Abstract
Parasitoids are an important mortality factor for insects. Susceptibility to parasitoids should thus be under strong negative selection. Nevertheless, ample genetic variation for susceptibility to parasitoids is commonly observed in natural populations, suggesting that trade-offs may constrain the evolution of reduced susceptibility. This can be studied by assessing genetic variation for susceptibility and its covariation with other components of fitness. In a set of 17 clones of the peach potato aphid, Myzus persicae, for which good estimates of heritable variation for life-history traits were available, we found significant clonal variation for susceptibility to two of their common parasitoids: Aphidius colemani and Diaeretiella rapae. One clone, the only one harbouring a facultative endosymbiotic bacterium, Regiella insecticola, was entirely resistant to both parasitoids. Susceptibilities to the two parasitoids exhibited a positive genetic correlation close to unity, implying a general mechanism of defence. However, the susceptibility to parasitoids was uncorrelated to the clones' fecundity or rate of increase, providing no evidence for costs of the ability to resist parasitoids.
Keywords: costs of resistance, endosymbionts, genetic correlations, aphids, parasitoids, Regiella insecticola
1. Introduction
The continuous threat from natural enemies shapes the evolution of all animals. In herbivorous insects in general (Hawkins et al. 1997), and in aphids in particular (Schmidt et al. 2003), the highest mortality appears to be caused by parasitoids. The ability to resist parasitoids should thus be under strong directional selection. In pea aphids (Acyrthosiphon pisum), however, this trait was found to show ample among-clone variation (Henter & Via 1995; Ferrari et al. 2001), and mean resistance did not increase over a summer of presumably strong selection by parasitoids (Henter & Via 1995). These observations could be explained if (i) resistance is negatively related to other components of fitness (e.g. fecundity, longevity), (ii) there are trade-offs among defences against different natural enemies or (iii) genotype specificity in the host–parasitoid interaction maintains variation through negative frequency-dependent selection. While there is little evidence from insect host–parasitoid systems for the latter hypotheses, there is some evidence for costs of resistance to parasitoids (Kraaijeveld et al. 2002). The first study to show this was on Drosophila melanogaster, in which lines selected for increased resistance against the endoparasitoid Asobara tabida suffered from reduced larval competitive ability under crowded conditions (Kraaijeveld & Godfray 1997). In the pea aphid, the evidence for trade-offs is ambiguous. Ferrari et al. (2001) detected no significant relationship between fecundity and susceptibility to the parasitoids, Aphidius ervi and Aphidius eadyi, but Gwynn et al. (2005) found that clones with a high fecundity were more susceptible to A. ervi.
Studies on pea aphids have also revealed that much of the ecologically relevant variation among clones may in fact be due to their harbouring certain facultative endosymbiotic bacteria that are collectively referred to as secondary symbionts (e.g. Oliver et al. 2003; Tsuchida et al. 2004; Ferrari et al. 2007). Three of these bacteria, all belonging to Enterobacteriaceae, have recently been named (Moran et al. 2005): Hamiltonella defensa; Regiella insecticola; and Serratia symbiotica. The latter two have only been found inside aphids, whereas H. defensa also occurs in psyllids and whiteflies (Moran et al. 2005). S. symbiotica and H. defensa were shown to increase the resistance of pea aphids to their parasitoid A. ervi (Oliver et al. 2003, 2006). This led Gwynn et al. (2005) to suggest that if harbouring such bacteria bears some cost (Chen et al. 2000), the negative relationship between resistance and fecundity in their pea aphid clones may be mediated by secondary symbionts, although the aphids were not tested for their presence.
Here, we address potential costs of resistance in another pest aphid, the peach potato aphid, Myzus persicae, using a set of 17 clones known to exhibit heritable variation for life-history traits resulting in strong fitness differences (Vorburger 2005). We screened them for secondary symbionts, quantified their genetic variation and covariation for susceptibility to two different parasitoid species and related their susceptibility to other components of fitness. These comparisons provided no evidence for a cost of resistance under the conditions of our experiment.
2. Material and methods
(a) Insects
The 17 clones of M. persicae were collected at Bacchus Marsh, Australia, in 2003. They have previously been used in two life-table experiments (Vorburger 2005; Vorburger & Ramsauer in press). Each clone has a different multilocus microsatellite genotype (published in Vorburger 2005) that was reconfirmed before the experiment. To assay the clones for the presence of secondary symbionts, the DNA was extracted from groups of five ethanol-preserved adults using the ‘DNeasy Blood & Tissue Kit’ (Qiagen, UK), following the manufacturer's instructions. The samples were then tested for symbiont infection using PCR. The bacterial 16S ribosomal RNA gene was amplified using a universal bacterial primer pair (10F, 35R; Sandström et al. 2001; Russell & Moran 2005), and the products were run on a 2% agarose gel. When a product was present, it was sequenced.
The parasitoid wasps, Aphidius colemani and Diaeretiella rapae, are solitary endoparasitoids and important natural enemies of M. persicae. Females oviposit a single egg into aphid nymphs. The larva develops inside the still-growing aphid until the last larval instar kills the host and pupates inside its dried remains, forming the characteristic mummy from which the adult wasp emerges. Aphidius colemani was obtained from a commercial supplier of biocontrol agents (Andermatt Biocontrol AG, Grossdietwil, Switzerland), and D. rapae was kindly provided by Martin Torrance from Rothamstead Research, UK, from a laboratory colony that was founded with animals from two English sites. Both species are maintained in our laboratory as large cage populations on Swiss clones of M. persicae.
(b) Experimental procedures
Following Henter & Via (1995), the basic assay to estimate aphid susceptibility to parasitoids was to expose groups of aphid nymphs to female wasps for a fixed period of time and determine the proportion of individuals that were mummified. We replicated this assay 14 times for each combination of aphid clone and parasitoid species.
Aphids exhibit what is termed telescoping of generations. The parthenogenetic females are viviparous and bear developing daughters that themselves already contain developing embryos. Environmental maternal or even grandmaternal effects may therefore be pronounced in aphids. To avoid inflation of our estimates of among-clone (thus genetic) variance by such carry-over effects from the stock culture, each clone was split into sublines that were maintained for several generations before aphids were tested. These sublines were maintained on seedlings of radish (Raphanus sativus) grown in small plastic pots fitted with cages made from clear plastic cylinders that have one end covered with gauze. Space constraints prevented us from keeping 14 sublines simultaneously; we therefore split each clone into only seven sublines that were tested twice to obtain the required number of replicates (once in the third generation and once in the fifth generation after the split). These replicates can still be regarded as independent and thus unconfounded by shared environmental effects because sublines were transferred twice to new plants between the third and fifth generations. Sublines were arranged at random positions within seven plastic trays, each containing a single subline of each clone, and kept in a plant growth chamber at 20°C and a photoperiod of 16 hours.
Individual assays were started by transferring five adult M. persicae from a subline to two new radish seedlings (one for exposure to D. rapae and the other for exposure to A. colemani). The adults were allowed to reproduce for 24 hours and then discarded. Three days later, when their offspring were between 72 and 96 hours old (second to third instar nymphs), we counted and reduced them to 30 individuals per plant. When fewer than 30 nymphs were available on a plant (15% of replicates), all were left on the plant. The mean number of nymphs per replicate was 28. Upon counting, we added two female wasps to each caged seedling and allowed them to attack the aphid nymphs for 6 hours. The wasps were between 24 and 48 hours old (time since emergence) and had access to mates and hosts before use. Nine days after exposure to wasps, mummies were clearly visible and counted.
Because the process of counting nymphs and exposing them to parasitoids was very time consuming, the experiment had to be temporally staggered such that either one or two trays (i.e. either one or two replicates of each clone×parasitoid species combination) were treated on the same day. To account for any uncontrolled environmental variation among trays, days and generations (each subline was tested in the third and fifth generations), we treated each tray that was tested on a given day as one experimental block in all analyses (i.e. a total of 14 complete randomized blocks).
(c) Statistical analyses of genetic variation
Susceptibility was expressed as the number of mummies divided by the total number of aphids exposed to parasitoids. These proportions were arcsin square-root transformed before analysis to better meet linear model assumptions (Sokal & Rohlf 1995). To analyse genetic variation for susceptibility to parasitoids, we used a linear mixed model with REML estimation as implemented in the lmer procedure of the lme4 library, a contributed package to the open source statistical software R (R Development Core Team 2006). The model included a block effect and two main effects: parasitoid species (fixed) and clone (random), as well as the parasitoid×clone interaction (random). The number of aphids exposed (some replicates had fewer than 30 nymphs) had no significant effect on the proportion mummified and was therefore omitted from the final model.
Significance tests for fixed effects in mixed models (normally based on t or F statistics) are a contentious issue, mainly because it is unclear how to calculate the appropriate degrees of freedom. A modern alternative is to use Markov Chain Monte Carlo sampling to obtain the fixed effect parameters' highest posterior density intervals and associated p-values (Baayen in press). We used this approach that is provided by the pvals.fnc function in the languageR library of R. The same function also provides the highest posterior density intervals for the variance components of random effects, but these intervals are constrained to never contain zero and cannot be used to test the significance of random effects (Baayen in press). We therefore tested their significance by running models with and without the effects and calculating likelihood ratio statistics.
The among-clone variance (Vclone) of susceptibility to each parasitoid species was estimated from separate models to calculate the susceptibility's broad-sense heritability, H2=Vclone/(Vclone+Vresidual). The genetic correlation between susceptibility to A. colemani and to D. rapae was calculated as rg=Vclone/(Vclone+Vclone×parasitoid) (Astles et al. 2006), where Vclone is the variance among clones over both parasitoids from the two-way analysis and Vclone×parasitoid is the interaction variance. The genetic correlation was jackknifed to obtain an estimate of the standard error (Roff & Preziosi 1994).
(d) Comparisons with reproductive traits
To detect potential trade-offs between defence against parasitoids and other components of fitness, we compared the clones' susceptibility to parasitoids with three other components of fitness, daily fecundity, total lifetime reproduction and (Service & Lenski 1982), an estimate of the clones' finite rate of increase. These estimates of life-history traits were obtained from a life-table experiment by Vorburger & Ramsauer (in press), using the same set of clones. All three life-history traits exhibited significant among-clone variation (Vorburger & Ramsauer in press).
It is known that the genetic covariation among traits can change across environments (see Sgrò & Hoffmann 2004 for a review), which means that the expression of trade-offs may be sensitive to the conditions under which they are measured. Therefore, we took great care to measure the susceptibility to parasitoids under the same environmental conditions as the life-history traits. Both experiments were carried out in the same laboratory at the same temperature and photoperiod, we used seedlings of the same host plant and variety (R. sativus, var. ‘Eiszapfen’; Samen Mauser AG, Winterthur, Switzerland) and the soil (H1 substrate; Tref B.V., Moerdijk, The Netherlands), pots and cages were also identical. Because the data to be compared came from two different experiments, we used correlations of clone means (rcm, Via 1991), i.e. standard Pearson product–moment correlations among the clones' mean trait values, as an approximation of the genetic correlation among traits.
3. Results
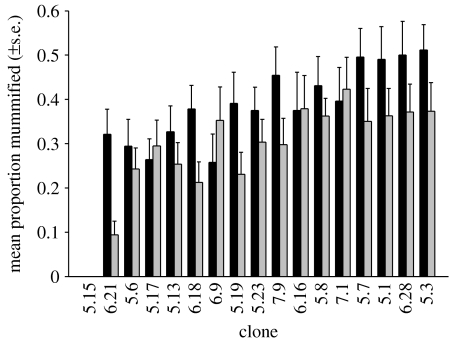
Myzus persicae was more susceptible to A. colemani (37% nymphs mummified on average) than to D. rapae (29% mummified; table 1; figure 1). There was significant variation among the 17 clones in their susceptibility to parasitoids (table 1; figure 1). One clone (5.15) was entirely resistant to both parasitoid species (figure 1). This was the only clone in which a facultative endosymbiont, R. insecticola, could be detected. The partial sequence of the endosymbiont's 16S ribosomal RNA gene has been deposited in GenBank (no. EF596788) and is identical to previously published sequences of this symbiont (e.g. AY136154.1). The entirely resistant clone 5.15 contributed strongly to the variance among clones, but the variance component for the effect of clone remained significant even when clone 5.15 was excluded from the model (LR Χ2=9.035, p=0.003). The variance component for the parasitoid species×clone interaction was small and not significantly different from zero (table 1).
Table 1.
Variance components (for random effects) and treatment effect (parasitoid species) from a linear mixed model of the proportion of aphids mummified by parasitoids. (Proportions were arcsin square-root transformed before analysis. *The p-value based on MCMC sampling.)
| source | var. comp. | LR Χ2 | p |
|---|---|---|---|
| block | 0.0059 | 14.725 | <0.001 |
| parasitoid species | — | — | 0.022* |
| clone | 0.0246 | 84.908 | <0.001 |
| parasitoid species×clone | 0.0013 | 0.153 | 0.696 |
| error | 0.0801 | 0.047 |
Figure 1.
Susceptibility of 17 clones of M. persicae to two species of parasitoids (black bars, A. colemani; grey bars, D. rapae). Clones are ordered by increasing overall susceptibility. Each bar represents mean (+s.e.) of 14 replicate assays.
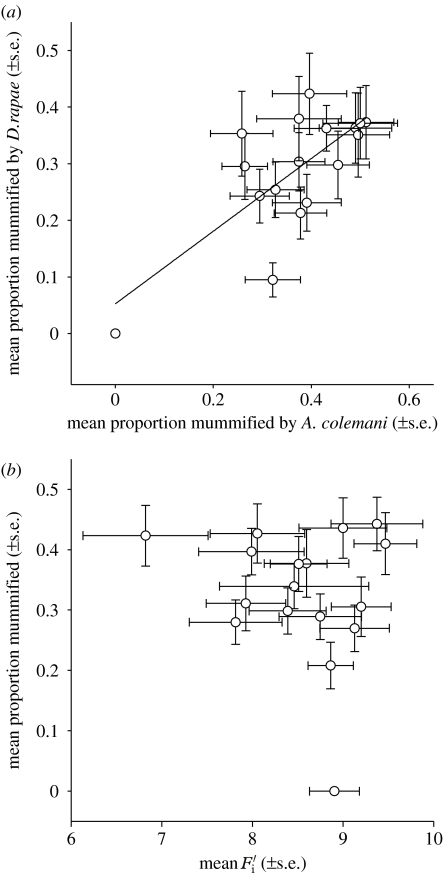
The broad-sense heritability of susceptibility to A. colemani was 0.235±0.081 (s.e.), and 0.298±0.090 to D. rapae. The genetic correlation among the susceptibilities to the two parasitoid species was very high (jackknifed rg=1.062±0.155; figure 2a). This estimate is significantly higher than zero (t16=6.836, p<0.001), but not significantly different from unity (t16=0.399, p=0.695), which is also supported by the lack of a significant parasitoid species×clone interaction. Again, this correlation is strongly influenced by the resistant clone 5.15. Yet even without this clone, the genetic correlation among susceptibilities to the two parasitoids remains significantly positive (jackknifed rg=0.774±0.327, t16=2.365, p<0.032). Also, this estimate is not significantly different from unity (t15=0.690, p=0.501).
Figure 2.
Relationship between (a) susceptibilities to the two parasitoid species tested and (b) the mean susceptibility to both parasitoids and mean (Service & Lenski 1982), a life-table estimate of the finite rate of increase for each clone. Susceptibility to parasitoids and were measured on the same host plant species, radish.
Correlations of clone means revealed no evidence for trade-offs between defence against parasitoids and other components of fitness (table 2). Given that the susceptibilities to the two parasitoids are highly correlated, the most relevant comparison is that between the mean susceptibility to both parasitoids and , the life-history trait most directly related to fitness (figure 2b). This correlation was not significantly different from zero and even slightly negative (table 2). So if anything, the clones with a higher rate of increase tended to be less susceptible to parasitoids. All additional comparisons between overall susceptibility or susceptibility to each individual parasitoid and the three life-history traits were also non-significant, independent of whether clone 5.15 was included in the comparison or not (table 2). Thus, at least in our collection of M. persicae clones, reproduction and defence against parasitoids appear to be genetically independent traits.
Table 2.
Correlations of clone means (rcm, Via 1991) between three fitness-related life-history traits and susceptibility to parasitoids in 17 clones of M. persicae. (The life-history traits for these clones were measured on the same host plant (R. sativus) as used in the present experiment and exhibit significant clonal variation (Vorburger & Ramsauer in press). Tests of correlations are with 15 d.f. for all clones and 14 d.f. when clone 5.15 is excluded from comparisons.)
| all clones | clone 5.15 excluded | |||||
|---|---|---|---|---|---|---|
| life-history trait | susceptibility to A. colemani | susceptibility to D. rapae | mean susceptibility | susceptibility to A. colemani | susceptibility to D. rapae | mean susceptibility |
| daily fecundity | 0.037 (p=0.889) | −0.022 (p=0.934) | 0.007 (p=0.977) | 0.069 (p=0.799) | −0.020 (p=0.942) | 0.025 (p=0.926) |
| lifetime fecundity | 0.055 (p=0.833) | 0.117 (p=0.655) | 0.089 (p=0.735) | −0.138 (p=0.609) | −0.015 (p=0.955) | −0.093 (p=0.731) |
| mean | −0.240 (p=0.353) | −0.054 (p=0.837) | −0.165 (p=0.527) | −0.210 (p=0.435) | 0.054 (p=0.842) | −0.092 (p=0.734) |
4. Discussion
In accordance with studies on pea aphids (Henter & Via 1995; Ferrari et al. 2001), we detected significant clonal variation for susceptibility to parasitoids in the peach potato aphid, M. persicae. In aphids, reduced susceptibility to parasitoids can be conferred by the endosymbiotic bacteria H. defensa and S. symbiotica (Oliver et al. 2003), yet neither of these secondary symbionts was detected in our collection of clones. Instead, we found that the one entirely resistant clone harboured R. insecticola. This secondary endosymbiont has so far not been implicated in defence against parasitoids, but it was shown to decrease susceptibility to a fungal pathogen and to affect host plant specialization in the pea aphid (Tsuchida et al. 2004; Scarborough et al. 2005). Our finding suggests that certain strains of R. insecticola may also provide protection against parasitoids, although the critical experiments of curing clone 5.15 from R. insecticola and/or transferring the symbiont to susceptible M. persicae clones are yet to be done. It is important to note, however, that we detected significant differences among clones even when the clone harbouring R. insecticola was excluded from the analysis. This indicates that in M. persicae, there is some genetic variation for susceptibility to parasitoids independent of that conveyed by endosymbionts.
Unlike Gwynn et al. (2005), we found no evidence that decreased susceptibility comes at a cost. Under the conditions of our experiments, less susceptible clones were neither less fecund, nor did they exhibit lower rates of increase, a trait more closely related to fitness than just fecundity. We are confident that a trade-off was not just obscured by the fact that reproductive traits and susceptibility to parasitoids had to be measured in two different experiments, because we did everything possible to keep the environmental conditions the same. However, these conditions may not have been ideal to detect an existing trade-off because they were rather benign. Radish is a very favourable host for M. persicae (Vorburger et al. 2003), and aphid densities were kept low. It has been argued that trade-offs are more likely to be expressed under conditions of limited resource availability (e.g. Blanckenhorn & Heyland 2005). Consistent with this suggestion, Kraaijeveld & Godfray (1997) found larvae of D. melanogaster selected for reduced susceptibility to parasitoids to be inferior competitors under low food conditions, but not under high food conditions. On the other hand, the fact that we assayed susceptibility of M. persicae to parasitoids under benign rearing conditions is unlikely to explain the difference between our study and that by Gwynn et al. (2005), because they also worked with a very favourable host plant and low numbers of aphids per plant.
The possibility that genotype×environment interactions might mask trade-offs also affects the extent to which findings from laboratory experiments can be generalized to the situation in the field. Service & Rose (1985) argued that laboratory experiments can be biased in the sense that they are more likely to detect positive genetic correlations among fitness components (and thus less likely to detect trade-offs) because genotypes may differ in their pre-adaptedness to the laboratory environment, resulting in general fitness differences. Again, this is a limitation that has to be acknowledged, yet we have some evidence that our result does not just reflect variation in general vigour specific to the one environment considered here. For the life-history traits, we have meanwhile conducted assays on several host plants of different quality and found the clones' fitness hierarchy to be surprisingly consistent (Vorburger & Ramsauer in press). Furthermore, it was found that laboratory estimates of clonal rates of increase of the same clones tend to be positively related to the abundance of these clones in the field, averaged over 1 year (Vorburger 2005), which would certainly have comprised a wide variety of environmental conditions.
The use of two different parasitoids also allowed us to compare the two parasitoid-specific susceptibilities. This comparison provided no evidence for a trade-off, either, because an increased ability to resist one wasp appeared to augment rather than compromise the ability to resist the other. In fact, the genetic correlation between the susceptibility to D. rapae and the susceptibility to A. colemani was indistinguishable from unity, suggesting that the variation observed is for a rather general mechanism of defence. Of course, this finding cannot exclude trade-offs with defences against enemies not considered here, for example, more distantly related parasitoids, predators or pathogens. However, Ferrari et al. (2001) found a positive trend between susceptibility to parasitoids and a fungal pathogen in the pea aphid.
By comparing susceptibility to parasitoids with other components of fitness, we did not detect any standing costs of defence, yet there may be additional costs of actually mounting the defence (Kraaijeveld et al. 2002). We never observed a complete failure to reproduce upon attack by parasitoids as reported by Ferrari et al. (2001), but we know that M. persicae that survive oviposition by parasitoids suffer from a reduction in fecundity (Vorburger et al. in press). This can be interpreted as a cost of actual defence or simply as a negative effect of parasitoid attack (e.g. venom injection). Whatever the interpretation, if this fecundity reduction differed among clones, it could affect the evolution of susceptibility levels in a population. How the fitness costs of successful defence against parasitoids relate to overall susceptibility is thus an important question to answer in the future.
Although we detected no trade-off between the ability to resist parasitoids and reproductive traits, there was no positive correlation either. Thus, depending on whether selection for increased reproduction or lower susceptibility to parasitoids is more important in a given environment, different clones may be favoured. We confirmed this prediction in another experiment (Herzog et al. 2007): experimental populations of M. persicae consisting of a subset of the clones tested here were allowed to evolve without parasitoids or in the presence of either A. colemani or D. rapae. Under strong selection by parasitoids, only the resistant clone 5.15 prevailed, whereas in the absence of parasitoids, populations became dominated by different clones (Herzog et al. 2007). This goes some way to explain the maintenance of genetic variation for traits with such relevance to fitness, but the challenge remains to explain how clones that combine very low fecundity with high susceptibility to parasitoids are maintained in the field.
Acknowledgments
We thank A. Hector and B. Schmid for their suggestions regarding the analyses and two reviewers for their helpful comments. This study was supported by grants from the Forschungskredit of the University of Zürich (57202802) and the Swiss National Science Foundation (3100A0-109266) to C.V.
References
- Astles P.A, Moore A.J, Preziosi R.F. A comparison of methods to estimate cross-environment genetic correlations. J. Evol. Biol. 2006;19:114–122. doi: 10.1111/j.1420-9101.2005.00997.x. doi:10.1111/j.1420-9101.2005.00997.x [DOI] [PubMed] [Google Scholar]
- Baayen, R. H. In press. Analyzing linguistic data. A practical introduction to statistics Cambridge, UK: Cambridge University Press. (Available as pre-print at http://www.ualberta.ca/∼baayen/publications.html)
- Blanckenhorn W.U, Heyland A. The quantitative genetics of two life history trade-offs in the yellow dung fly in abundant and limited food environments. Evol. Ecol. 2005;18:385–402. doi:10.1007/s10682-004-2680-z [Google Scholar]
- Chen D.Q, Montllor C.B, Purcell A.H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 2000;95:315–323. doi:10.1023/A:1004083324807 [Google Scholar]
- Ferrari J, Müller C.B, Kraaijeveld A.R, Godfray H.C.J. Clonal variation and covariation in aphid resistance to parasitoids and a pathogen. Evolution. 2001;55:1805–1814. doi: 10.1554/0014-3820(2001)055[1805:CVACIA]2.0.CO;2. doi:10.1111/j.0014-3820.2001.tb00829.x [DOI] [PubMed] [Google Scholar]
- Ferrari J, Scarborough C.L, Godfray H.C.J. Genetic variation in the effect of a facultative symbiont on host–plant use by pea aphids. Oecologia. 2007;153:323–329. doi: 10.1007/s00442-007-0730-2. doi:10.1007/s00442-007-0730-2 [DOI] [PubMed] [Google Scholar]
- Gwynn D.M, Callaghan A, Gorham J, Walters K.F.A, Fellowes M.D.E. Resistance is costly: trade-offs between immunity, fecundity and survival in the pea aphid. Proc. R. Soc. B. 2005;272:1803–1808. doi: 10.1098/rspb.2005.3089. doi:10.1098/rspb.2005.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.A, Cornell H.V, Hochberg M.E. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology. 1997;78:2145–2152. doi:10.1890/0012-9658(1997)078[2145:PPAPAM]2.0.CO;2 [Google Scholar]
- Henter H.J, Via S. The potential for coevolution in a host–parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution. 1995;49:427–438. doi: 10.1111/j.1558-5646.1995.tb02275.x. doi:10.2307/2410267 [DOI] [PubMed] [Google Scholar]
- Herzog J, Müller C.B, Vorburger C. Strong parasitoid-mediated selection in experimental populations of aphids. Biol. Lett. 2007;3:667–669. doi: 10.1098/rsbl.2007.0362. doi:10.1098/rsbl.2007.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. doi:10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Ferrari J, Godfray H.C.J. Costs of resistance in insect–parasite and insect–parasitoid interactions. Parasitology. 2002;125:S71–S82. doi: 10.1017/s0031182002001750. doi:10.1017/S0031182002001762 [DOI] [PubMed] [Google Scholar]
- Moran N.A, Russell J.A, Koga R, Fukatsu T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005;71:3302–3310. doi: 10.1128/AEM.71.6.3302-3310.2005. doi:10.1128/AEM.71.6.3302-3310.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.M, Russell J.A, Moran N.A, Hunter M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. doi:10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.M, Moran N.A, Hunter M.S. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. B. 2006;273:1273–1280. doi: 10.1098/rspb.2005.3436. doi:10.1098/rspb.2005.3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team 2006 R: a language and environment for statistical computing. See http://cran.r-project.org
- Roff D.A, Preziosi R. The estimation of the genetic correlation—the use of the jackknife. Heredity. 1994;73:544–548. [Google Scholar]
- Russell J.A, Moran N.A. Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel aphid host. Appl. Environ. Microbiol. 2005;71:7987–7994. doi: 10.1128/AEM.71.12.7987-7994.2005. doi:10.1128/AEM.71.12.7987-7994.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström J.P, Russell J.A, White J.P, Moran N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001;10:217–228. doi: 10.1046/j.1365-294x.2001.01189.x. doi:10.1046/j.1365-294X.2001.01189.x [DOI] [PubMed] [Google Scholar]
- Scarborough C.L, Ferrari J, Godfray H.C.J. Aphid protected from pathogen by endosymbiont. Science. 2005;310:1781. doi: 10.1126/science.1120180. doi:10.1126/science.1120180 [DOI] [PubMed] [Google Scholar]
- Schmidt M.H, Lauer A, Purtauf T, Thies C, Schaefer M, Tscharntke T. Relative importance of predators and parasitoids for cereal aphid control. Proc. R. Soc. B. 2003;270:1905–1909. doi: 10.1098/rspb.2003.2469. doi:10.1098/rspb.2003.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service P.M, Lenski R.E. Aphid genotypes, plant phenotypes, and genetic diversity: a demographic analysis of experimental data. Evolution. 1982;36:1276–1282. doi: 10.1111/j.1558-5646.1982.tb05496.x. doi:10.2307/2408159 [DOI] [PubMed] [Google Scholar]
- Service P.M, Rose M.R. Genetic covariation among life-history components—the effect of novel environments. Evolution. 1985;39:943–945. doi: 10.1111/j.1558-5646.1985.tb00436.x. doi:10.2307/2408694 [DOI] [PubMed] [Google Scholar]
- Sgrò C.M, Hoffmann A.A. Genetic correlations, tradeoffs and environmental variation. Heredity. 2004;93:241–248. doi: 10.1038/sj.hdy.6800532. doi:10.1038/sj.hdy.6800532 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. Freeman; New York, NY: 1995. Biometry. [Google Scholar]
- Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science. 2004;303:1989. doi: 10.1126/science.1094611. doi:10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- Via S. The genetic structure of host plant adaptation in a spatial patchwork—demographic variability among reciprocally transplanted pea aphid clones. Evolution. 1991;45:827–852. doi: 10.1111/j.1558-5646.1991.tb04353.x. doi:10.2307/2409692 [DOI] [PubMed] [Google Scholar]
- Vorburger C. Positive genetic correlations among major life-history traits related to ecological success in the aphid Myzus persicae. Evolution. 2005;59:1006–1015. doi:10.1111/j.0014-3820.2005.tb01039.x [PubMed] [Google Scholar]
- Vorburger, C. & Ramsauer, N. In press. Genetic variation and covariation of life-history traits across unrelated host plants in the aphid Myzus persicae Bull. Entomol. Res [DOI] [PubMed]
- Vorburger C, Sunnucks P, Ward S.A. Explaining the coexistence of asexuals with their sexual progenitors: no evidence for general-purpose genotypes in obligate parthenogens of the peach-potato aphid, Myzus persicae. Ecol. Lett. 2003;6:1091–1098. doi:10.1046/j.1461-0248.2003.00536.x [Google Scholar]
- Vorburger, C., Gegenschatz, S. E., Ranieri, G. & Rodriguez, P. In press. Limited scope for maternal effects in aphid defence against parasitoids. Ecol. Entomol (doi:10.1111/j.1365-2311.2007.00949.x)