989). Assuming a similar time window for female black coucals, we compared steroid hormone levels of these 7 STI females to those of 17 unchallenged territorial females either caught passively without playback (n=7) or with a short playback of less than 8-min duration (n=10; mean catch time±s.e.m.=3.9±0.9 min). Because hormone levels of the two categories of control females did not differ, we pooled the data into one control category. In addition, as a third category, we included four non-territorial female floaters that were caught passively in the comparison of hormones (none of the results change when this third category is excluded). The plasma hormone levels of these behaviourally very cryptic birds supposedly reflect the levels of a completely non-territorial context. Blood samples of all birds (STI-challenged and controls) were usually taken within 3 min after capture (mean±s.e.m.=2.8±0.3 min). For the analysis of corticosterone, we included only those 11 controls and all 7 STI-challenged birds that were bled within less than 3 min after capture (1.7±0.1 min). The STI and control birds did not differ in body mass (separate variance t-test: t10.0=0.525, p=0.525), tarsus (t14.9=0.853, p=0.407) or wing length (t13.1=0.765, p=0.458), indicating that we did not select for a specific class of females when we conducted the STIs.
(c) Effect of progesterone on territorial aggression of female black coucals
In 2007, we caught 16 female black coucals using mist nets and playback, which were alternately assigned either to the control or the experimental group. Because only territorial females respond to playback we could exclude capturing non-territorial females. Upon capture they were measured (Goymann et al. 2004) and ringed with a numbered aluminium ring (Vogelwarte Radolfzell; size G) and coloured plastic rings for individual identification. Females (n=8) assigned to the progesterone group and those assigned to the control group (n=8) did not differ in body mass (t13.6=0.867, p=0.401), tarsus (t13.4=0.165, p=0.871) or wing length (t13.4=−0.950, p=0.359). Also, the latency between the onset of playback and capture was not different between birds assigned to the progesterone- and control group (t14.0=1.308, p=0.212), suggesting that birds were equally likely to respond to a STI before the treatment. Females were implanted with time release pellets from Innovative Research of America (Sarasota, FL). Individuals of the control group received a placebo pellet and individuals of the progesterone group received a 21-day constant release pellet containing 7.5 mg of progesterone with a release rate 0.35 mg d−1. Pellets were applied through a small incision of the skin on the lower back which was closed with tissue glue (Histoacryl, Braun Surgical; GmbH). Because coucals spend a lot of time hidden in dense vegetation they cannot be located reliably based on sight. Hence, before release, black coucals were fitted with a radio transmitter as described in Goymann et al. (2004). All females were then monitored on a daily basis for at least three weeks to observe their territorial and mating status. After 3–4 days of implantation, territorial aggression between control- and progesterone-implanted female black coucals was tested by STIs as described above and the behaviour was recorded for 15 min. The initial distance between the focal bird and the decoy did not differ between progesterone-treated and control birds (t10.5=1.3, p=0.223), i.e. the possibility for each bird to detect and respond to the auditory and visual stimuli was similar for both treatment groups. Vocalization data were only available for seven control and seven progesterone-treated females. Territory sizes were calculated as described in Goymann et al. (2004) using 13.2±0.8 GPS data points for the calculation.
The effectiveness of the progesterone pellets was tested by implanting them into female Japanese quail (Coturnix japonica) that are slightly larger than black coucals. We used quail instead of coucals, owing to logistic and animal welfare considerations. It would have been necessary to keep coucals in captivity for at least 3 days. In quail, the progesterone pellets significantly increased levels of progesterone from 0.8±0.5 ng ml−1 (range: 0.5–3.0 ng ml−1) before implantation to 2.0±0.5 ng ml−1 (1.4–4.0 ng ml−1) after 3 days of implantation (one-tailed paired t-test, n=5, t=3.523, p=0.012), indicating that the increase was within a physiological range. The maximum value of progesterone we measured in a female black coucal was 10 ng ml−1 (C. Muck & W. Goymann 2005, unpublished data).
(d) Hormone assays
Radioimmunoassays of progesterone (P4), testosterone (T), dihydrotestosterone (DHT), oestradiol (E2) and corticosterone (Cort) were performed using a modification (Goymann et al. 2001) of the method established by Wingfield & Farner (1975). All samples were analysed in two assays. The detection limits were 4.9–6.7 pg per tube for P4, 0.6–0.8 pg per tube for T, 1.1–1.4 pg per tube for DHT, 0.5 pg per tube for E2 and 6.1–6.8 pg per tube for Cort. The intra-assay variations were 10.4 and 5.7%, 3.2 and 8.9%, 9.3 and 3.4%, 15.8 and 16.4%, 11.2 and 10.0% for P4, T, DHT, E2 and Cort, respectively. Inter-assay variations were 2.4, 13.1, 4.7, 16.7 and 10.8% for P4, T, DHT, E2 and Cort, respectively. All except three samples for oestradiol were non-detectable and thus not included in the statistical evaluation.
(e) Statistical analyses
Statistical analyses were performed with Systat v. 12 (Systat Software, Inc., Erkrath, Germany). Data and model residuals were tested for normality. If necessary, data were transformed to meet criteria for parametric statistical tests (general linear models or separate variance t-tests). Data on closest approach, territory size, T, DHT and Cort levels were log-transformed, P4 data were transformed using a reciprocal square root transformation (Lamprecht 1992). If transformation was not feasible (catch latency, approach latency and k'tuc rate) we followed the recommendation of Ruxton (2006) and performed the separate variance t-test on ranks. Data are presented as mean±standard error (s.e.m.) or median±quartiles; the significance level was set at α=0.05. The p values are two-tailed for comparison of hormones during the STI tests. Based on the results of the STI tests, we expected a decrease in aggression when manipulating progesterone and thus used one-tailed tests for the comparison between placebo and progesterone-implanted female black coucals.
3. Results
Female African black coucals showed a clear aggressive response immediately after the onset of the STI. They immediately flew towards the stimulus (mean±s.e.m. latency to respond 98±26 s; n=11) and approached the decoy (closest approach, 0.6±0.3 m). On average, females spent 5.3±1.5 min (one-third of the 15 min observation period) within 1 m distance of the decoy. They flew over the decoy 1.2±0.3 times, and 7 of the 11 tested females physically attacked the decoy by repeatedly picking its head from 8 to more than 30 times. They mainly attacked head, eyes and the back of the respective decoy. Attacks were so intense that the skullcaps of the decoys were damaged within the first trials and the glass eyes had to be fixed frequently. Challenged females called 84.5±13.5 times whoot and 53.3±14.2 times k'tuc.
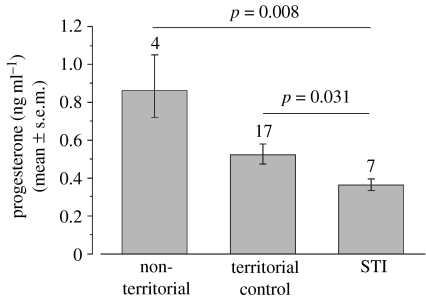
After a 15-min observation period, we caught 7 of the 11 challenged females within 26.7±3.1 min after onset of the STI. Females challenged with a STI had significantly lower levels of progesterone than unchallenged territorial females (caught either passively without decoy and playback (n=7) or within 3.9±0.9 min (n=10) after a short playback presentation), and passively caught non-territorial females (figure 1), suggesting that acute territorial challenges led to a decrease in progesterone levels. Also, unchallenged territorial females tended to have lower levels of progesterone than non-territorial females (p=0.10). The model (F2,25=6.906, p=0.004) explained 35.6% of the variance in the data. None of the other hormones, including testosterone, differed between challenged territorial females and unchallenged territorial or non-territorial females (table 1).
Figure 1.
Mean±s.e.m. concentrations of progesterone in ng ml−1. Females subjected to STIs had significantly lower levels of progesterone than territorial females not subjected to STIs and non-territorial females. For graphical presentation, data were back-transformed resulting in asymmetrical standard error bars. Numbers above bars indicate sample sizes.
Table 1.
Hormone concentrations (ng ml−1) following territorial challenges (concentrations refer to back-transformed mean concentrations in ng ml−1 (lower s.e.m.; upper s.e.m.)) of testosterone and 5α-dihydrotestosterone (DHT) of 7 females challenged with a STI, 17 unchallenged territorial females and 4 non-territorial female black coucals. (For corticosterone, we considered only those individuals for which a blood sample was obtained within 3 min after capture (7 STI and 11 control females). 17β-Oestradiol concentrations were non-detectable.)
| hormone | STI-females | unchallenged females | non-territorial females | statistics |
|---|---|---|---|---|
| testosterone | 0.23 (0.17; 0.30) | 0.18 (0.13; 0.23) | 0.23 (0.12; 0.44) | F2,25=0.19; p>0.8 |
| DHT | 0.08 (0.07; 0.09) | 0.09 (0.08; 0.10) | 0.11 (0.09; 0.14) | F2,25=0.75; p>0.4 |
| corticosterone | 13.4 (10.3; 17.6) | 13.8 (11.6; 16.6) | t13.9=0.5; p>0.6 |
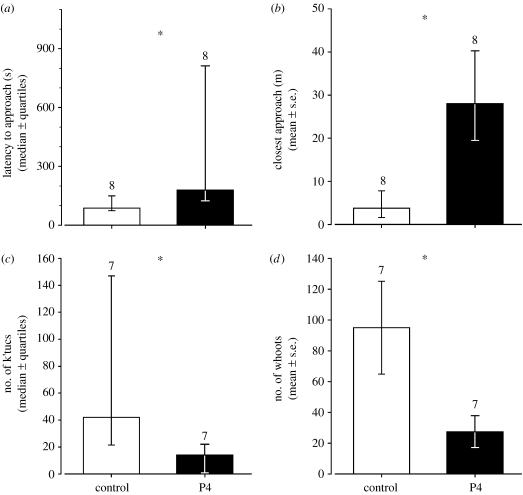
To establish whether the decrease in progesterone levels during STIs was causally related to acute territorial challenges, we then manipulated circulating progesterone concentrations of territorial females. All females implanted with either a control or progesterone pellet remained territorial and were paired to at least one male after the treatment and until the end of the study. Three to four days after the manipulation, we tested whether progesterone implants decreased the aggressive response to STIs. As predicted, we found that progesterone-treated birds responded to STIs less strongly than control birds: their latency to approach the decoy was significantly longer than that of controls (t12.5=−2.181, p=0.024; figure 2a), they did not come as close to the decoy (t11.2=−2.564, p=0.013; figure 2b), and they uttered fewer territorial vocalizations (‘k'tucs’ (t12=1.898, p=0.041; figure 2c) and ‘whoots’ (t7.4=2.121, p=0.034; figure 2d)) than control birds. The cooing call that expresses the highest level of vocal aggressive display (Goymann et al. 2004) was heard only five times and only from two control females. However, territory sizes did not differ between progesterone-treated (mean±s.e.m.=4.1±0.6 ha) and control birds (5.8±1.7 ha, t8.5=0.932, p=0.189), although the variance was higher in the control group (F7.7=9.247, p=0.009), mainly due to the very large territory of one female.
Figure 2.
Behavioural responses of control- and progesterone-implanted (P4) female black coucals during STIs. (a) P4-implanted females take longer to approach the decoy, (b) P4-implanted females do not come as close to the decoy, and (c,d) they vocalize less than control-implanted female black coucals during a 15-min STI. Numbers above bars indicate sample sizes and asterisk indicates significant differences. Please note that the bars in (a,c) represents the median and error bars indicate the interquartile range, whereas bars in (b,d) show back-transformed means and standard errors. Numbers above bars indicate sample sizes.
4. Discussion
Free-living female African black coucals showed a clear aggressive response to STIs with stuffed decoys and playback. We found evidence that progesterone is directly involved in the modulation of this kind of territorial aggression. First, females subjected to territorial challenges had lower levels of progesterone than unchallenged females, indicating that acute territorial aggression leads to a decrease in progesterone levels. Second, external progesterone decreased territorial aggression. To our knowledge, this is the first demonstration of a potential hormonal mechanism that modulates resource-defence aggression in females of a sex-role reversed species.
Recently, Davis & Marler (2003) conducted STIs in captive female California mice (Peromyscus californicus) and, like our study, found that progesterone levels decreased during territorial encounters. In contrast, progesterone and oestradiol levels were higher in female Galápagos marine iguanas (Amblyrhynchus cristatus) during fights for places suitable for egg-laying (Rubenstein & Wikelski 2005). We are aware of just one other study investigating progesterone levels during STIs in birds: Elekonich & Wingfield (2000) did not find changes in progesterone concentrations after STIs in female song sparrows (Melospiza melodia). Thus, progesterone may be affected differently in different species or depending on the context of aggression. Nevertheless, these studies and our results suggest that behavioural feedback mechanisms on progesterone secretion may be of similar importance for the fine-tuning of territorial aggression in females as it has been postulated for testosterone in males (Wingfield et al. 1990).
None of the other hormones measured, including testosterone, differed between territorially challenged and unchallenged female black coucals. With regard to testosterone, female black coucals may secrete this hormone at a maximal level that cannot be further modulated during territorial challenges. This idea is supported by earlier results demonstrating that the female black coucals do not further increase testosterone levels after injections of gonadotrophin-releasing hormone (GnRH; Goymann & Wingfield 2004). With respect to the predictions of the challenge hypothesis (Wingfield et al. 1990), the dynamics of plasma testosterone levels of polyandrous female black coucals represent an interesting analogue to males of polygynous bird species: polygynous males that do not provide parental care express testosterone levels close to maximum throughout the breeding season with no further modulation during territorial conflicts. Female black coucals may represent the polyandrous equivalent of such polygynous males: they do not provide parental care and they may secrete testosterone on a female specific maximal level that is not further modulated during territorial challenges.
Similar to black coucals, there was no association between territorial aggression and testosterone in female white-browed sparrow weavers (Plocepasser mahali: Wingfield & Lewis 1993), dark-eyed juncos (Junco hyemalis; Jawor et al. 2006), California mice (Davis & Marler 2003) and spiny damselfish (Acanthochromis polyacanthus: Hay & Pankhurst 2005). In female song sparrows (Elekonich & Wingfield 2000), eastern bluebirds (Sialia sialis; Navara et al. 2006) and Galápagos marine iguanas (Rubenstein & Wikelski 2005), testosterone even decreased during STIs. To our knowledge, only female buff-breasted wrens (Thryothorus leucotis) in prebreeding condition (Gill et al. 2007) and females of the cichlid Neolamprologus pulcher (Desjardins et al. 2006) increased testosterone levels during STIs. High levels of testosterone have been shown to be detrimental for the fecundity of females (Rutkowska et al. 2005), possibly preventing many female vertebrates to use this hormone for the modulation of aggression.
Most previous studies have found a suppressive effect of progesterone on territorial aggression in females (Fraile et al. 1988; Meisel et al. 1988; Meisel & Sterner 1990; Albert et al. 1992; Kohlert & Meisel 2001, but see Kapusta 1998). These studies were innovative and new, but unlike our study they were done on gonadectomized females in artificial environments, and often used higher than physiological doses of progesterone. Given that the effects of progesterone are dose-dependent and differ between intact and gonadectomized animals (Young et al. 1991; Witt et al. 1994), the implications of such laboratory studies are limited. We are convinced that our treatment was physiological, even if we did not use coucals for the validation, but quails (see §2). Since the pellets used should have a constant release rate regardless of the species, we expect that the release rate in coucals was similar to that in quails. The female quails we used were slightly larger (253±9.8 g) than the female black coucals (174±2.9 g) and in quail, the average increase in progesterone was 1.2 ng ml−1. Adjusting for the difference in size, this should have resulted in an approximate increase of progesterone in the range of 1.8 ng ml−1 in coucals. Based on the average value of 0.7 ng ml−1 (figure 1), this would have increased levels to approximately 2.5 ng ml−1 in female black coucals. The highest level of progesterone we ever measured in a female black coucal during breeding was 10 ng ml−1 (C. Muck & W. Goymann, unpublished data). We are thus confident that the treatment was well in the physiological range of black coucals. Our current results thus provide evidence that physiological levels of progesterone may exert suppressive effects on territorial aggression in an ecologically relevant context.
Currently we do not know how progesterone may reduce territorial aggression in female black coucals. Because external progesterone attenuates aggression, it is unlikely that the hormone is locally converted to testosterone during territorial challenges. Progesterone could act indirectly via interactions of progesterone metabolites with γ-aminobutyric acid type A (GABAA) receptors (Rupprecht & Holsboer 1999; Miczek et al. 2003) or more directly by binding to the progesterone receptor or even to the androgen receptor (Bullock et al. 1978; Crews et al. 1996). Progesterone has been demonstrated to affect the expression of androgen receptor mRNA in male guinea-pigs (Cavia porcellus; Connolly & Resko 1989) and whiptail lizards (Cnemidophorus inornatus: Crews et al. 1996). Further, progesterone attenuates testosterone-induced behaviours in male birds (Erickson et al. 1967; Bottoni et al. 1985) and female mammals (Barfield 1984; Albert et al. 1992), and it protects female guinea-pigs from the masculinizing actions of testosterone (Diamond & Young 1963). Thus, testosterone and progesterone may be team players in the regulation of androgen-induced territorial aggression. In a previous study, we have shown that female black coucals express higher levels of androgen receptor mRNA than males in the nucleus taeniae—a brain area known to be involved in the control of aggressive behaviour (Voigt & Goymann 2007). In combination with the results of the current study, we propose that testosterone and progesterone interact to modulate territorial aggression in female black coucals. Constant, but relatively low levels of testosterone in combination with high expression of androgen receptors in the nucleus taeniae may be sufficient to sustain a default level of territorial aggression during the breeding season. Progesterone may be used by black coucals to fine-tune, i.e. decrease this default level of aggressive behaviour. We propose that progesterone may represent a key hormone in the modulation of territorial aggression in sex-role reversed female black coucals and possibly in other female vertebrates, thus substituting testosterone as the main modulator of resource-defence aggression (but see Sandell 2007). However, while testosterone in general facilitates resource-defence aggression, the effects of progesterone appear to be mainly suppressive instead. Thus, in a reproductive context, females may use this steroid hormone to decrease a testosterone-induced default level of aggressive behaviour.
Currently we know little about the dynamics of progesterone during the reproductive cycle of female black coucals. As in other birds, progesterone is most likely to be involved in ovulation with higher levels during the period around laying (Silver et al. 1974; Sharp 1980; Sockman & Schwabl 1999). If the suppressive effect of progesterone on territorial aggression is not counterbalanced by other mechanisms, this means that female black coucals should be least aggressive when they lay a clutch. The most detailed information about how different sex steroids interact to modulate aggression in females is available from a study by Albert et al. (1992). In rats, these authors found quite complex interactions between oestradiol, testosterone and progesterone. Compared with ovariectomized female rats implanted with high levels of oestradiol and testosterone (mimicking oestrus), female rats implanted with high levels of oestradiol, testosterone and progesterone (mimicking late pregnancy) were less aggressive towards a female intruder. This indicated that progesterone suppresses aggressive behaviour induced by testosterone and oestradiol. However, when the high-concentration implants of oestradiol and testosterone were exchanged with low-concentration implants, and the progesterone implant was removed completely (mimicking the situation after parturition), aggression decreased in females previously implanted with high levels of oestradiol and testosterone. In contrast, those females that were previously implanted with high levels of oestradiol, testosterone and progesterone showed an increase in aggression after switching to low-level implants of testosterone and oestradiol (Albert et al. 1992). These results suggest complex interactions between the sex steroids during the oestrus cycle of rats and indicate that progesterone buffers aggression induced by oestradiol and testosterone. Generally, such interactions between different sex hormones could represent a means by which female vertebrates may maintain appropriate aggressive responses to challenges while at the same time sex hormone levels change depending on the oestrus state.
The complexity of hormonal effects on behaviour may be mirrored by similar impacts of behaviour on hormones. Such impacts could, for example, be responsible for the differences that were observed in the hormonal response to STIs in female Galápagos marine iguanas, California mice, buff-breasted wrens, song sparrows or black coucals (see above). Recent work suggests that even males of many bird species do not show the expected rise in testosterone after territorial challenges and that the differences between species may be related to natural history parameters (Goymann et al. 2007; Landys et al. 2007). Possibly, similar factors may be responsible for variation in the testosterone and progesterone response of females to territorial challenges (see Moore 2007). Furthermore, a closer look at other steroids such as progesterone may provide some answers regarding so far unexplained patterns in the testosterone response to territorial challenges in males (see also Crews 2005; Andersen & Tufik 2006).
Acknowledgments
This study complies with the laws of Tanzania.
We thank the Tanzanian Commission for Science and Technology (COSTECH) and the Tanzania Wildlife Research Institute (TAWIRI) for the permission to conduct this study. Furthermore, we thank Nicole Geberzahn for sound recordings of black coucals, Christina Muck for preparing the dummies, Sam Bostock and Raimund Barth for their assistance in the field, Monika Trappschuh for assistance with the hormone assays, and also Redouan Bshary, Manfred Gahr, Michaela Hau, Barbara Helm, Stefan Leitner, Martin Wikelski and two anonymous referees for reviewing earlier versions of this manuscript. This study was funded by grant Go 985/5-1 from Deutsche Forschungsgemeinschaft (DFG) and by the Max Planck Society. The sequence of authors was determined using the sequence-determines-credit approach (Tscharntke et al. 2007).
References
- Albert D.J, Jonik R.H, Walsh M.L. Interaction of estradiol, testosterone, and progesterone in the modulation of hormone-dependent aggression in the female rat. Physiol. Behav. 1992;52:773–779. doi: 10.1016/0031-9384(92)90413-v. doi:10.1016/0031-9384(92)90413-V [DOI] [PubMed] [Google Scholar]
- Andersen M.L, Tufik S. Does male sexual behavior require progesterone? Brain Res. Rev. 2006;51:136–143. doi: 10.1016/j.brainresrev.2005.10.005. doi:10.1016/j.brainresrev.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Balthazart J. Hormonal correlates of behavior. In: Farner D.S, King J.R, Parker K.C, editors. Avian biology, vol. VII. Academic Press; San Diego, CA: 1983. pp. 221–365. [Google Scholar]
- Barfield R.J. Reproductive hormones and aggressive behavior. In: Flannelly K.J, Blanchard R.J, Blanchard D.C, editors. Biological perspectives on aggression. Alan R. Liss; New York, NY: 1984. pp. 105–134. [PubMed] [Google Scholar]
- Bottoni L, Lucini V, Massa R. Effect of progesterone on the sexual behavior of the male Japanese quail. Gen. Comp. Endocrinol. 1985;57:345–351. doi: 10.1016/0016-6480(85)90213-8. doi:10.1016/0016-6480(85)90213-8 [DOI] [PubMed] [Google Scholar]
- Bullock I.P, Bardin C.W, Sherman M.R. Androgenic, antiandrogenic, and synandrogenic actions of progestins: role of steric and allosteric interactions with androgen receptors. Endocrinology. 1978;103:1768–1782. doi: 10.1210/endo-103-5-1768. [DOI] [PubMed] [Google Scholar]
- Connolly P.B, Resko J.A. Progestins affect reproductive behavior and androgen receptor dynamics in male guinea pig brain. Brain Res. 1989;503:312–316. doi: 10.1016/0006-8993(89)91681-8. doi:10.1016/0006-8993(89)91681-8 [DOI] [PubMed] [Google Scholar]
- Crews D. Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trend Endocrinol. Metab. 2005;16:354–361. doi: 10.1016/j.tem.2005.08.007. doi:10.1016/j.tem.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Crews D, Godwin J, Hartman V, Grammer M, Prediger E.A, Sheppherd R. Intrahypothalamic implantation of progesterone in castrated male whiptail lizards (Cnemidophorus inornatus) elicits courtship and copulatory behavior and affects androgen receptor- and progesterone receptor-mRNA expression in the brain. J. Neurosci. 1996;16:7347–7352. doi: 10.1523/JNEUROSCI.16-22-07347.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.S, Marler C.A. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm. Behav. 2003;44:185–198. doi: 10.1016/s0018-506x(03)00128-4. doi:10.1016/S0018-506X(03)00128-4 [DOI] [PubMed] [Google Scholar]
- Desjardins J.K, Hazelden M.R, Van der Kraak G.J, Balshine S. Male and female cooperatively breeding fish provide support for the “challenge hypothesis”. Behav. Ecol. 2006;17:149–154. doi:10.1093/beheco/arj018 [Google Scholar]
- Diamond M, Young W.C. Differential responsiveness of pregnant and nonpregnant guinea pigs to the masculinizing action of testosterone propionate. Endocrinology. 1963;72:429–438. doi: 10.1210/endo-72-3-429. [DOI] [PubMed] [Google Scholar]
- Eens M, Pinxten R. Sex-role reversal in vertebrates: behavioural and endocrinological accounts. Behav. Process. 2000;51:135–147. doi: 10.1016/s0376-6357(00)00124-8. doi:10.1016/S0376-6357(00)00124-8 [DOI] [PubMed] [Google Scholar]
- Elekonich M.M, Wingfield J.C. Seasonality and hormonal control of territorial aggression in female song sparrows (Melospiza melodia; Passeriformes, Emberizidae) Ethology. 2000;106:493–510. doi:10.1046/j.1439-0310.2000.00555.x [Google Scholar]
- Erickson C.J, Bruder R.H, Komisaruk B.R, Lehrman D.S. Selective inhibition by progesterone of androgen-induced behavior in male ring doves (Streptopelia risoria) Endocrinology. 1967;81:39–44. doi: 10.1210/endo-81-1-39. [DOI] [PubMed] [Google Scholar]
- Fraile I.G, McEwen B.S, Pfaff D.W. Comparative effects of progesterone and alphaxalone on aggressive, reproductive and locomotor behaviors. Pharmacol. Biochem. Behav. 1988;30:729–735. doi: 10.1016/0091-3057(88)90091-3. doi:10.1016/0091-3057(88)90091-3 [DOI] [PubMed] [Google Scholar]
- Fusani L, Metzdorf R, Hutchison J.B, Gahr M. Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J. Neurobiol. 2003;54:370–379. doi: 10.1002/neu.10141. doi:10.1002/neu.10141 [DOI] [PubMed] [Google Scholar]
- Gill S.A, Alfson E.D, Hau M. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis) Proc. R. Soc. B. 2007;274:2187–2194. doi: 10.1098/rspb.2007.0457. doi:10.1098/rspb.2007.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Wingfield J.C. Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim. Behav. 2004;68:733–740. doi:10.1016/j.anbehav.2003.12.012 [Google Scholar]
- Goymann W, East M.L, Hofer H. Androgens and the role of female “hyperaggressiveness” in spotted hyenas (Crocuta crocuta) Horm. Behav. 2001;39:83–92. doi: 10.1006/hbeh.2000.1634. doi:10.1006/hbeh.2000.1634 [DOI] [PubMed] [Google Scholar]
- Goymann W, Wittenzellner A, Wingfield J.C. Competing females and caring males. Polyandry and sex-role reversal in African black coucals, Centropus grillii. Ethology. 2004;110:807–823. doi:10.1111/j.1439-0310.2004.01015.x [Google Scholar]
- Goymann W, Kempenaers B, Wingfield J. Breeding biology, sexually dimorphic development and nestling testosterone concentrations of the classically polyandrous African black coucal, Centropus grillii. J. Ornithol. 2005;146:314–324. doi:10.1007/s10336-005-0004-x [Google Scholar]
- Goymann W, Landys M.M, Wingfield J.C. Distinguishing seasonal androgen responses from male–male androgen responsiveness—revisiting the challenge hypothesis. Horm. Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. doi:10.1016/j.yhbeh.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Harding C.F. Social modulation of circulating hormone levels in the male. Am. Zool. 1981;21:223–232. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. doi:10.1002/bies.20524 [DOI] [PubMed] [Google Scholar]
- Hay A.C, Pankhurst N.W. Effect of paired encounters on plasma androgens and behaviour in males and females of the spiny damselfish Acanthochromis polyacanthus. Mar. Freshw. Behav. Physiol. 2005;38:127–138. doi:10.1080/10236240500125528 [Google Scholar]
- Hirschenhauser K, Oliveira R.F. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 2006;71:265–277. doi:10.1016/j.anbehav.2005.04.014 [Google Scholar]
- Jawor J.M, Young R, Ketterson E.D. Females competing to reproduce: dominance matters but testosterone may not. Horm. Behav. 2006;49:362–368. doi: 10.1016/j.yhbeh.2005.08.009. doi:10.1016/j.yhbeh.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Kapusta J. Gonadal hormones and intrasexual aggressive behavior in female bank voles (Clethrionomys glareolus) Aggr. Behav. 1998;24:63–70. doi:10.1002/(SICI)1098-2337(1998)24:1<63::AID-AB6>3.0.CO;2-T [Google Scholar]
- Ketterson E.D, Nolan V, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166:S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Kohlert J.G, Meisel R.L. Inhibition of aggression by progesterone and its metabolites in female Syrian hamsters. Aggr. Behav. 2001;27:372–381. doi:10.1002/ab.1022 [Google Scholar]
- Kriner E, Schwabl H. Control of winter song and territorial aggression of female robins (Erithacus rubecula) by testosterone. Ethology. 1991;87:37–44. doi: 10.1016/0018-506x(91)90049-n. [DOI] [PubMed] [Google Scholar]
- Lamprecht J. Verlag Paul Parey; Berlin, Germany: 1992. Biologische Forschung: Von der Planung bis zur Publikation. [Google Scholar]
- Landys M.M, Goymann W, Raess M, Slagsvold T. Hormonal responses to male–male social challenge in the blue tit Cyanistes caeruleus: single-broodedness as an explanatory variable. Physiol. Biochem. Zool. 2007;80:228–240. doi: 10.1086/510564. doi:10.1086/510564 [DOI] [PubMed] [Google Scholar]
- Meisel R.L, Sterner M.R. Progesterone inhibition of sexual behavior is accompanied by an activation of aggression in female Syrian hamsters. Physiol. Behav. 1990;47:415–417. doi: 10.1016/0031-9384(90)90102-a. doi:10.1016/0031-9384(90)90102-A [DOI] [PubMed] [Google Scholar]
- Meisel R.L, Sterner M.R, Diekman M.A. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm. Behav. 1988;22:453–466. doi: 10.1016/0018-506x(88)90050-5. doi:10.1016/0018-506X(88)90050-5 [DOI] [PubMed] [Google Scholar]
- Miczek K.A, Fish E.W, De Bold J.F. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm. Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. doi:10.1016/j.yhbeh.2003.04.002 [DOI] [PubMed] [Google Scholar]
- Moore I.T. Advancing the challenge hypothesis. Horm. Behav. 2007;51:461–462. doi: 10.1016/j.yhbeh.2007.02.009. doi:10.1016/j.yhbeh.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Navara K.J, Sieferman L.M, Hill G.E, Mendonca M.T. Yolk androgens vary inversely to maternal androgens in eastern bluebirds: an experimental study. Funct. Ecol. 2006;20:449–456. doi:10.1111/j.1365-2435.2006.01114.x [Google Scholar]
- Oliveira R.F. Social modulation of androgens in vertebrates: mechanisms and function. Adv. Stud. Behav. 2004;34:165–239. doi:10.1016/S0065-3454(04)34005-2 [Google Scholar]
- Rubenstein D.R, Wikelski M. Steroid hormones and aggression in female Galapagos marine iguanas. Horm. Behav. 2005;48:329–341. doi: 10.1016/j.yhbeh.2005.04.006. doi:10.1016/j.yhbeh.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trend Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. doi:10.1016/S0166-2236(99)01399-5 [DOI] [PubMed] [Google Scholar]
- Rutkowska J, Cichon M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm. Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. doi:10.1016/j.yhbeh.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Ruxton G.D. The unequal variance t-test is an underused alternative to Student's t-test and the Mann–Whitney U test. Behav. Ecol. 2006;17:688–690. doi:10.1093/beheco/ark016 [Google Scholar]
- Sandell M.I. Exogenous testosterone increases female aggression in the European starling (Sturnus vulgaris) Behav. Ecol. Sociobiol. 2007;62:255–262. doi:10.1007/s00265-007-0460-9 [Google Scholar]
- Sharp, P. J. 1980 The endocrine control of ovulation in birds. In Proc. 17th Int. Ornithological Congress (ed. R. Nohring), pp. 245–248. Berlin, Germany: Verlag der Deutschen Ornithologen Gesellschaft.
- Silver R, Reboulleau C, Lehrman D.S, Feder H.H. Radioimmunoassay of plasma progesterone during the reproductive cycle of male and female ring doves (Streptopelia risoria) Endocrinology. 1974;94:1547–1554. doi: 10.1210/endo-94-6-1547. [DOI] [PubMed] [Google Scholar]
- Simon N.G. Hormonal processes in the development and expression of aggressive behavior. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior vol. 1. Academic Press; San Diego, CA: 2002. pp. 339–392. [Google Scholar]
- Sockman K.W, Schwabl H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen. Comp. Endocrinol. 1999;114:257–268. doi: 10.1006/gcen.1999.7252. doi:10.1006/gcen.1999.7252 [DOI] [PubMed] [Google Scholar]
- Staub N.L, de Beer M. The role of androgens in female vertebrates. Gen. Comp. Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. doi:10.1006/gcen.1997.6962 [DOI] [PubMed] [Google Scholar]
- Tscharntke T, Hochberg M.E, Rand T.A, Resh V.H, Krauss J. Author sequence and credit for contributions in multiauthored publications. PLoS Biol. 2007;5:e18–e19. doi: 10.1371/journal.pbio.0050018. doi:10.1371/journal.pbio.0050018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon C.J. Notes on the biology of the black coucal. Ostrich. 1971;42:242–258. [Google Scholar]
- Voigt C, Goymann W. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii) Dev. Neurobiol. 2007;67:1560–1573. doi: 10.1002/dneu.20528. doi:10.1002/dneu.20528 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Lewis D.M. Hormonal and behavioural responses to simulated territorial intrusion in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Anim. Behav. 1993;45:1–11. doi:10.1006/anbe.1993.1001 [Google Scholar]
- Wingfield J.C, Silverin B. Ecophysiological studies of hormone–behavior relations in birds. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior, vol. 2. Academic Press; San Diego, CA: 2002. pp. 587–647. [Google Scholar]
- Wingfield J.C, Wada M. Changes in plasma levels of testosterone during male–male interactions in the song sparrow, Melospiza melodia: time course and specifity of response. J. Comp. Physiol. A. 1989;166:189–194. doi:10.1007/BF00193463 [Google Scholar]
- Wingfield J.C, Hegner R.E, Dufty A.M, Ball G.F. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. doi:10.1086/285134 [Google Scholar]
- Wingfield J.C, Moore I.T, Goymann W, Wacker D, Sperry T. Contexts and ethology of vertebrate aggression: implications for the evolution of hormone–behavior interactions. In: Nelson R, editor. Biology of aggression. Oxford University Press; New York, NY: 2006. pp. 179–210. [Google Scholar]
- Witt D.M, Young L.J, Crews D. Progesterone and sexual behavior in males. Psychoneuroendocrinology. 1994;19:553–562. doi: 10.1016/0306-4530(94)90040-x. doi:10.1016/0306-4530(94)90040-X [DOI] [PubMed] [Google Scholar]
- Young L.J, Greenberg N, Crews D. The effects of progesterone on sexual behavior in male green anole lizards (Anolis carolinensis) Horm. Behav. 1991;25:477–488. doi: 10.1016/0018-506x(91)90015-a. doi:10.1016/0018-506X(91)90015-A [DOI] [PubMed] [Google Scholar]