Abstract
For their size, barnacles possess the longest penis of any animal (up to eight times their body length). However, as one of few sessile animals to copulate, they face a trade-off between reaching more mates and controlling ever-longer penises in turbulent flow. We observed that penises of an intertidal barnacle (Balanus glandula) from wave-exposed shores were shorter than, stouter than, and more than twice as massive for their length as, those from nearby protected bays. In addition, penis shape variation was tightly correlated with maximum velocity of breaking waves, and, on all shores, larger barnacles had disproportionately stouter penises. Finally, field experiments confirmed that most of this variation was due to phenotypic plasticity: barnacles transplanted to a wave-exposed outer coast produced dramatically shorter and wider penises than counterparts moved to a protected harbour. Owing to the probable trade-off between penis length and ability to function in flow, and owing to the ever-changing wave conditions on rocky shores, intertidal barnacles appear to have acquired the capacity to change the size and shape of their penises to suit local hydrodynamic conditions. This dramatic plasticity in genital form is a valuable reminder that factors other than the usual drivers of genital diversification—female choice, sexual conflict and male–male competition—can influence genital form.
Keywords: genitalia, phenotypic plasticity, trade-off, sexual selection, morphology, marine invertebrate
1. Introduction
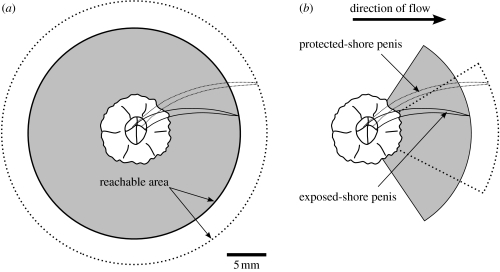
To cope with a sessile lifestyle and an evolutionary legacy of mandatory internal fertilization (Ruppert et al. 2004), barnacles have evolved a remarkable mechanism for copulating with distant neighbours: penises that can extend up to eight times their body length (Darwin 1854; table 1). However, as one of very few sessile animals to copulate (Ruppert et al. 2004), barnacles face a delicate trade-off. Although longer penises greatly increase the number of potential mates—because the searchable area expands as the square of penis length—the benefits of larger penises may be outweighed by increased drag as water turbulence intensifies, particularly in species that live on wave-exposed shores (figure 5).
Table 1.
Extended penis length in animals.
| common name | scientific name | penis length relative to body length | references |
|---|---|---|---|
| burrowing barnacle | Cryptophialus minutus | 8.0 | Darwin (1854) |
| Japanese acorn barnacle | Tetraclita japonica | 3.9 | Murata et al. (2001) |
| Pacific acorn barnacle | Balanus glandula | 3.6 | C. J. Neufeld (unpublished data) |
| rove beetle | Aleochara tristis | 2.0 | Gack & Peschke (2005) |
| Argentine lake duck | Oxyura vittata | 1.0 | McCracken et al. (2001) |
| hat snail | Calyptraea morbida | 1.0 | Chen & Soong (2000) |
| seed bug | Lygaeus simulans | 1.0 | Tadler (1999) |
| feather mite | Proterothrix sp. | 1.0 | H. Proctor (2007, personal communication) |
| sand flea | Tunga penetrans | 1.0 | H. Proctor (2007, personal communication) |
| slipper limpet | Crepidula spp. | 0.6 | Brown & Olivares (1996) |
| spider | Tidarren spp. | 0.5 | Ramos et al. (2004) |
| ostracod | Candona suburbana | 0.3 | Cohen & Morin (1990) |
Figure 5.
Proposed trade-off between penis form and flow environment. (a) In quiet water, the 25% longer penis of a protected-shore barnacle relative to its exposed-shore counterpart (figure 2a) may increase the reachable area (the area within which to find mates) by as much as 90% (compare the area encompassed by the dotted line with the shaded area). (b) In high flow, a small increase in the ability of the stouter exposed-shore penis form to resist bending due to drag would enable the penis of an exposed-shore barnacle to extend across more streamlines and yield an effective reachable area (shaded region) greater than that of a similar-sized quiet water barnacle with a penis more prone to downstream deflection due to drag (area bounded by the dotted line).
Curiously, because most free-living barnacles are simultaneous hermaphrodites (Charnov 1987), they could potentially self-fertilize, yet in most species self-fertilization is rare or non-existent (Barnes & Crisp 1956; Furman & Yule 1990). Instead, most barnacles reproduce by extending impressively long penises (table 1) to find and fertilize distant mates (Klepal 1990). However, such long penises pose a major challenge because many intertidal barnacle species live under a wide range of wave conditions where water velocities can span up to three orders of magnitude (Denny 1988) and reach extremes of up to 20 m s−1 (Helmuth & Denny 2003). Consequently, individuals with penises well suited for mating in quiet waters may be poorly suited for copulating on wave-exposed shores. Indeed, other structures that must extend into flow, like the long feathery feeding legs of barnacles, differ dramatically in form between protected harbours and nearby wave-exposed sites (Arsenault et al. 2001). Furthermore, in one species, these differences in feeding leg form arise primarily due to phenotypic plasticity (Marchinko 2003).
Therefore, because penises of barnacles probably face hydrodynamic constraints similar to those experienced by feeding legs, we predicted that (i) penis size and shape should vary among sites with different wave-force regimes: penises should be shorter (i.e. smaller) on wave-exposed shores owing to more turbulent hydrodynamic conditions, and, for a given length, penises should be stouter at wave-exposed sites to better resist bending in turbulent flow, and (ii) because wave action varies so dramatically in space and time, differences in penis form should arise primarily due to phenotypic plasticity.
2. Material and methods
(a) Artificial inflation of barnacle penises
To ensure that relaxed penis length was a valid proxy for extended penis length, we mechanically inflated penises of barnacles from an exposed shore (Prasiola Point, 48°49′01″ N, 125°10′02″ W; velocity of breaking waves approx. 5 m s−1; C. J. Neufeld 2006, unpublished data) and a protected shore (Grappler Inlet; 0.05 m s−1; Marchinko & Palmer 2003). To avoid potential density-induced differences in penis form, 25 solitary barnacles—barnacles with plates not touching another barnacle but with an approximately equal number of neighbours within 1–2 cm—were collected in the middle of the Balanus glandula zone at each site on 14 December 2007. After freezing for 24 hours at −8°C, barnacles were thawed in seawater and the soma was removed, photographed under a dissecting microscope at 6–8×, blotted dry and weighed to the nearest 0.1 mg following Arsenault et al. (2001). The soma was then cut between the first and second pair of thoracic legs, inserted onto the tapered end of a seawater-filled plastic capillary tube (1.09 mm in outside diameter, 0.38 mm in inside diameter and approx. 25 mm long) and carefully glued in place (Krazy Glue, Elmer's Products, Columbus, OH) while keeping the penis tissue moist. The capillary tube was then inserted onto the end of a hypodermic needle (0.5 mm in outside diameter and 0.2 mm in inside diameter) and fitted onto a 10 ml plastic syringe filled with seawater. The penis and remaining feeding legs were positioned in seawater under a dissecting microscope. The penis was oriented perpendicular to the field of view, and pressure was applied to the syringe to slowly inflate the penis until (i) the glue failed, (ii) the soma tissue or cuticle ruptured, or (iii) the penis inflated fully. Full inflation was recorded when additional pressure on the syringe failed to extend the penis further and all annulations of the penis cuticle had disappeared. At this point, the penis was photographed again. This process was repeated for approximately 20 individuals per site until we had achieved full penis extension for three individuals from each population.
(b) Field survey
Balanus glandula (Darwin) were collected from eight sites that spanned a wide range of maximum water velocities within Barkley Sound, BC, Canada. Six sites in the order of decreasing wave exposure (Seppings Island, SI, Bordelais Island, Wizard Islets, Kelp Bay, Self Point and Ross Islets, RI) are described in Arsenault et al. (2001) and two additional low-velocity sites (Bamfield Inlet, BI and Grappler Inlet) are described in Marchinko & Palmer (2003). Barnacles were collected between 21 and 24 February 2006, when the majority of individuals in this area are reproductively active (Strathmann 1987) and have fully developed penises (Barnes 1992). Twenty solitary barnacles—barnacles with plates not touching another barnacle but with an approximately equal number of neighbours within 1–2 cm—were collected in the middle of the B. glandula zone at each site. Owing to errors during dissection, sample sizes used to determine site means differed slightly (from n=17 to 20) among sites and analyses. Owing to the difficulty of measuring winter water velocity using standard techniques, we used the known relationship between leg length and maximum velocity of breaking waves (Arsenault et al. 2001) to estimate the maximum water velocity at each site. In addition, we used empirically determined summer velocities at these sites (Arsenault et al. 2001; Marchinko & Palmer 2003) for comparison (figure 1).
Figure 1.
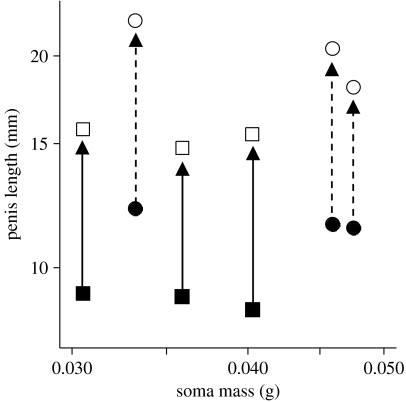
Relaxed penis length (filled symbols) and corresponding manually inflated penis length (open symbols) of barnacles from an exposed shore (Prasiola Point; squares, solid lines) and a protected shore (Grappler Inlet; circles, dashed lines) in Barkley Sound, BC, Canada. Paired points represent individual barnacles and arrows denote transition from relaxed to inflated penis length (mean proportional inflation±s.e.m.: protected shore=1.82±0.082; exposed shore=1.75±0.044; p=0.77).
(c) Field transplant experiment
To differentiate between genetic control (differential settlement and/or selective mortality) and environmental control (phenotypic plasticity) of penis form, we transplanted barnacles from two source populations to each of two destination sites. On 25 September 2006, adult B. glandula were collected on mussel shells (Mytilus californianus and Mytilus trossulus) from two source populations chosen for a substantial difference in wave force between sites (Arsenault et al. 2001) and for a sizeable supply of adult barnacles growing on mussels: a protected shore (RI; Arsenault et al. 2001) and an exposed shore (SI; Arsenault et al. 2001) in Barkley Sound, BC, Canada. Mussel shells were cut using a variable speed rotary tool so that one barnacle occupied each mussel shell fragment. Mussel shell fragments were spaced approximately 15 mm apart and glued to two 10×13 cm Plexiglas plates using marine epoxy putty (Z-spar Splash Zone Compound) in a 5×8 grid alternating between protected- and exposed-shore source populations. Plates were kept overnight in flowing seawater and then bolted to the rock in the middle of the B. glandula zone in two outplant locations chosen for a more than fivefold variation in wave force: a protected shore (BI; Marchinko & Palmer 2003) and an exposed shore (SI; Arsenault et al. 2001). On 13 February 2007 (after 20 weeks), during the local reproductive period, intact transplanted barnacles were collected to measure barnacle penis form. During the outplant period, barnacles suffered some mortality at all sites. The final numbers used in the analyses were as follows: protected (site RI) to protected (site BI), n=18; protected (RI) to exposed (SI), n=6; exposed (SI) to protected (BI), n=16; exposed (SI) to exposed (SI), n=13. Furthermore, owing to an omission during dissection, soma mass was not measured for one individual in the protected to exposed group (RI to BI). Consequently, data from this individual were excluded from analyses involving soma mass.
(d) Sample processing
Samples were frozen at −8°C and processed within 30 days of collection. Barnacles were thawed in seawater and the soma was removed, blotted dry and weighed to the nearest 0.1 mg following Arsenault et al. (2001). The penis and the sixth thoracic leg from the left side were removed, wet mounted in seawater and photographed under a dissecting microscope at 15–31×. Photographs were measured to the nearest 10 μm using ImageJ (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MA, USA, http://rsb.info.nih.gov/ij/, 1997–2008). For the field survey barnacles, after being photographed, penises were dried to a constant mass and weighed to the nearest 0.001 mg on a Cahn C-31 analytical microbalance (Thermo Electronic Corporation, Waltham, MA).
(e) Statistical analyses
All statistics were calculated using R (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). Assumptions of equal variance (using Levene's test) and normal distribution (using Shapiro–Wilk test) were met in all cases. To test for differences in extended length and proportional change of artificially inflated penises, we used two-sample t-tests on log-transformed data. For the field survey, least-square means of body size (figure 2a,b) or penis length (figure 2c,d) were calculated using analysis of covariance (ANCOVA) assuming an equal slope among sites. Regression and ANCOVA analyses were calculated on log-transformed data. Where slopes varied significantly among groups (table S2, electronic supplementary material), we recalculated least-square means at first, second and third quartiles of penis length assuming unequal slopes (table S5, electronic supplementary material). In all cases, the conclusions were not affected (figure S1, electronic supplementary material). For the field transplant experiment, we computed 2 two-way ANCOVAs on log-transformed data with source population and transplant location as factors and soma mass (figure 4a) or penis length (figure 4b) as the covariate.
Figure 2.
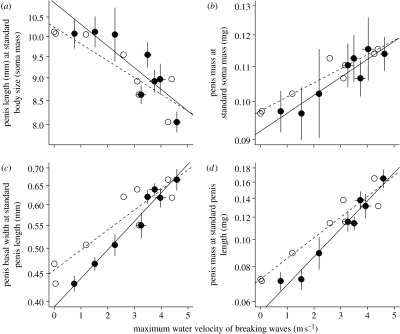
Variation in penis form of the intertidal barnacle Balanus glandula as a function of wave exposure among eight sites in Barkley Sound, BC, Canada. (a) Penis length of a standard-sized barnacle (soma wet mass=0.0172 g, approx. 8 mm basal width; F1,6=16.476, p=0.007). (b) Penis mass of a standard-sized barnacle (soma wet mass=0.0172 g, approx. 8 mm basal width; F1,6=37.857, p<0.001). (c) Basal width of a standard-length barnacle penis (penis length=9.21 mm; F1,6=154.05, p<0.001, r2=0.96). (d) Mass of a standard-length barnacle penis (penis length=9.21 mm; F1,6=149.05, p<0.001, r2=0.95). Filled circles (solid line) represent velocities calculated from the known relationship between leg length and water velocity at each site (see §2). Open circles (dashed line) are empirically determined summer velocities at these sites (Arsenault et al. 2001; Marchinko & Palmer 2003). All points are mean±s.e.m. (error bars are only shown for filled circles for clarity; in some cases, error bars are smaller than the symbol size).
Figure 4.
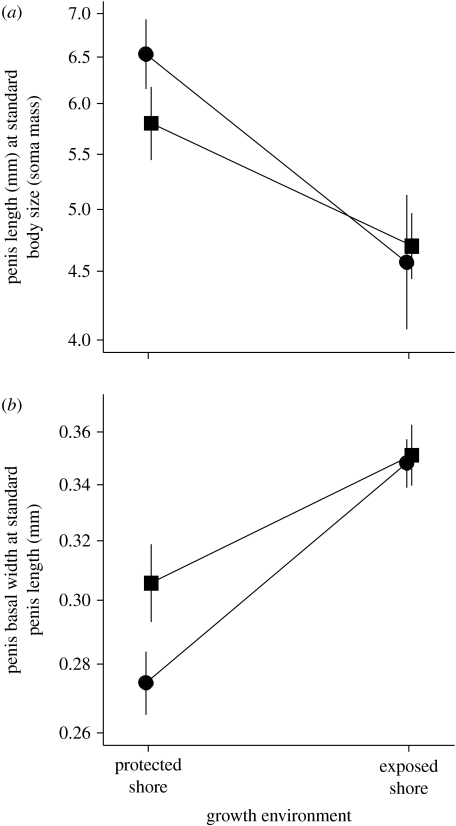
Relaxed penis form of the barnacle Balanus glandula from a protected shore (RI; filled circles) and an exposed shore (SI; filled squares) 20 weeks after the transplant to a protected shore (BI) and an exposed shore (SI) in Barkley Sound, BC, Canada. (a) Penis length of a standard-sized barnacle (soma wet mass=0.0058 g, basal width approx. 6 mm). (b) Basal width of a standard-length barnacle penis (penis length=5.59 mm). All points are mean±s.e.m.
3. Results
(a) Artificial inflation of barnacle penises
Relaxed penis length was a consistent indicator of maximum penis length for both protected- and exposed-shore barnacles (figure 1). Mean proportional inflation did not differ between source populations (p=0.772) and erect penises of protected-shore barnacles remained significantly longer than those of exposed-shore barnacles (p=0.039).
(b) Field survey
We observed differences in both penis size and shape. When corrected for body size (soma mass), penises from the most wave-exposed site were 25% shorter than those from the most protected bay and penis length was strongly negatively correlated with velocity when compared across all eight sites (figure 2a). Furthermore, penis mass (corrected for body size) was 16% higher in the most exposed site when compared with the most protected site and this trait was positively correlated with water velocity across sites (figure 2b).
In contrast to the modest differences in penis size, we observed substantial differences in penis shape among the same sites. Relative to penis length, penis basal width at the most exposed site was more than 50% larger than that at the most protected site (figures 2c and 3). Furthermore, penis mass (standardized for penis length) was more than twice as high in the most wave-exposed site compared with the most protected bay (figure 2d). In both cases, water velocity explained a high percentage of among-site variation in penis shape: 96% for penis basal width at standard length (figure 2c) and 95% for penis mass (figure 2d).
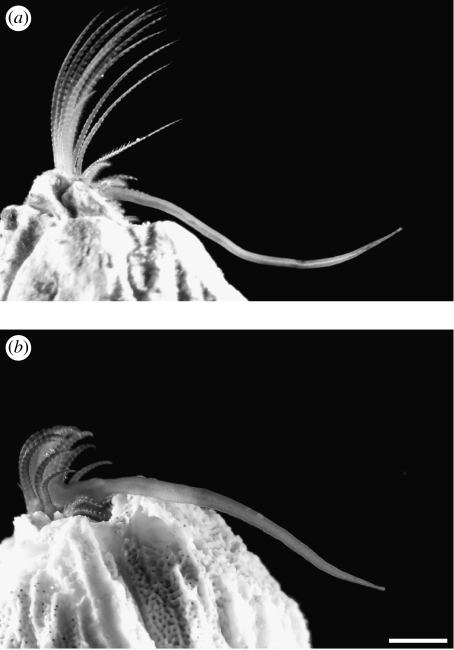
Figure 3.
Relaxed penis form of similar-sized barnacles (Balanus glandula) from Barkley Sound, BC, Canada. (a) Protected shore (Grappler Inlet; maximum velocity of breaking waves=0.75 m s−1, soma wet mass=0.019 g, basal width=8.5 mm). (b) Wave-exposed outer coast (SI; maximum velocity of breaking waves=4.5 m s−1, soma wet mass=0.024 g, basal width=8.9 mm). Scale bar, 2 mm.
(c) Field transplant experiment
When corrected for body size, barnacles transplanted to the wave-exposed shore produced 25% shorter (analysis of covariance on log-transformed data, F1,45=15.46, p<0.001; figure 4a) and 20% wider (F1,46=11.57, p=0.001; figure 4b) penises than counterparts moved to the protected harbour after a period of 20 weeks. In addition, penis length exhibited a disproportionately greater response to the local growth environment in larger barnacles than in smaller ones (F1,45=4.59, p=0.038). Finally, regardless of whether barnacles originated from exposed- or protected-shore populations, both penis size (penis length relative to soma mass; F1,45=0.39, p=0.534) and penis shape (penis basal width relative to penis length; F1,46=1.90, p=0.175) converged to similar values at both transplant sites (figure 4).
4. Discussion
(a) Adaptive significance of penis form variation
The remarkably close fit between wave force and penis form (figure 2c,d) suggests that even slight deviations from an optimal shape in a particular environment drastically reduce opportunities to mate. Two lines of argument suggest that the spatial variation in penis form we observed is adaptive.
First, in the absence of hydrodynamic effects, longer penises should exponentially increase the number of potential mates because the reachable area increases as the square of penis length (figure 5a). Second, the penis form variation we observed is consistent with two predictions from engineering theory. Beam theory (Vogel 2003) predicts that stouter, heavier penises should better resist bending caused by drag, so stouter penises should enable more successful copulations on wave-exposed shores (figure 5b). In addition, hydrodynamic theory predicts that, under turbulent flow conditions, larger penises should be disproportionately stouter than smaller ones because drag, and hence the overall bending force, increases to the second power of length (Vogel 2003). Indeed, we observed that penis basal width exhibited significant and similar positive allometry relative to body size and penis length at all sites (table S1, electronic supplementary material), and penises of larger barnacles exhibited a disproportionately greater response when transplanted to different flow conditions (table S6, electronic supplementary material).
Although the observed variation in penis form seems most likely to be adaptive, one non-adaptive explanation that cannot be ruled out is developmental pleiotropy (Ronemus et al. 1996): mechanisms controlling the development of leg length and penis length may not be independent. Nonetheless, pleiotropy could be an elegant developmental mechanism for ensuring that penis form varied appropriately in response to wave action. Because legs are exposed to flow more frequently, they would be a more reliable sensor of flow conditions and could ‘signal’ to the penis to change form. In other words, developmental pleiotropy itself could represent an adaptive evolutionary response that couples the developmental control of leg and penis form.
(b) Is barnacle penis form plastic?
A close fit between penis form and environment could arise in three ways: (i) differential settlement, where genetically different larvae settle in a specific environment appropriate to their genotype, (ii) selective mortality, where larvae of all genotypes settle independent of environmental conditions but mismatched genotypes suffer higher mortality, or (iii) phenotypic plasticity, where different phenotypes are directly induced by different growth conditions. We found that barnacles transplanted to quiet water produced longer and thinner penises than those moved to wave-exposed shores and converged on the same phenotype at a given transplant site regardless of source population; therefore, penis size and shape appear to vary among sites primarily due to phenotypic plasticity, and little if at all due to genetic differences (via differential settlement or selective mortality) among populations.
Phenotypic plasticity in penis form may have arisen as an adaptive strategy to cope with spatial and/or temporal variation in flow conditions that developing barnacles experience. Because barnacles have a long pelagic larval duration (Strathmann 1987) and probably travel long distances from their source population prior to settlement, offspring may end up in a vastly different flow environment than that experienced by their parents. Furthermore, independent of where they end up, barnacles may face significant temporal variation in flow. In addition to seasonal patterns in swell-generating storm events (swell increases dramatically in the winter months at our study sites), the settlement, growth and mortality of other animals may also cause persistent changes in flow between successive years. Therefore, the ability to alter penis form after settlement may be advantageous to cope with aspects of spatial as well as temporal variation in flow conditions.
(c) Constraints on penis length
Regardless of how they develop, longer penises are probably constrained by turbulent flow (figures 1 and 5b). However, at low-flow sites where drag is less likely to limit penis form, several other ‘braking’ effects may also restrict the development of ever-longer penises in barnacles. First, although oversized male genitals are thought to entail few costs (Eberhard 1985; Chapman et al. 2003), oversized reproductive organs may increase susceptibility to predators by significantly reducing escape velocities (Ramos et al. 2004; Langerhans et al. 2005). Given that even the agile feeding legs of barnacles are eaten by fishes (Barnes 1999), barnacles' unwieldy penises are probably even more vulnerable while exposed during copulation. Second, as length increases, the ability to control such a long appendage may become difficult: in one beetle species, a similar cost appears to have promoted an elaborate behaviour to retract its outsized intromittent organ without causing irreversible damage (Gack & Peschke 2005). Finally, beyond a certain length, the benefit of greater reach may be outweighed by the cost of producing and housing the large genitalia and ample sperm necessary to fertilize a growing number of distant mates. Collectively, any number of these effects could reduce the advantage of ever-longer penises and may account for the levelling-off of penis length with decreasing water flow that we observed at the most protected sites (figure 2a).
In summary, our results suggest that penis size and shape in B. glandula are strongly influenced by a trade-off between length and manoeuvrability that varies with wave force. Through the capacity to grow wider and heavier penises (for their length) on wave-exposed shores, B. glandula may improve their mating success under turbulent flow. This study provides a rare example of conspicuous phenotypic plasticity in animal genitalia and reveals how factors other than the usual drivers of genital diversification—female choice, sexual conflict and male–male competition (Eberhard 1985; Arnqvist 1998; Eberhard et al. 1998; Hosken & Stockley 2004)—can influence genital form.
Acknowledgments
All work conducted in this study complied with the current Canadian Council on Animal Care guidelines.
We thank Mike Hart for stimulating our thinking about barnacle penises; C. Gruman and H. Parkis for their field assistance; the director and staff of the Bamfield Marine Sciences Centre for their logistical support; D. Schindler and S. Leys for their laboratory support; H. Proctor for the unpublished arthropod penis lengths the Huu-ay-aht First Nation for continued access to the Prasiola Point site; and C. Bergstrom, L. Hammond, A. Eaves and A. Churcher for their comments on earlier drafts. C.J.N. designed and performed the transplant experiment, collected and measured the samples, inflated the penises, analysed the data and wrote the majority of the manuscript. A.R.P. provided the initial idea, consulted on experimental design and aided in writing the manuscript. This research was funded by a University of Alberta Graduate Research Assistantship to C.J.N. and a Natural Sciences and Engineering Research Council Discovery grant (A7245) to A.R.P.
Supplementary Material
Fig. S1 provides support for the use of equal slopes when standardizing for body size in the Field Survey comparisons; The supplementary discussion describes potential sources of variation in the Field Survey and sources of mortality in the Field Transplant experiment; tables S1–S5 provide additional statistical data to support the results and discussion in the main body of the paper
References
- Arnqvist G. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 1998;393:784–786. doi:10.1038/31689 [Google Scholar]
- Arsenault D.J, Marchinko K.B, Palmer A.R. Precise tuning of barnacle leg length to coastal wave action. Proc. R. Soc. B. 2001;268:2149–2154. doi: 10.1098/rspb.2001.1776. doi:10.1098/rspb.2001.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. The reproductive periods and condition of the penis in several species of common Cirripedes. Oceanogr. Mar. Biol. 1992;30:483–525. [Google Scholar]
- Barnes M. The mortality of intertidal cirripedes. Oceanogr. Mar. Biol. 1999;37:153–244. [Google Scholar]
- Barnes H, Crisp D.J. Evidence of self-fertilization in certain species of barnacles. J. Mar. Biol. Assoc. UK. 1956;35:631–639. [Google Scholar]
- Brown D.I, Olivares C.A. A new species of Crepidula (Mollusca: Mesogastropoda: Calyptraeidae) from Chile: additional characters for the identification of eastern Pacific planar Crepidula group. J. Nat. Hist. 1996;30:1443–1458. doi:10.1080/00222939600770821 [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. doi:10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Charnov E.L. Sexuality and hermaphroditism in barnacles: a natural selection approach. In: Southward A.J, editor. Barnacle biology. A. A. Balkema; Rotterdam, The Netherlands: 1987. pp. 89–102. [Google Scholar]
- Chen M.H, Soong K. Sex change in the hat snail, Calyptraea morbida (Reeve) (Gastropoda: Calyptraeidae): an analysis of substratum, size, and reproductive characteristics. Veliger. 2000;43:210–217. [Google Scholar]
- Cohen A.C, Morin J.G. Patterns of reproduction in ostracods - A Review. J. Crustac. Biol. 1990;10:184–211. doi:10.2307/1548480 [Google Scholar]
- Darwin, C. 1854 The Balanidae (or Sessile Cirripedes); the Verrucidae, etc A Monograph of the Sub-class Cirripedia, with figures of all the species. London, UK: The Ray Society.
- Denny M.W. Princeton University Press; Princeton, NJ: 1988. Biology and the mechanics of the wave-swept environment. [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, UK: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Eberhard W.G, Huber B.A, Rodriguez R.L, Briceno R.D, Salas I, Rodriquez V. One size fits all? Relationships between the size and degree of variation in genitalia and other body parts in twenty species of insects and spiders. Evolution. 1998;52:415–431. doi: 10.1111/j.1558-5646.1998.tb01642.x. doi:10.2307/2411078 [DOI] [PubMed] [Google Scholar]
- Furman E.R, Yule A.B. Self-fertilization in Balanus improvisus Darwin. J. Exp. Mar. Biol. Ecol. 1990;144:235–239. doi:10.1016/0022-0981(90)90030-G [Google Scholar]
- Gack C, Peschke K. ‘Shouldering’ exaggerated genitalia: a unique behavioural adaptation for the retraction of the elongate intromittant organ by the male rove beetle (Aleochara tristis Gravenhorst) Biol. J. Linn. Soc. 2005;84:307–312. doi:10.1111/j.1095-8312.2005.00432.x [Google Scholar]
- Helmuth B, Denny M.W. Predicting wave exposure in the rocky intertidal zone: do bigger waves always lead to larger forces. Limnol. Oceanogr. 2003;48:1338–1345. [Google Scholar]
- Hosken D.J, Stockley P. Sexual selection and genital evolution. Trends Ecol. Evol. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. doi:10.1016/j.tree.2003.11.012 [DOI] [PubMed] [Google Scholar]
- Klepal W. The fundamentals of insemination in Cirripedes. Oceanogr. Mar. Biol. 1990;28:353–379. [Google Scholar]
- Langerhans R.B, Layman C.A, DeWitt T.J. Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc. Natl Acad. Sci. USA. 2005;102:7618–7623. doi: 10.1073/pnas.0500935102. doi:10.1073/pnas.0500935102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchinko K.B. Dramatic phenotypic plasticity in barnacle legs (Balanus glandula Darwin): Magnitude, age dependence, and speed of response. Evolution. 2003;57:1281–1290. doi: 10.1111/j.0014-3820.2003.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Marchinko K.B, Palmer A.R. Feeding in flow extremes: dependence of cirrus form on wave-exposure in four barnacle species. Zoology. 2003;106:127–141. doi: 10.1078/0944-2006-00107. doi:10.1078/0944-2006-00107 [DOI] [PubMed] [Google Scholar]
- McCracken K.G, Wilson R.E, McCracken P.J, Johnson K.P. Sexual selection—are ducks impressed by Drakes' display? Nature. 2001;413:128. doi: 10.1038/35093160. doi:10.1038/35093160 [DOI] [PubMed] [Google Scholar]
- Murata A, Imafuku M, Abe N. Copulation by the barnacle Tetraclita japonica under natural conditions. J. Zool. 2001;253:275–280. doi:10.1017/S0952836901000243 [Google Scholar]
- Ramos M, Irschick D.J, Christenson T.E. Overcoming an evolutionary conflict: removal of a reproductive organ greatly increases locomotor performance. Proc. Natl Acad. Sci. USA. 2004;101:4883–4887. doi: 10.1073/pnas.0400324101. doi:10.1073/pnas.0400324101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronemus M.J, Galbiati M, Ticknor C, Chen J.C, Dellaporta S.L. Demethylation-induced developmental pleiotropy in Arabidopsis. Science. 1996;273:654–657. doi: 10.1126/science.273.5275.654. doi:10.1126/science.273.5275.654 [DOI] [PubMed] [Google Scholar]
- Ruppert E, Fox R, Barnes R. Brooks Cole; Belmont, CA: 2004. Invertebrate zoology: a functional evolutionary approach. [Google Scholar]
- Strathmann M.F. University of Washington Press; Seattle, WA: 1987. Reproduction and development of marine invertebrates of the Northern Pacific Coast. [Google Scholar]
- Tadler A. Selection of a conspicuous male genitalic trait in the seedbug Lygaeus simulans. Proc. R. Soc. B. 1999;266:1773–1777. doi:10.1098/rspb.1999.0845 [Google Scholar]
- Vogel S. Princeton University Press; Princeton, NJ: 2003. Comparative biomechanics: life's physical world. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 provides support for the use of equal slopes when standardizing for body size in the Field Survey comparisons; The supplementary discussion describes potential sources of variation in the Field Survey and sources of mortality in the Field Transplant experiment; tables S1–S5 provide additional statistical data to support the results and discussion in the main body of the paper