Abstract
Polyandry, i.e. mating with multiple males within one reproductive event, is a common female mating strategy but its adaptive function is often unclear. We tested whether polyandrous females gain genetic benefits by comparing fitness traits of monandrous (mated twice with a single male) and polyandrous (mated twice with two different males) female bank voles Clethrionomys glareolus. We raised the offspring in the laboratory until adulthood and measured their body size, before releasing them to outdoor enclosures to overwinter. At the onset of the breeding season in the following spring, we found that offspring of polyandrous females performed significantly better at reproduction than those of monandrous females. This was mainly due to sons of polyandrous females producing significantly more offspring than those of monandrous females. No significant differences were found for offspring body mass or winter survival between the two treatments. Our results appear to provide evidence that bank vole females gain long-term benefits from polyandry.
Keywords: polyandry, genetic benefits, intrinsic male quality, genetic incompatibility, Clethrionomys glareolus=Myodes glareolus
1. Introduction
Sexual dimorphism in gamete size, costs involved in mating and differential parental investment predict different mating strategies for males and females of most species. Males are expected to increase their reproductive success by mating with multiple females. Females, on the other hand, are expected to increase their reproductive success by choosing a high-quality male and should mate only as often as is needed to assure fertilization, which is typically once (Bateman 1948; Trivers 1972). However, polyandry is a common reproductive strategy among many species (Birkhead & Møller 1998) and its adaptive significance is currently one of the most debated subjects in sexual selection research. In resource-based mating systems, material benefits to the female seem to outweigh the fitness costs of multiple mating (Arnquist & Danielsson 1999; Hosken & Stockley 2003). However, for many polyandrous species no obvious material benefits are detectable. In the absence of such direct benefits, polyandry is explained by genetic benefits, such as the ‘intrinsic male quality hypothesis’ and the ‘genetic incompatibility hypothesis’ (Jennions & Petrie 2000).
The intrinsic male quality hypothesis states that multiple mating increases the probability of fertilization by males with superior genetic quality. This can be achieved in two ways. First, if females are able to assess the genetic quality of males prior to mating, they may remate (i.e. trade up) if they encounter a male that is of superior genetic quality to a previous mate (Halliday 1983). Second, if females are not able to assess the genetic quality of a male pre-copulation, they may benefit from mating multiply via post-copulatory mechanisms. If females assess male quality during mating, they may selectively choose the sperm of the best mate (cryptic choice; Eberhard 1996). If there is, on the other hand, a link between sperm competitiveness and offspring viability, the promotion of sperm competition will increase offspring viability (Yasui 1997). Additionally, the ‘sexy-sperm hypothesis’ proposes that fertilizing efficiency under sperm competition may be paternally heritable, and therefore polyandrous females will produce sons that are successful at sperm competition (Keller & Reeve 1995).
The genetic incompatibility hypothesis states that multiple mating increases the probability of reproducing with genetically compatible males (Zeh & Zeh 1996). Because offspring viability is dependent on parental genetic compatibility, females may benefit from choosing a compatible male for fertilization. By mating with multiple males, females can use post-copulatory mechanisms, such as choice of sperm or assortative abortion of embryos, to bias paternity towards the most compatible mate (Zeh & Zeh 1996, 1997). The avoidance of inbreeding, a special case of the genetic incompatibility hypothesis, is likely to be the most widespread of all potential sources of genetic incompatibility (Tregenza & Wedell 2000).
The aim of this study is to test whether bank vole Clethrionomys glareolus females gain genetic benefits from mating with multiple males. The bank vole is a common boreal rodent with a promiscuous and non-resource-based mating system. Bank vole females gain direct benefits from multiple mating in the form of an increased probability of pregnancy initiation due to increased stimulation (Klemme et al. 2007). However, this direct benefit may not explain the occurrence of polyandry, because the stimulus needed to initiate pregnancy can be achieved by repeated matings with the same male (Klemme et al. 2007) and such repeated matings are common in bank voles (Milligan 1979). Furthermore, when presented sequentially to two males, a majority of bank vole females mated polyandrously and did not base their remating decision on male social status (Klemme et al. 2006).
A possible experimental set-up for testing whether multiple mating brings genetic benefits is to compare the fitness of females for which the number of mates is varied experimentally (monandrous versus polyandrous), while mating frequency is controlled for (reviewed in Neff & Pitcher (2005) and Simmons (2005)). Most of the reviewed studies use invertebrates as a model and test genetic fitness benefits under laboratory conditions. In our experiment under semi-natural conditions, we worked with small mammals whose offspring reproduced in a subsequent season, separated by a long harsh winter that exerts strong selection on the offspring. Moreover, most of the previous studies focused on early offspring survival or offspring number as a measure of fitness to be compared between females with different mating regimes. In this study, we looked, in addition to offspring winter survival and body mass, on reproductive performance. We used offspring from females that were experimentally assigned to be either monandrous or polyandrous and conducted a long-term outdoor enclosure experiment to test whether polyandry increases these fitness traits.
2. Material and methods
(a) Experimental animals
The study was conducted from summer 2004 to spring 2005. The animals used were laboratory-born descendants of wild captured bank voles, originally trapped in 2002 in Konnevesi, Central Finland (62°37′ N, 26°20′ E). The colony was replenished every year with wild males and females trapped at the same site. All animals were housed individually in standard mouse cages (43×26×15 cm) with saw dust and hay as bedding. The temperature was kept constant at 22±1°C and a 16 L : 8 D photoperiod was maintained. All animals were individually marked with small mammal ear tags. Bank voles are seasonal breeders and most animals breed only during one breeding season (Kaikusalo 1972; Prevot-Julliard et al. 1999). Female bank voles are territorial during breeding (Bujalska 1994). Young are weaned at the age of three weeks.
(b) Experimental procedure
For this experiment, we used F1 progeny of a laboratory experiment conducted during June–July 2004, in which females were randomly mated either two times with a single male (monandrous mating=M-treatment) or once with each of two different males (polyandrous mating=P-treatment; Klemme et al. 2007). The males used in both treatments were the same, so that a pair of males was mated to one single female (P-treatment) and each male was also mated alone to a different female (M-treatment). Initially, we had 30 females in each treatment and 30 males altogether. Therefore, each male was used twice in the P-treatment, always with a different male partner. Using the same set of males in both treatments controls for inherent male genetic-quality effects and thus assures that average male quality is identical in the treatments (Tregenza & Wedell 1998; Zeh & Zeh 2001). Not all of the 30 females bred successfully and not all offspring survived until adulthood in the laboratory. Therefore, we selected for this experiment only those litters that were complete and for which we had used identical males in the P- and M-treatments, i.e. one complete litter from the P-treatment plus two complete litters from the M-treatment sired by the same two males. In total, we used 14 litters delivered by M-females and 10 litters delivered by P-females, resulting from matings with a set of 14 males. Unequal litter numbers between treatments are caused by using only a selection of litters from the original dataset. Among this selection, 6 of the 14 males were used twice in the P-treatment, and each of these 6 males has only one ‘counterpart litter’ in the M-treatment.
In the original dataset, we found no differences in the number of breeding females, in litter sizes and progeny survival until weaning between the treatments (Klemme et al. 2007). Considering the selected litters, litters from the M-treatment (mean±s.d., 5.1±1.3) appeared to be larger than those from the P-treatment (4.3±1.4; t-test; t22=1.513, p=0.144). Litters of both the treatments did not differ in sex ratio (males per female; mean±s.d.: M: 1.1±1.0, P: 1.2±1.2; t-test, t22=−0.358, p=0.724). The progeny were raised in the laboratory until they were at least five weeks old. Litters were weaned at the age of 21 days and progeny were subsequently kept either with one (half-) sibling of the same sex or alone. Offspring from both the treatments were homogeneously distributed over the single and the double housing options. Among female offspring, 6.1% in the M-treatment and 9.1% in P-treatment were housed alone (Χ2=0.18, N=55, p=0.672) and among male offspring, 71.8 and 61.9% were housed alone, respectively (Χ2=0.62, N=60, p=0.432). We measured the body mass of all individuals before the field experiment with an electronic scale to the nearest 0.1 g.
Our field experiment aimed to collect data on offspring winter survival and reproductive performance at the onset of breeding in spring. The field experiment was carried out from October 2004 to April 2005 in eight 0.25 ha vole proof outdoor enclosures situated in an old field with a grass and herb vegetation layer, willow bushes and young trees. Each enclosure held a grid of 25 multiple-capture live traps (Ugglan Special) at regular intervals of 10 m. We released altogether 115 individuals, 72 F1 progeny from the M-treatment and 43 F1 progeny from the P-treatment. Five enclosures were filled with 14 progeny each and three enclosures with 15 progeny each, mixed from both the treatments. The number of progeny originating from the M-treatment varied from 8 to 10 per enclosure, and that from the P-treatment varied from 4 to 7 per enclosure. The number of male and female progeny of either treatment released to the same enclosure was nearly the same. Consequently, the overall sex ratio (males per females) per enclosure varied from 1.0 to 1.3 and was on average 1.1. The age at release was on average 10 weeks (range 5–12 weeks) for the M-treatment and on average 11 weeks (range 5–12 weeks) for the P-treatment. All individuals within the same enclosure were unrelated to each other, i.e. no full or half siblings.
We released the individuals on 1 October 2004 to the centre of each enclosure. At the onset of breeding, we conducted a 4 day trapping series from 26 to 29 April 2005 to estimate the survival of the progeny. All the survived individuals were transferred back to the laboratory and parturition of pregnant females was monitored.
(c) Paternity analysis
We collected tissue samples from all surviving individuals of our enclosure experiment and the resulting offspring and conducted paternity analyses to estimate the number of F2 progeny for each experimental female. DNA was extracted from a small piece of ear (2 mm in diameter) with use of KingFisher silicate magnetic beads and a KingFisher magnetic particle processor (ThermoLabsystems) according to the manufacturer's protocol. All females, offspring and potential fathers were genotyped at six microsatellite loci developed for bank voles: MSCg4; MSCg7; MSCg9; MSCg15; MSCg18; and MSCg24 (Gockel et al. 1997; Gerlach & Musolf 2000). DNA fragments were amplified using polymerase chain reaction as described in Gockel et al. (1997). Genotypes were scored on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems) and analysed with GeneMapper v. 3.7 software. Paternity was assigned manually by comparing the alleles of each offspring with those of the known mother and all potential fathers. All offspring could be unambiguously assigned to one of the potential fathers in all six loci. Paternity for all other potential sires could be excluded in at least two loci.
(d) Statistics
Statistical analyses were performed using SAS v. 9.1. Unless stated otherwise, means are given with their (+/−) standard deviations, probability values are two-tailed and the level of significance was set at α=0.05. We used generalized linear mixed models (GLMMs) with either a normal (offspring body mass before the field study), a binomial (offspring survival) or a Poisson error distribution (offspring reproductive performance) to analyse the data. For body mass, treatment and sex were entered as fixed effects, age at weighing as covariate and mother as random factor to control for the non-independence of data from siblings. For survival and reproductive performance, treatment and sex were entered as fixed effects, body mass and age at release as covariates, and enclosure as well as mother as random factors to control for the non-independence of data from individuals sharing the same enclosure and from siblings.
3. Results
(a) Offspring body mass
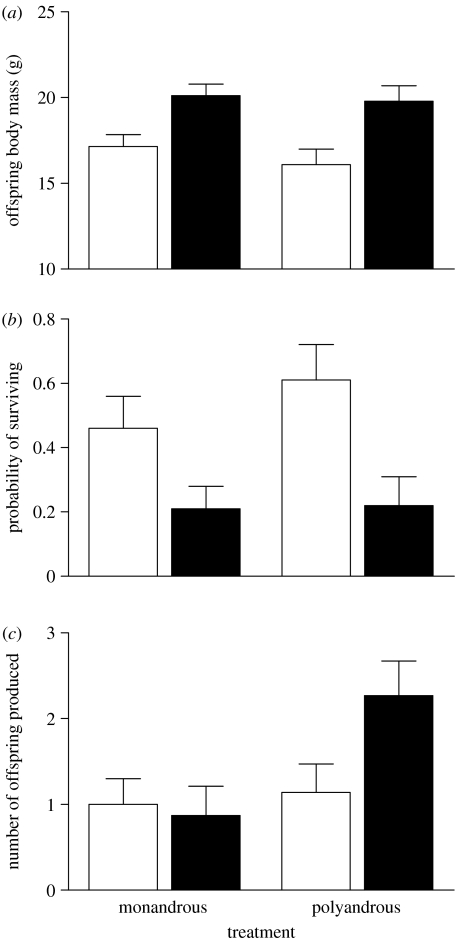
Offspring body mass at the age of 5–12 weeks did not differ between treatments (GLMM, F1,19.1=0.47, p=0.501; figure 1a). Females were significantly lighter than males (F1,92.4=39.99, p<0.001) in both the treatments (treatment×sex F1,92.4=0.46, p=0.499; figure 1a). Age at weighing had no effect on offspring body mass (F1,17.6=2.51, p=0.131).
Figure 1.
Effect of mating treatment and sex on (a) offspring body mass before the field study (N=115), (b) winter survival (N=115) and (c) the number of F2 offspring produced by all surviving F1 offspring (N=41). All values are adjusted means (±s.e.) from the final models. White bars, daughters; black bars, sons.
(b) Offspring survival
Of 115 F1 progeny introduced to the field, 41 (26 females and 15 males) survived until the next breeding season. Per enclosure, the number of survivors ranged from 0 to 9 and was on average 5.9±2.1 individuals. Among all enclosures with survivors, at least one female from each treatment survived. The total number of females survived per enclosure ranged from 2 to 7 and was on average 3.7±1.6. In five out of seven enclosures with survivors, at least one male from each treatment survived. In the remaining two enclosures, only one male survived in each, one from the M-treatment and one from the P-treatment, respectively (the number of surviving females in both the enclosures was three). Thus, the total number of males survived per enclosure ranged from 1 to 4 and was on average 2.2±1.1.
Offspring of monandrous and polyandrous females did not differ in survival (GLMM, F1,29.5=0.56, p=0.460; figure 1b). Female offspring survived significantly better than male ones (F1,109=7.96, p=0.006) and this pattern was the same in both the treatments (treatment×sex F1,109=0.46, p=0.499; figure 1b). Age at release and body mass at release did not affect survival (F1,25.9=0.91, p=0.348 and F1,104.4=2.58, p=0.111, respectively).
(c) Offspring reproductive performance
The surviving F1 individuals produced 18 litters with 84 F2 progeny among all seven enclosures in which voles survived. Offspring of polyandrous females performed significantly better at reproduction than those of monandrous females (GLMM, F1,17.5=4.52, p=0.048; figure 1c). There was no significant difference between sexes (F1,22.5=1.97, p=0.174). Though the treatment×sex interaction was not statistically significant (F1,18.2=3.80, p=0.066), we explored it more closely because in the final model, male offspring contribute much more to the significant main effect of the treatment than female offspring (figure 1c). Post hoc LSmeans significance tests showed that sons of polyandrous females sired more offspring than those of monandrous females (t15.9=−2.73, p=0.015), but there was no significant difference in the production of offspring between daughters of monandrous and polyandrous females (t20.6=−0.31, p=0.760). Age at release or body mass at release did not affect reproductive performance (F1,24.1=0.24, p=0.505 and F1,35=0.04, p=0.630, respectively).
4. Discussion
We were able to show that bank vole females gained long-term fitness benefits from multiple mating with different males. Offspring of polyandrous females performed significantly better at reproduction than those of monandrous females, caused mainly by sons of polyandrous females producing more offspring than those of monandrous females. Offspring body mass and survival were not affected by mating treatment. To our knowledge, this is the first experimental study on mammals measuring offspring reproductive performance as fitness component potentially affected by polyandry.
Recent studies have demonstrated an increase in female fitness due to polyandry that can be attributed to genetic effects (Neff & Pitcher 2005; Simmons 2005). Most of these studies, including two on mammals, have focused on early offspring survival as a component of fitness. For example, a field study on the brown antechinus Antechinus stuartii showed that polyandry significantly increased offspring survival until weaning (Fisher et al. 2006). A similar result was found in a laboratory study on yellow-toothed cavies Galea musteloides (Keil & Sachser 1998, mating frequency not controlled for). An effect of polyandry on offspring reproductive performance has been previously examined in only four studies. Daughter fecundity was found to be increased in bulb mites Rhizoglyphus robini (Konior et al. 2001, mating frequency not controlled for) and increased and decreased in red flour beetles Tribolium castaneum, depending on the competitive environment (Pai & Yan 2002, mating frequency not controlled for). Son reproductive success was enhanced by polyandry again in red flour beetles (Bernasconi & Keller 2001; Pai & Yan 2002) but decreased in black field crickets Teleogryllus commodus (Jennions et al. 2007).
The increased reproductive performance of polyandrous offspring in our study could be explained by both the intrinsic male quality and the genetic incompatibility hypotheses. Post-copulatory processes, as the inevitable result of polyandry, may have secured offspring with an improved genetic quality due to fertilization by ‘good sperm’, ‘sexy sperm’, ‘compatible sperm’ or a combination of these (Jennions & Petrie 2000). We found that sons of polyandrous females contributed much more than daughters to the increased reproductive performance, suggesting that polyandry may have had a positive effect on son fertilization efficiency or attractiveness. The sexy-sperm hypothesis assumes an increase in female fitness solely due to the competitive ability of sons under sperm competition, for example, via the quantity or quality of sperm or other mechanisms preventing competitors from fertilizing (e.g. sperm displacement; Keller & Reeve 1995). Even though daughters also contributed to the increased reproductive success of offspring from the polyandrous treatment (the interaction term treatment×sex was insignificant), the sexy-sperm hypothesis could potentially at least partly explain the 2.5-fold higher reproductive success of sons of polyandrous females. Alternatively, sons of polyandrous females may have been more attractive to females. ‘Attractiveness’ in bank vole males may be, for example, characterized by social status. Laboratory experiments have shown that female bank voles prefer dominant over subordinate males pre-copulatory (Horne & Ylönen 1996), paternity is skewed towards dominant males when females mate with both a dominant and a subordinate male (Klemme et al. 2006), and traits reflecting dominance are highly heritable (Horne & Ylönen 1998). On the other hand, also a more heterozygote male may be more attractive. Fennoscandian bank vole populations undergo multiannual population density cycles (Krebs & Myers 1974) and thus often pass through genetic bottle necks. Inbreeding, as a special case of genetic incompatibility, may reduce progeny fitness due to a decrease in size, vigour and reproductive success (reviewed in Shields 1993) and thus females are expected to choose sperm of dissimilar males or embryos sired by dissimilar males among multiple partners to promote offspring heterozygosity at many loci (Pusey & Wolf 1996). However, with our experimental design, we are not able to estimate whether the sons of polyandrous females had a higher access to females than those of monandrous females or, given that polyandry was common among the F1 generation, whether the resulting paternity pattern stemmed from sperm competition, female choice or both. Future studies should therefore address, for example, fertilizing efficiencies of sons from polyandrous and monandrous females.
Are there alternative explanations for the enhanced fitness of polyandrous females? The increased reproductive performance of polyandrous offspring may also, or additionally, be explained by maternal effects. If females of polyandrous species have no opportunity to mate with multiple males and as a consequence are likely to suffer from fitness costs, they may invest less in their offspring (Simmons 2001). In bank voles, male infanticide is a common phenomenon (Ylönen et al. 1997) and polyandry may serve as a confusion of paternity to prevent males from killing potential offspring (Ebensperger 1998). Therefore, females who are not able to mate polyandrously may invest less in their embryos during pregnancy due to the likelihood that they are killed post-partum. Here, we found no differences in body mass supporting such a differential allocation. However, body mass has only been measured at adulthood and possible compensatory growth effects (Oksanen et al. 2001) may have obscured potential differences in body mass at birth. Additionally, females may derive direct benefits from multiple mating. Females of both the treatments potentially differed in the amount of sperm received because in successive matings with the same female, males may allocate progressively smaller ejaculates. In mammals it is unclear though, whether potential differences in the amount of sperm received can have long-term consequences on female reproduction or offspring performance.
Polyandry did not seem to affect offspring body mass at adulthood nor long-term offspring survival. However, it is important to note that good-genes effects can be very small (though considerable on an evolutionary time scale) due to a generally small heritability of fitness (Alatalo et al. 1997; Møller & Alatalo 1999). Thus, often large sample sizes are needed to demonstrate such an effect. Additionally, polyandrous females had only a choice of two males. These may have been very similar in genetic quality, making it difficult to detect small genetic effects. In future, it will be crucial to conduct experiments in which male genetic quality is varied and controlled for.
Female offspring survived significantly better than male in both the treatments. A decreased winter survival of males has been reported in earlier studies on voles. For instance, a higher mortality rate of bank vole males compared with females has been demonstrated in an enclosed population in Fennoscandia (Ylönen & Viitala 1985) and in one of three wild populations studied in the French Alps (Yoccoz & Mesnager 1998). As also shown in this study, bank vole males are larger than females during the non-reproductive season (Bondrup-Nielsen & Ims 1990). A reduced winter survival of male voles could be explained by their larger size, because both large and very small individuals do not meet their physiological optimum in terms of winter survival (Aars & Ims 2002 and references therein).
The benefit hypotheses proposed to explain polyandry are not mutually exclusive and our results on bank voles, together with previous findings (Klemme et al. 2007), show that females can gain diverse benefits from multiple mating. Although our data on offspring reproductive performance are restricted to the onset of breeding and we were not able to compare the lifetime reproductive success of offspring from both mating treatments, our findings may represent a clear fitness advantage for polyandrous females; if offspring of polyandrous females perform better at reproduction, polyandrous females may gain significantly more grand offspring than monandrous females. The mechanisms underlying our results are not clear and we cannot rule out maternal effects. However, our observations are consistent with the hypothesis that females can increase their fitness by multiple mating with different males and may therefore explain the maintenance of polyandry in bank voles.
Acknowledgments
We would like to thank E. Puolakka for help with the fieldwork, T. Solismaa for assistance with paternity analyses and L. Trebatická for kind introduction to SAS. R. Alatalo, M. Jennions, J. Loehr, M. Puurtinen and one anonymous referee provided helpful comments on earlier drafts of this manuscript. Konnevesi Research Station is thanked for the friendly atmosphere and excellent working conditions. This research was financed by the Academy of Finland and I.K. was supported by a grant from the Graduate School in Evolutionary Ecology.
References
- Aars J, Ims R.A. Intrinsic and climatic determinants of population demography: the winter dynamics of tundra voles. Ecology. 2002;83:3449–3456. doi:10.2307/3072093 [Google Scholar]
- Alatalo R.V, Mappes J, Elgar M.A. Heritabilities and paradigm shifts. Nature. 1997;385:402–403. doi:10.1038/385402a0 [Google Scholar]
- Arnquist G, Danielsson I. Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav. Ecol. 1999;10:358–365. doi:10.1093/beheco/10.4.358 [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Bernasconi G, Keller L. Female polyandry affects their sons' reproductive success in the red flour beetle Tribolium castaneum. J. Evol. Biol. 2001;14:186–193. doi: 10.1046/j.1420-9101.2001.00247.x. doi:10.1046/j.1420-9101.2001.00247.x [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; New York, NY: 1998. Sperm competition and sexual selection. [Google Scholar]
- Bondrup-Nielsen S, Ims R. Reversed sexual size dimorphism in microtines: are females larger than males or are males smaller than females? Evol. Ecol. 1990;4:261. doi:10.1007/BF02214334 [Google Scholar]
- Bujalska G. Female and male territoriality in the bank vole. In: Jarman P.J, Rossiter A, editors. Animal societies individuals, interactions and organisation. Kyoto University Press; Kyoto, Japan: 1994. pp. 56–69. [Google Scholar]
- Ebensperger L.A. Strategies and counterstrategies to infanticide in mammals. Biol. Rev. 1998;73:321–346. doi:10.1017/S0006323198005209 [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Fisher D.O, Double M.C, Blomberg S.P, Jennions M.D, Cockburn A. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature. 2006;444:89–92. doi: 10.1038/nature05206. doi:10.1038/nature05206 [DOI] [PubMed] [Google Scholar]
- Gerlach G, Musolf K. Fragmentation of landscape as a cause for genetic subdivision of bank vole populations. Conserv. Biol. 2000;14:1–10. doi:10.1046/j.1523-1739.2000.98519.x [Google Scholar]
- Gockel J, Harr B, Schlötterer C, Arnolds W, Gerlach G, Tautz D. Isolation and characterization of microsatellite loci from Apodemus flavicollis (rodentia, muridae) and Clethrionomys glareolus (rodentia, cricetidae) Mol. Ecol. 1997;6:597–599. doi: 10.1046/j.1365-294x.1997.00222.x. doi:10.1046/j.1365-294X.1997.00222.x [DOI] [PubMed] [Google Scholar]
- Halliday T.R. The study of mate choice. In: Bateson P, editor. Mate choice. Cambridge University Press; Cambridge, UK: 1983. pp. 3–32. [Google Scholar]
- Horne T.J, Ylönen H. Female bank voles (Clethrionomys glareolus) prefer dominant males; but what if there is no choice? Behav. Ecol. Sociobiol. 1996;38:401–405. doi:10.1007/s002650050257 [Google Scholar]
- Horne T.J, Ylönen H. Heritabilities of dominance-related traits in male bank voles (Clethrionomys glareolus) Evolution. 1998;52:894–899. doi: 10.1111/j.1558-5646.1998.tb03714.x. doi:10.2307/2411284 [DOI] [PubMed] [Google Scholar]
- Hosken D.J, Stockley P. Benefits of polyandry: a life history perspective. J. Evol. Biol. 2003;33:173–194. [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Philos. Soc. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Drayton J.M, Brooks R, Hunt J. Do female black field crickets Teleogryllus commodus benefit from polyandry? J. Evol. Biol. 2007;20:1469–1477. doi: 10.1111/j.1420-9101.2007.01333.x. doi:10.1111/j.1420-9101.2007.01333.x [DOI] [PubMed] [Google Scholar]
- Kaikusalo A. Population turnover and wintering of the bank vole, Clethrionomys glareolus (schreb.), in southern and central Finland. Ann. Zool. Fenn. 1972;9:219–224. [Google Scholar]
- Keil A, Sachser N. Reproductive benefits from female promiscuous mating in a small mammal. Ethology. 1998;104:897–903. [Google Scholar]
- Keller L, Reeve H.K. Why do females mate with multiple males? The sexually selected sperm hypothesis. In: Slater P.J.B, Rosenblatt J.S, Snowden C.T, Milinski M, editors. Advances in the study of behaviour. Academic Press; New York, NY: 1995. pp. 291–315. [Google Scholar]
- Klemme I, Eccard J.A, Ylönen H. Do female bank voles (Clethrionomys glareolus) mate multiply to improve on previous mates? Behav. Ecol. Sociobiol. 2006;60:415–421. doi:10.1007/s00265-006-0181-5 [Google Scholar]
- Klemme I, Eccard J.A, Ylönen H. Why do female bank voles, Clethrionomys glareolus, mate multiply? Anim. Behav. 2007;73:623–628. doi:10.1016/j.anbehav.2006.07.010 [Google Scholar]
- Konior M, Radwan J, Kolodziejczyk M. Polyandry increases offspring fecundity in the bulb mite. Evolution. 2001;55:1893–1896. doi: 10.1111/j.0014-3820.2001.tb00838.x. doi:10.1554/0014-3820(2001)055[1893:PIOFIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krebs C.J, Myers J.H. Population cycles in small mammals. In: Macfadyen A, editor. Advances in ecological research. Academic Press; London, UK: 1974. pp. 268–401. [Google Scholar]
- Milligan S.R. The copulatory pattern of the bank vole (Clethrionomys glareolus) and speculation on the role of penile spines. J. Zool. 1979;188:279–300. [Google Scholar]
- Møller A.P, Alatalo R.V. Good-genes effects in sexual selection. Proc. R. Soc. B. 1999;266:85–91. doi:10.1098/rspb.1999.0607 [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Oksanen T.A, Jonsson P, Koskela E, Mappes T. Optimal allocation of reproductive effort: manipulation of offspring number and size in the bank vole. Proc. R. Soc. B. 2001;268:661–666. doi: 10.1098/rspb.2000.1409. doi:10.1098/rspb.2000.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai A, Yan G. Polyandry produces sexy sons at the cost of daughters in red flour beetles. Proc. R. Soc. B. 2002;269:361–368. doi: 10.1098/rspb.2001.1893. doi:10.1098/rspb.2001.1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot-Julliard A.C, Henttonen H, Yoccoz N.G, Stenseth N.C. Delayed maturation in female bank voles: optimal decision or social constraint? J. Anim. Ecol. 1999;68:684–697. doi:10.1046/j.1365-2656.1999.00307.x [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Evol. Ecol. 1996;11:201. doi: 10.1016/0169-5347(96)10028-8. doi:10.1016/0169-5347(96)10028-8 [DOI] [PubMed] [Google Scholar]
- Shields W.M. The natural and unnatural history of inbreeding and outbreeding. In: Thornhill N.W, editor. The natural history of inbreeding and outbreeding: theoretical and empirical perspectives on population structure. University of Chicago Press; Chicago, IL: 1993. pp. 143–169. [Google Scholar]
- Simmons L.W. The evolution of polyandry: an examination of the genetic incompatiblity and good-sperm hypotheses. J. Evol. Biol. 2001;14:585–594. doi:10.1046/j.1420-9101.2001.00309.x [Google Scholar]
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;36:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Tregenza T, Wedell N. Benefits of multiple mates in the cricket Gryllus bimaculatus. Evolution. 1998;52:1726–1730. doi: 10.1111/j.1558-5646.1998.tb02252.x. doi:10.2307/2411345 [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Heinemann; London, UK: 1972. pp. 136–179. [Google Scholar]
- Yasui Y. A “good-sperm” model can explain the evolution of costly multiple mating by females. Am. Nat. 1997;149:573–584. doi:10.1086/286006 [Google Scholar]
- Ylönen H, Viitala J. Social organization of an enclosed winter population of the bank vole Clethrionomys glareolus. Ann. Zool. Fenn. 1985;22:353–358. [Google Scholar]
- Ylönen H, Koskela E, Mappes T. Infanticide in the bank vole (Clethrionomys glareolus): occurrence and the effect of familiarity on female infanticide. Ann. Zool. Fenn. 1997;34:259–266. [Google Scholar]
- Yoccoz N.G, Mesnager S. Are alpine bank voles larger and more sexually dimorphic because adults survive better? Oikos. 1998;82:85–98. doi:10.2307/3546919 [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc. R. Soc. B. 1996;263:1711–1717. doi:10.1098/rspb.1996.0250 [Google Scholar]
- Zeh J.A, Zeh D.W. The evolution of polyandry II: post-copulatory defences against genetic incompatibility. Proc. R. Soc. B. 1997;264:69–75. doi:10.1098/rspb.1997.0010 [Google Scholar]
- Zeh J.A, Zeh D.W. Reproductive mode and genetic benefits of polyandry. Anim. Behav. 2001;61:1051–1063. doi:10.1006/anbe.2000.1705 [Google Scholar]