Abstract
Sauropod dinosaurs, the dominant herbivores throughout the Jurassic, challenge general rules of large vertebrate herbivory. With body weights surpassing those of any other megaherbivore, they relied almost exclusively on pre-angiosperm plants such as gymnosperms, ferns and fern allies as food sources, plant groups that are generally believed to be of very low nutritional quality. However, the nutritive value of these taxa is virtually unknown, despite their importance in the reconstruction of the ecology of Mesozoic herbivores. Using a feed evaluation test for extant herbivores, we show that the energy content of horsetails and of certain conifers and ferns is at a level comparable to extant browse. Based on our experimental results, plants such as Equisetum, Araucaria, Ginkgo and Angiopteris would have formed a major part of sauropod diets, while cycads, tree ferns and podocarp conifers would have been poor sources of energy. Energy-rich but slow-fermenting Araucaria, which was globally distributed in the Jurassic, was probably targeted by giant, high-browsing sauropods with their presumably very long ingesta retention times. Our data make possible a more realistic calculation of the daily food intake of an individual sauropod and improve our understanding of how large herbivorous dinosaurs could have flourished in pre-angiosperm ecosystems.
Keywords: herbivorous dinosaurs, Mesozoic food plants, herbivory, nutrition
1. Introduction
Many attempts have been made to reconstruct the nutritional ecology of dinosaurian megaherbivores such as the giant sauropod Brachiosaurus brancai, but all are hampered by the tremendous body weights (BW) of up to 70 t (Mazzetta et al. 2004) in combination with a restriction of potential food plants to pre-angiosperm taxa until the Mid-Cretaceous (Weaver 1983; Farlow 1987). Both factors push sauropods out of the ecological framework that has been established for extant herbivores (Van Soest 1994). In principle, an increase in body size has been considered beneficial with regard to digestive capacity (see Clauss et al. 2007 for a review and revision); however, it also implies different constraints such as a very high absolute energy requirement or a low degree of selectivity (Owen-Smith 1988).
The kinds of food plants that were available is another major difference between extant herbivores and sauropods. While it was recently reported that sauropods ingested grass during the Late Cretaceous (Prasad et al. 2005), their food plants must have consisted exclusively of ferns, fern allies, such as horsetails, and gymnosperms during most of their existence, namely in the Late Triassic, throughout the Jurassic and into the Early Cretaceous. Nevertheless, it is commonly believed that all non-angiosperm forages are of exceptionally low nutritional quality (Coe et al. 1987; Wing & Tiffney 1987; Van Soest 1994; Taggart & Cross 1997; Midgley et al. 2002; Farlow 2007). Furthermore, palaeobotanists have hypothesized that herbivorous dinosaurs preferred soft-tissued plants such as ferns, ginkgoes and the extinct Cheirolepidiaceae over the woodier, spinier and phytochemically less palatable conifers (Coe et al. 1987; Tiffney 1997), and others have advocated ferns as the prime sauropod food (Dodson 1990; Taggart & Cross 1997). Krassilov (1981) put forth ferns and horsetails as diplodocid and cycads and conifers as camarasaurid food plants. Based on the reconstructions of the sauropod neck position, Stevens & Parrish (2005) state that only brachiosaurids and camarasaurids would have been able to feed on tall trees, while other taxa should have focused on low-growing ferns and fern allies. By contrast, Fiorillo (1998) dismissed ferns and horsetails as suitable food plants for sauropods due to the lower gross energy content of these plants, while Engelmann et al. (2004) accepted ferns and horsetails as the sauropod fodder, despite their presumed low energy content. It should be noted here that this energetic ranking of taxa was based on gross energy measurements by Weaver (1983) on extant relatives of potential sauropod food plants. However, using gross energy measurements as an estimate of the energy available to herbivores, e.g. metabolizable energy (ME), may not yield accurate or even reasonable results for the plant material (GfE 2003). The example of coal explains the concept: coal is high in combustion energy, but its energy is virtually indigestible and hence inaccessible to animals.
Standard feed evaluation techniques, such as in vitro fermentation methods, make possible a ‘semi-biological’ estimation of the energy content of leafy plant tissue available to herbivores (=degradability; Van Soest 1994). The application of this approach to sauropods here was made on the grounds that most authors agree that the basic physiological and anatomical set-up of fibrous plant digestion in herbivorous dinosaurs followed the same general rules as in extant herbivores with a gut fermentation chamber (Farlow 1987; Dunham et al. 1989; Marshall & Stevens 2000; Mackie 2002). Specifically, the energy yield from the fibrous plant material is determined by the rate and extent of its digestion and fermentation (plant factors), in combination with the duration of retention in the digestive tract (animal factor; Waldo et al. 1972; Van Soest 1994), and not by the biological affinity of the herbivore. The use of a standardized inoculum, namely from a mammalian donor, is acceptable here, as the gut microbe populations of different herbivores are comparable in their biochemical characteristics (Van Soest 1994). In other words, in regard to the metabolic energy yield, it is secondary whether the microbial process occurs in the gut of an herbivorous reptile, bird or mammal.
The aim of our study is to estimate the nutritional quality of the extant relatives of potential sauropod food plants in regard to energy content using modern feed evaluation techniques. Comparison of the experimental data is used here to deduce sauropod food preferences and to shed light on the general nutritional ecology of herbivorous dinosaurs.
2. Material and methods
Foliage samples of nearest living relatives of major plant groups in the Mesozoic were taken, some of which are identical to their Mesozoic relatives at the genus level. These included Equisetum, ferns such as the Dicksoniaceae, Matoniaceae and Osmundaceae, cycads, Ginkgo and conifers such as the Araucariaceae, Podocarpaceae and Taxodiaceae. Angiosperm forage groups (browse, forbs and grasses) were included for reference (Hummel et al. 2006), and the results of the living Mesozoic flora were ranked within this framework.
Foliage was collected between May and July 2004 from botanical gardens and parks in Germany. In the laboratory, the samples were dried at 60°C and milled through a 1 mm sieve in preparation for experimental trials using an in vitro fermentation method, a modified Hohenheim gas test (Menke et al. 1979). The microbes were obtained from the rumen liquid of sheep fed on a standardized diet. The milled plant tissue was weighed in airtight glass syringes and placed in an incubator at 39°C for 72 hours. Gas production (Gp) was recorded after 4, 8, 12, 24, 32, 48, 56 and 72 hours. The gas produced during the fermentation represents a measure of feed degradation and consists of nearly equal parts of the CO2 evolving from the buffer (bicarbonate) reaction with the volatile fatty acids developing during fermentation and the waste gases of fermentation (Blümmel et al. 1999). Nonlinear regression on cumulative Gp curves was run using an exponential model (Blümmel & Ørskov 1993). Dry matter (DM), crude protein (CP; N×6.25), cell wall (neutral detergent fibre, NDF; ash corrected) and ether extract (EE) contents were quantified as well.
An estimation of the ME content was performed using a standard regression for grasses and forbs (using Gp at 24 hours, CP and EE to predict ME according to Menke & Huss 1987), and by calculating a regression between Gp and ME from this database (n=40), which was used to estimate the ME content of the gymnosperm and fern samples based on their Gp during 72 hours ((ME (MJ kg−1 DM)=0.1842×Gp (ml per 200 mg DM)+1.9649); R2=0.85; s.d. of the residuals Sy.x=0.474). Values calculated in this way represent ME for ruminants (MEr); these values can be extrapolated to ME for a hindgut fermenter (the horse, MEh) using the formula of Jansson (2004): MEh=1.12×MEr−1.1. Since MEr and MEh differed only slightly from one another, only MEr values are given in table 1.
Table 1.
Nutrient and metabolizable energy (ME) contents of potential dinosaur food plants. (ME for the data of Weaver (1983) was calculated by multiplying gross energy with a factor of 0.5 (digestible energy, according to Weaver 1983), and consequently with a factor of 0.76 to obtain ME (according to Robbins 1993). Gp, gas production; DM, dry matter; NDF, neutral detergent fibre.)
| sample type (no. of spp.) | ME (Gp at 72 hours) | ME (Weaver 1983) | crude protein | NDF |
|---|---|---|---|---|
| (MJ kg−1 DM) | (% DM) | |||
| grasses (16) | 11.3 (9.3–13.6) | 15.3 | 62.8 | |
| forbs (11) | 10.4 (9.1–11.9) | 19.8 | 37.8 | |
| dicot browse (13) | 7.5 (5.5–10.0) | 20.7 | 43.2 | |
| Ginkgo (1) | 8.6 | 6.7 | 15.6 | 27.5 |
| Araucariaceae (5) | 9.4 (8.0–11.6) | 7.0 | 4.4 | 65.2 |
| Podocarpaceae (3) | 5.9 (5.0–6.1) | 6.6 | 62.3 | |
| various conifers (13) | 8.3 (6.3–10.8) | 7.0 (6.4–7.5) | 10.0 | 51.3 |
| cycads (7) | 6.1 (4.4–7.7) | 7.6 (7.1–8.6) | 11.4 | 65.3 |
| various ferns (9) | 7.7 (4.7–11.7) | 6.6 (5.4–7.4) | 11.5 | 62.8 |
| tree ferns (5) | 6.4 (3.6–9.3) | 6.9 (6.6–7.2) | 11.3 | 63.6 |
| Equisetum (3) | 11.6 (10.8–12.9) | 5.3 | 11.7 | 48.4 |
3. Results
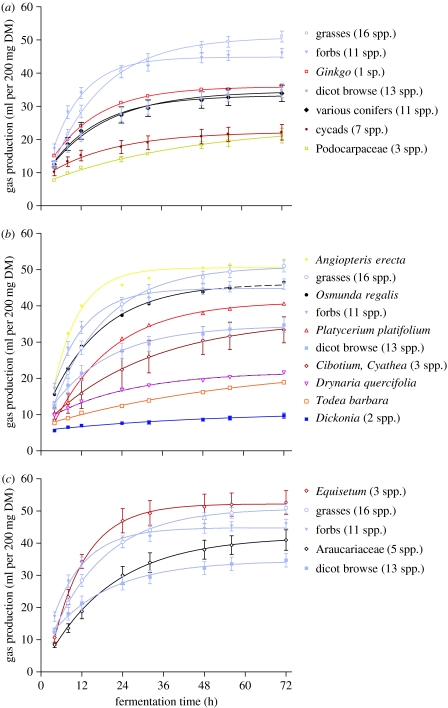
In general, fern and gymnosperm foliage yielded levels of energy that were only moderately lower than for forbs and grasses. Ginkgo and some conifers performed at a level similar to temperate browse. However, another group of conifers with an extensive Mesozoic record, the Podocarpaceae, and the cycads both yielded rather low amounts of energy (figure 1a). The ferns were quite variable; Angiopteris or the royal fern Osmunda were highly digestible, while others, such as the tree fern Dicksonia, were very poor energy sources (figure 1b).
Figure 1.
Fermentative behaviour of potential dinosaur food plants compared with that of angiosperms. Gp in the Hohenheim gas test is plotted versus fermentation time. (a) Various gymnosperms compared with angiosperms. Note that Ginkgo and some conifers (Cephalotaxaceae, Taxodiaceae, Pinaceae and Taxaceae) performed at the level of angiosperm browse, whereas podocarp conifers and cycads fared poorly. (b) Ferns compared with angiosperms. Note the great variability among ferns, including the very poor performance of the tree fern Dicksonia. (c) Araucariaceae and horsetails (Equisetum spp.) compared with angiosperms. Note that horsetails even surpass grasses and that araucarias outperform browse after 72 hours (DM, dry matter; means and standard error of the mean (s.e.m.) are indicated).
Interestingly, the Araucariaceae showed a pattern reminiscent of grasses by starting out slowly, but in the end attaining higher values than those of browse, Ginkgo or other conifers after 72 hours. Equisetum, representing the most basal plant group in the study, yielded the highest fermentative energy output, exceeding even that of grasses, especially in the initial phases of fermentation (figure 1c).
The resulting ME content was high in Equisetum (11.6 MJ kg−1 DM) and also in Araucariaceae (9.4 MJ per kg DM; table 1). Crude protein level was found to be high in Ginkgo (15.6% DM) and low in Araucariaceae (4.4% DM). Several of the plants investigated showed high cell wall (NDF) contents of more than 60% DM, while Ginkgo was low in NDF (27.5% DM).
4. Discussion
In general, the degradability of the pre-angiosperm plants investigated was surprisingly high in many taxa. This is true despite the use of a non-specific (but therefore standardized) inoculum, the rumen liquid from sheep. Since a comprehensive discussion of this topic is beyond the scope of this paper (but see Hummel et al. 2006 for a review), we note that using better adapted inoculum would only increase the degradability values of the pre-angiosperm flora.
From a nutritional point of view, the data predict that herbivorous dinosaurs in a pre-angiosperm world would have preferred Equisetum above all other food sources. The crude protein content of 11.7% DM supports the view that Equisetum was a staple food resource for smaller herbivores with a greater need for a higher quality diet. Equisetum may have also been an important food source for sauropods such as specialized low browsers like dicraeosaurids, as Mesozoic sphenophytes are thought to have produced large amounts of biomass by forming dense thickets in open or disturbed moist habitats, much as they do today (Wing & Sues 1992). Since Equisetum has changed very little in its morphology, anatomy or growth habits since the Mesozoic (Gould 1968), it is reasonable to assume that it accumulated silica in its outermost cells then as well. However, Wing & Sues (1992) comment that there is no direct evidence of similar quantities of silica phytoliths in Mesozoic horsetails. While it is generally thought that the large quantity of silica in horsetails has an inhibitory effect on digestion (Van Soest 1994) and wears down the teeth of herbivores excessively (Massey et al. 2006), herbivorous taxa that are not dependent on intensive oral processing of their food, such as sauropods (Upchurch & Barrett 2000) and other dinosaur groups such as prosauropods (Crompton & Attridge 1986), stegosaurs (Galton & Upchurch 2004) and ankylosaurs (Coombs 1978), would have accepted horsetails much more readily. Extant species feeding on horsetails are found among the birds (Thomas & Prevett 1982)–-herbivores independent of dental food processing. Although not as nutritious as Equisetum, the ginkgoes, conifers and ferns such as Angiopteris would also have fulfilled nutritional needs of smaller herbivorous dinosaurs.
It has long been believed that the long neck of sauropods evolved in connection with high browsing on tall trees, which would include conifers of the Araucariaceae. With increased gut retention times, Araucaria foliage would have become especially attractive as an energy-efficient food source. Such long retention times were most probably typical of adult individuals of sauropod species owing to their low mass-specific energy requirements and enormous body size (Farlow 1987; Wings & Sander 2007).
The crude protein content of 4.4% DM in Araucaria spp. falls below the requirements of extant large herbivores such as ruminants, and therefore makes the exclusive use of Araucaria by young, actively growing animals with higher requirements for this nutrient unlikely. By contrast, it would have been feasible for adult sauropods to have relied on Araucaria as a major food source. Moreover, it should be noted that nutritional requirements of herbivores depend and develop in concert with the quality of their food (Grubb 1992; Midgley 2005), and that low dietary protein contents can lead to the evolution of low requirements for this nutrient. For marsupials such as the sugar glider (Petaurus breviceps), protein requirements as low as 1.4% DM have been described (Smith & Green 1987).
In megaherbivores like the sauropods, it is most likely that a wide range of food plants was consumed (Owen-Smith 1988), thereby compensating for the low nutrient content in single forages. However, given the global distribution of Araucaria in the Mid-Mesozoic and its tall, arborescent, forest-forming habit in conjunction with its high energy yield, it is well probable that Araucaria was targeted as commonly available nutritious food source by many high-browsing megaherbivores. The cosmopolitan distribution of Araucaria in the Jurassic, for example, extended as far north as present-day northern England (Harris 1979) and as far south as the Antarctic Peninsula (Gee 1989), as well as into the mid-latitudes of various continents (e.g. Mildenhall & Johnston 1971; Sharma & Bohra 1977; Stockey 1978; Aberhan et al. 2002). Recently, a large, virtually monospecific compression flora of Araucaria was found in a Late Jurassic bone bed in Wyoming, where it occurs alongside articulated skeletons of a diverse megaherbivore fauna (Ayer 1999), suggesting a close relationship between Araucaria and these herbivorous dinosaurs. For low-browsing taxa such as Dicraeosaurus and other diplodocids, Equisetum may have been a favoured food source. By contrast, cycads and podocarps, despite their abundance in the Jurassic record, are inferred by our data to have been of very low nutritional quality and therefore probably played a lesser role in the diet of herbivorous dinosaurs. It may be possible, using coprolites/fossilized gut contents of Mesozoic herbivores (e.g. Stokes 1964; Chin & Gill 1996; Prasad et al. 2005), to test these hypotheses on food choice, although the assignment of these remains to sauropods is difficult to prove.
We note that the hypothesis that particularly low-quality forage in Mesozoic ecosystems led directly to gigantism in dinosaurs (Midgley et al. 2002) is not substantiated by our data because the energy yield from many potential sauropod food plants is comparable to that measured in extant browse species. Estimates of the ME content of some samples even reached above 10 MJ ME/kg DM. The assumption of Tiffney (1997) that fern foliage is generally more nutritious than gymnosperm foliage is also not supported. Furthermore, our data also show a considerably higher energy yield for most taxa than indicated by the results of Weaver (1983), which were simply based on a measurement of gross energy and the assumption of constant digestibility. The ranking of potential dinosaur food plants in our study is thus considerably different from that of Weaver (1983), who ranked cycads best and Equisetum worst. We emphasize again that measuring the gross energy of plant material provides little information (if any at all) on its ME content (GfE 2003).
If the nutritional quality of some potential dinosaur food plants is higher than expected, then why do few herbivores feed on pteridophytes and gymnosperms today? Possible explanations include (i) increased plant defences in extant compared with the Mesozoic taxa (Tiffney 1997), e.g. due to severe competition with angiosperms in addition to feeding pressure from herbivores, (ii) lower tolerance to indigestible or toxic secondary plant compounds in extant herbivores (although the range of secondary compounds in pteridophytes and gymnosperms is considered to be narrower than in angiosperms; Swain 1978), and (iii) low accessibility of these plant groups to extant herbivores, owing to the low frequency of pteridophytes and gymnosperms in angiosperm-dominated ecosystems.
A major question in any discussion on giant sauropod feeding ecology is the quantities of food that must have been ingested by an individual on a daily basis (Farlow 2007). These values are strongly influenced by the level of metabolism assumed for the animal and the energy yield of the food plants. Table 2 presents our calculations of the daily dietary intake of a sauropod of either average (30 t) or maximal (70 t) BW based on different metabolic rates: 100, 75, 50 and 10% of extant tachymetabolic animals (birds and mammals), the latter percentage being at the level of the metabolic rate of extant true bradymetabolic animals (such as reptiles). Energy requirements for extant tachymetabolic animals were calculated to be 1.75 times the basal metabolic rate, plus a supplement of 15% for free-ranging conditions (Blaxter 1989; Robbins 1993). These values, in turn, were compared with those of modern tachymetabolic megaherbivore analogues, 7 t elephants (Colbert 1993). As an extreme example for extant elephants, the exceptional 10 t African elephant bull mentioned in Owen-Smith (1988) was also included in the calculations.
Table 2.
Estimates of daily dry-matter food consumption of a sauropod and an elephant with respect to differing energy densities of fodder and differing energy requirements on the part of the herbivore. (ME, metabolizable energy; DM, dry matter; BW, body weight.)
| energy requirement (kJ ME/kg BW0.75×d) | food energy (ME) content | |||
|---|---|---|---|---|
| 10 MJ/kg DM | 8 MJ/kg DM | 6 MJ/kg DM | ||
| estimates of daily dietary intake (DM) (kg) | ||||
| 30 t sauropod | 55 | 14 | 17 | 23 |
| 280 | 64 | 80 | 106 | |
| 410 | 93 | 117 | 156 | |
| 550 | 125 | 157 | 209 | |
| 70 t sauropod | 55 | 26 | 32 | 43 |
| 280 | 120 | 151 | 201 | |
| 410 | 176 | 221 | 294 | |
| 550 | 237 | 296 | 394 | |
| 7 t elephant | 550 | 42 | 53 | 70 |
| 10 t elephant | 550 | 55 | 69 | 92 |
Using the unrealistically low metabolic rate of extant reptiles (55 kJ ME/kg BW0.75), the resulting necessary food intake for a sauropod is rather unspectacular, even for a 70 t animal (26–43 kg DM per day). For an average-sized sauropod of 30 t, a metabolism of 50% of today's true tachymetabolic animals (280 kJ ME/kg BW0.75) would result in 64–106 kg DM for an animal per day, while a true tachymetabolic metabolism (550 kJ ME/kg BW0.75) results in the intakes of 125–209 kg DM. When compared with the dietary requirements of elephants, a 70 t sauropod would have had to ingest 4.3 times the amount of dry plant matter necessary for a 10 t elephant and 5.6 times the amount necessary for a 7 t elephant. However, in regard to the actual ingestion of foodstuffs, this might not have posed much of a problem for the sauropods, since adaptations such as the lack of oral food comminution of the plant matter, a wide mouth opening and the lack of cheeks (Paul 1998; Christiansen 1999) would have facilitated a high intake capacity. In regard to preferences for certain food plants based on nutritional quality, a giant sauropod of 70 t with a high energy requirement that fed only on low-energy yielding tree ferns and cycads would have needed to ingest 394 kg of dry plant matter daily. The same giant sauropod would need only approximately 237 kg of dry plant matter if browsing on a mixture of horsetails and araucarias, a total of 40% less. Another important aspect in regard to food preferences is the differing amounts of nutrients in the potential food plants. Araucaria, for example, yields high amounts of energy over a long retention time, yet it offers very little in the way of protein, especially when compared with the low energy/high protein content of tree ferns. Ginkgo offers both moderate amounts of energy and high amounts of protein, and Equisetum supplies both high protein and high energy.
5. Conclusions
In summary, our study indicates that the energy supply for the large sauropods was not as problematic as commonly thought and helps to explain how a non-angiosperm flora could have nourished a diverse fauna of megaherbivores. It is thus quite plausible that pteridophyte and gymnosperm floras could have even sustained huge dinosaurs. In particular, the pattern of fermentative behaviour in Araucaria foliage suggests that these globally widespread, tall, forest-forming trees provided the largest high-browsing sauropods with a widely available and energy-rich source of food.
Acknowledgements
We thank S. Anhalt (Botanical Gardens Cologne, Germany) and W. Lobin (Botanical Gardens University of Bonn, Germany) for their support in obtaining plant samples. This is contribution no. 42 of the DFG Research Unit 533 ‘Biology of the Sauropod Dinosaurs’.
References
- Aberhan M, Bussert R, Heinrich W.-D, Schrank E, Schultka S, Sames B, Kriwet J, Kapilima S. Palaeoecology and depositional environments of the Tendaguru Beds (Late Jurassic to Early Cretaceous, Tanzania) Mitteilungen Museum für Naturkunde Berlin, Geowissenschaftliche Reihe. 2002;5:19–44. [Google Scholar]
- Ayer J. Sauriermuseum Aathal; Aathal, Switzerland: 1999. The Howe Ranch dinosaurs. [Google Scholar]
- Blaxter K.L. Cambridge University Press; Cambridge, UK: 1989. Energy metabolism in animals and man. [Google Scholar]
- Blümmel M, Ørskov E.R. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Technol. 1993;40:109–119. doi:10.1016/0377-8401(93)90150-I [Google Scholar]
- Blümmel M, Aiple K.-P, Steingaß H, Becker K. A note on the stoichiometrical relationship of short chain fatty acid production and gas formation in vitro in feedstuffs of widely differing quality. J. Anim. Physiol. Anim. Nutr. 1999;81:157–167. doi:10.1046/j.1439-0396.1999.813205.x [Google Scholar]
- Chin K, Gill B.D. Dinosaurs, dung beetles, and conifers: participants in a Cretaceous food web. Palaios. 1996;11:280–285. doi:10.2307/3515235 [Google Scholar]
- Christiansen P. On the head size of sauropodomorph dinosaurs: implications for ecology and physiology. Hist. Biol. 1999;13:269–297. [Google Scholar]
- Clauss M, Schwarm A, Ortmann S, Streich J.W, Hummel J. A case of non-scaling in mammalian physiology? Body size, digestive capacity, food intake, and ingesta passage in mammalian herbivores. Comp. Biochem. Physiol. A. 2007;148:249–265. doi: 10.1016/j.cbpa.2007.05.024. doi:10.1016/j.cbpa.2007.05.024 [DOI] [PubMed] [Google Scholar]
- Coe M.L, Dilcher D.L, Farlow J.O, Jarzen D.M, Russell D.A. Dinosaurs and land plants. In: Friis E.M, Chaloner W.G, Crane P.R, editors. The origins of angiosperms and their biological consequences. Cambridge University Press; Cambridge, UK: 1987. pp. 225–258. [Google Scholar]
- Colbert E.H. Feeding strategies and metabolism in elephants and sauropod dinosaurs. Am. J. Sci. 1993;293:1–19. [Google Scholar]
- Coombs W.P. The families of the ornithischian dinosaur order Ankylosauria. Palaeontology. 1978;21:143–170. [Google Scholar]
- Crompton A.W, Attridge J. Masticatory apparatus of the larger herbivores during Late Triassic and Early Jurassic times. In: Padian K, editor. The beginning of the age of dinosaurs. Cambridge University Press; Cambridge, UK: 1986. pp. 223–236. [Google Scholar]
- Dodson P. Sauropod paleoecology. In: Weishampel D.B, Dodson P, Osmólska H, editors. The Dinosauria. University of California Press; Berkeley, CA: 1990. pp. 402–407. [Google Scholar]
- Dunham E, Overall K.I, Porter W.P, Forster C.A. Implications of ecological energetics and biophysical and developmental constraints for life-history variation in dinosaurs. In: Farlow J, editor. Paleobiology of the dinosaurs. Geological Society of America; Boulder, CO: 1989. pp. 1–19. [Google Scholar]
- Engelmann G.F, Chure D.J, Fiorillo A.R. The implications of a dry climate for the paleoecology of the fauna of the Upper Jurassic Morrison formation. Sediment. Geol. 2004;167:297–308. doi:10.1016/j.sedgeo.2004.01.008 [Google Scholar]
- Farlow J.O. Speculations about the diet and digestive physiology of herbivorous dinosaurs. Paleobiology. 1987;13:60–72. [Google Scholar]
- Farlow J.O. A speculative look at the paleoecology of large dinosaurs of the Morrison formation, or, life with Camarasaurus and Allosaurus. In: Kvale E.P, Brett-Surman M.K, Farlow J, editors. Dinosaur paleoecology and geology: the life and times of Wyoming's Jurassic dinosaurs and marine reptiles. Geoscience Adventure Workshop; Shell, WY: 2007. pp. 98–151. [Google Scholar]
- Fiorillo A.R. Dental microwear patterns from the sauropod dinosaurs Camarasaurus and Diplodocus: evidence for resource partitioning in the Late Jurassic of North America. Hist. Biol. 1998;13:1–16. [Google Scholar]
- Galton, P. M., & Upchurch, P. 2004 Stegosauria In The Dinosauria (eds D. B. Weishampel, P. Dodson & H. Osmólska), pp. 343–362, 2nd edn. Berkeley, CA: University of California Press.
- Gee C.T. Revision of the Late Jurassic/Early Cretaceous flora from Hope Bay, Antarctica. Palaeontogr. B. 1989;213:149–214. [Google Scholar]
- Gesellschaft für Ernährungsphysiologie (GfE) DLG-Verlag; Frankfurt, Germany: 2003. Recommendations for the supply of energy and nutrients to goats. [Google Scholar]
- Gould R.E. Morphology of Equisetum laterale Philips, 1829, and E. bryanii sp. nov. from the Mesozoic of south-eastern Queensland. Aust. J. Bot. 1968;16:153–176. doi:10.1071/BT9680153 [Google Scholar]
- Grubb P.J. A positive distrust in simplicity—lessons from plant defences and from competition among plants and among animals. J. Ecol. 1992;80:585–610. doi:10.2307/2260852 [Google Scholar]
- Harris T.M. Coniferales. vol. V. British Museum (Natural History); London, UK: 1979. The Yorkshire Jurassic flora. [Google Scholar]
- Hummel J, Südekum K.-H, Streich W.J, Clauss M. Comparative in vitro fermentative behaviour of temperate forage plant classes—potential consequences for herbivore ingesta retention times. Funct. Ecol. 2006;20:989–1002. doi:10.1111/j.1365-2435.2006.01206.x [Google Scholar]
- Jansson A. Feed recommendations for horses. Department of Equine Studies, Swedish University of Agricultural Sciences; Uppsala, Sweden: 2004. [Google Scholar]
- Krassilov V.A. Changes of Mesozoic vegetation and the extinction of dinosaurs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1981;34:207–224. doi:10.1016/0031-0182(81)90065-1 [Google Scholar]
- Mackie R.I. Mutualistic fermentative digestion in the gastrointestinal tract: diversity and evolution. Integr. Comp. Biol. 2002;42:319–326. doi: 10.1093/icb/42.2.319. doi:10.1093/icb/42.2.319 [DOI] [PubMed] [Google Scholar]
- Marshall C.L, Stevens C.E. Yes Virginia, there were foregut fermenting dinosaurs. Proc. Comp. Nutr. Soc. 2000:138–142. [Google Scholar]
- Massey F.P, Ennos A.R, Hartley S.E. Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006;75:595–603. doi: 10.1111/j.1365-2656.2006.01082.x. doi:10.1111/j.1365-2656.2006.01082.x [DOI] [PubMed] [Google Scholar]
- Mazzetta G.A, Christiansen P, Farina R. Giants and bizarres: body size of some southern South American Cretaceous dinosaurs. Hist. Biol. 2004;16:71–83. [Google Scholar]
- Menke K.H, Huss K.H. Ulmer Verlag; Stuttgart, Germany: 1987. Tierernährung und Futtermittelkunde. [Google Scholar]
- Menke K.H, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of the digestibility and metabolisable energy content of ruminant feedingstuffs from the gas production when incubated with rumen liquor in vitro. J. Agr. Sci. Camb. 1979;93:217–222. [Google Scholar]
- Midgley J.J. Why don't leaf-eating animals prevent the formation of vegetation? Relative vs absolute dietary requirements. New Phytol. 2005;168:271–273. doi: 10.1111/j.1469-8137.2005.01564.x. doi:10.1111/j.1469-8137.2005.01564.x [DOI] [PubMed] [Google Scholar]
- Midgley J.J, Midgley G, Bond W.J. Why were dinosaurs so large? A food quality hypothesis. Evol. Ecol. Res. 2002;4:1093–1095. [Google Scholar]
- Mildenhall D.C, Johnston M.R. A megastrobilus belonging to the genus Araucarites from the Upper Motuan (Upper Albian), Wairarapa, North Island, New Zealand. New Zeal. J. Bot. 1971;9:67–79. [Google Scholar]
- Owen-Smith N. Megaherbivores. Cambridge University Press; Cambridge, UK: 1988. [Google Scholar]
- Paul G.S. Terramegathermy and Cope's rule in the land of titans. Mod. Geol. 1998;23:179–217. [Google Scholar]
- Prasad V, Strömberg C.A.E, Alimohammadian H, Sahni A. Dinosaur coprolites and the early evalution of grasses and grazers. Science. 2005;310:1177–1180. doi: 10.1126/science.1118806. doi:10.1126/science.1118806 [DOI] [PubMed] [Google Scholar]
- Robbins C.T. Wildlife feeding and nutrition. San Diego Academic Press; San Diego, CA: 1993. [Google Scholar]
- Sharma B.D, Bohra D.R. Petrified araucarian megastrobili from the Jurassic of the Rajmahal Hils, India. Acta Palaeobot. 1977;18:31–36. [Google Scholar]
- Smith A.P, Green S.W. Nitrogen requirements of the sugar glider (Petaurus greviceps), an omnivorous marsupial, on a honey-pollen diet. Physiol. Zool. 1987;60:82–92. [Google Scholar]
- Stevens K.A, Parrish J.M. Digital reconstructions of sauropod dinosaurs and implications for feeding. In: Curry Rogers K.A, Wilson J.A, editors. The sauropods: evolution and paleobiology. University of California Press; Berkeley, CA: 2005. pp. 178–200. [Google Scholar]
- Stockey R.A. Reproductive biology of Cerro Cuadrado (Jurassic) conifers: ontogeny and reproductive strategies in Araucaria mirabilis (Sepegazzini) Windhausen. Palaeontographica B. 1978;166:1–15. [Google Scholar]
- Stokes W.L. Fossilized stomach contents of a sauropod dinosaur. Science. 1964;143:576–577. doi: 10.1126/science.143.3606.576. doi:10.1126/science.143.3606.576 [DOI] [PubMed] [Google Scholar]
- Swain T. Plant–animal coevolution: a synoptic view of the Paleozoic and Mesozoic. In: Harborne J.B, editor. Biochemical aspects of plant and animal coevolution. Academic Press; London, UK: 1978. pp. 3–19. [Google Scholar]
- Taggart R.E, Cross A.T. The relationship between land plant diversity and productivity and patterns of dinosaur herbivory. In: Wolberg D.L, Stump E, Rosenberg G.D, editors. Dinofest international. Academy of Natural Sciences; Philadelphia, PA: 1997. pp. 403–416. [Google Scholar]
- Thomas V.G, Prevett J.P. The role of horsetails (Equisetaceae) in the nutrition of northern-breeding geese. Oecologia. 1982;53:359–363. doi: 10.1007/BF00389014. doi:10.1007/BF00389014 [DOI] [PubMed] [Google Scholar]
- Tiffney B.H. Land plants as food and habitat in the age of dinosaurs. In: Farlow J, Brett-Surman M.K, editors. The complete dinosaur. Indiana University Press; Bloomington, MN: 1997. pp. 352–370. [Google Scholar]
- Upchurch P, Barrett P.M. The evolution of sauropod feeding mechanisms. In: Sues H.D, editor. Evolution of herbivory in terrestrial vertebrates. Cambridge University Press; Cambridge, UK: 2000. pp. 79–122. [Google Scholar]
- Van Soest P.J. Nutritional ecology of the ruminant. Cornell University Press; Ithaca, NY: 1994. [Google Scholar]
- Waldo D.R, Smith L.W, Cox E.L. Model of cellulose disappearance from the rumen. J. Dairy Sci. 1972;55:125–129. doi: 10.3168/jds.S0022-0302(72)85442-0. [DOI] [PubMed] [Google Scholar]
- Weaver J.C. The improbable endotherm: the energetics of the sauropod dinosaur Brachiosaurus. Paleobiology. 1983;9:173–182. [Google Scholar]
- Wing S.L, Sues H.D. Mesozoic and Early Cenozoic terrestrial ecosystems. In: Behrensmeyer A.K, Damuth J.D, DiMichele W.A, Potts R, Sues H.-D, Wing S.L, editors. Terrestrial ecosystems through time. University of Chicago Press; Chicago, IL: 1992. pp. 326–416. [Google Scholar]
- Wing S.L, Tiffney B.H. Interactions of angiosperms and herbivorous tetrapods through time. In: Friis E.M, Chaloner W.G, Crane P.R, editors. The origins of angiosperms and their biological consequences. Cambridge University Press; Cambridge, UK: 1987. pp. 203–224. [Google Scholar]
- Wings O, Sander P.M. No gastric mill in sauropod dinosaurs: new evidence from analysis of gastrolith mass and function in ostriches. Proc. R. Soc. B. 2007;274:635–640. doi: 10.1098/rspb.2006.3763. doi:10.1098/rspb.2006.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]