Abstract
Objective To investigate the influence of increasing age on the incidence and remaining lifetime risk of cardiovascular disease and cancer in a cohort of older men.
Design Prospective cohort study.
Setting United States.
Participants 22 048 male doctors aged 40-84 who were free of major disease in 1982.
Main outcome measures Incidence and remaining lifetime risk of major cardiovascular disease (myocardial infarction, stroke, and death from cardiovascular disease) and cancer.
Results 3252 major cardiovascular events and 5400 incident cancers were confirmed over 23 years of follow-up. The incidence of major cardiovascular disease continued to increase to age 100. Beginning at age 80, however, major cardiovascular disease was more likely to be diagnosed at death. The incidence of cancer peaked in those aged 80-89 and then declined. Cancers detected by screening accounted for most of the decline, whereas most cancers for which there was no screening continued to increase to age 100. Unadjusted cumulative incidence overestimated the risk of cardiovascular disease by 16% and cancer by 8.5%. The remaining lifetime risk of cancer at age 40 was 45.1% (95% confidence interval 43.8% to 46.3%) and at age 90 was 9.6% (7.2% to 11.9%). The remaining lifetime risk of major cardiovascular disease at age 40 was 34.8% (33.1% to 36.5%) and at age 90 was 16.7% (12.9% to 20.6%).
Conclusions In this prospective cohort of men, the incidence of new cardiovascular disease continued to increase after age 80 but was most often diagnosed at death. The decrease in incidence of cancer late in life seemed largely due to a decline in cancers usually detected by screening. These findings suggest that people aged 80 and older have a substantial amount of undiagnosed disease. The remaining lifetime risk of both diseases approached a plateau in the 10th decade. This may be due to decreased detection of disease and reporting of symptoms and increased resistance to disease in those who survive to old age. Accurate estimates of disease risk in an aging population require adjustment for competing risks of mortality.
Introduction
Heart disease, cancer, and stroke are the leading causes of morbidity and mortality in developed nations.1 Their burden is predicted to grow substantially over the next generation as the result of increased life expectancy.2 3 Age is perhaps the most powerful risk factor for cardiovascular disease and cancer, both of which increase exponentially between ages 40 and 80. However, there is some evidence that risk may decline after age 80.4 5 6 Data on incidence in the ninth and 10th decades are sparse, particularly in men. As people of advanced age (80 and older) are the fastest growing segment of most populations worldwide,7 a clearer understanding of their true experience of illness is critical to determining the impact of increased life expectancy on overall burden from disease.
The measurement and interpretation of the incidence of disease in advanced age is complex. Lower incidence in late life may reflect decreased screening and medical surveillance rather than decreased risk. Exposures to environmental and behavioural risk factors may also differ between younger and older people. In addition, traditional measures of cumulative incidence overestimate the true risk of disease when competing risks of death from other causes are high.8 To investigate further the incidence of disease in advanced age, we estimated the age specific incidence and remaining lifetime risk of cardiovascular disease and cancer up to age 100 in a large prospective cohort of men with 23 years of follow-up. This cohort of health conscious doctors has several advantages for studying the incidence of disease in men of advanced age, as it has a large proportion of participants surviving to age 90 and beyond,9 as well as a higher level of screening for disease and diagnosis than in a general population.
Methods
The Physicians’ Health Study is a completed randomised trial of aspirin (325 mg every other day) and β carotene (50 mg on alternate days) for the primary prevention of cardiovascular disease and cancer among 22 071 male doctors in the United States. Participants provided written informed consent. Detailed descriptions of the study design and findings have been published previously.10 11 At study entry in 1982, participants were aged between 40 and 84 and had no history of cardiovascular disease, cancer (with the exception of non-melanoma skin cancer), or other serious illnesses. In total, 92.2% of the participants identified their race as white. Baseline information was self reported and collected by a postal questionnaire that asked about many lifestyle variables and other risk factors for cardiovascular disease and cancer. Participants were sent follow-up questionnaires asking about study outcomes and other medical information twice in the first year and yearly thereafter. Follow-up after the trial is ongoing.12 In this analysis we used follow-up information up to 30 March 2007.
Ascertainment of cancer and cardiovascular diseases
Non-fatal cases of cancer and cardiovascular diseases were self reported by participants on follow-up questionnaires, and fatal cases were reported by family members or next of kin. Reports of coronary revascularisation procedures (coronary bypass surgery and percutaneous coronary angioplasty) and new onset angina were also recorded. We obtained the medical records for all reported cancers (excluding non-melanoma skin cancers) and major cardiovascular events, defined as non-fatal myocardial infarction, non-fatal stroke, or death from ischaemic heart disease or stroke. Cases of cancer, major cardiovascular disease, and death were confirmed by review of the participants’ medical records by the endpoints committee of the Physicians’ Health Study, which included two internists, a cardiologist, and a neurologist. Review of pathology reports was required for the confirmation of reported malignancies. Non-fatal myocardial infarction was confirmed using the World Health Organization criteria, and if the event was associated with diagnostic electrocardiograms or increased levels of cardiac enzymes.13 Non-fatal stroke was defined as a typical neurological deficit, sudden or rapid in onset and lasting more than 24 hours, that was attributed to a cerebrovascular event. Death due to ischaemic heart disease or stroke was confirmed by convincing evidence based on available information from medical records or death certificates, and for deaths outside the hospital from family members or next of kin. For this analysis we used only confirmed events. Major cardiovascular disease was defined as non-fatal myocardial infarction, non-fatal stroke, or death from cardiovascular disease. For the end point all cardiovascular disease, we also considered self reported angina and revascularisation procedures. If a participant reported more than one end point, we used the first event to occur to define the onset of disease.
Statistical analysis
We excluded participants who reported a history of cancer (n=19) or of major cardiovascular disease (n=4) before receiving the baseline questionnaire, leaving 22 048 men for this analysis. Age (in years) was used as the time scale. Follow-up began at baseline age and we censored participants at the age they developed the end point of interest, died, or reached the end of follow-up. Since few participants lived beyond the 10th decade, we censored data for when patients reached 100 years of age. We calculated one year crude incidence (per 100 000 person years) for each age and then collapsed these into 10 year age groups. We stratified age specific incidence by smoking status (ever v never). Using a modified Kaplan-Meier method we estimated the cumulative incidence of major cardiovascular disease or cancer conditional on survival to age 40.14 To understand the influence of mortality on estimates of disease incidence in very old people, we calculated remaining lifetime risk using a method described previously.15 We calculated the remaining lifetime risk of cancer, major cardiovascular disease, and overall cardiovascular disease for those who reached the ages of 50, 60, 70, 80, and 90 free of the disease of interest.
We produced incidence estimates using the practical incidence estimators macro, which has been described in detail8 and used in several previous analyses.5 6 16 17 Statistical calculations were done using SAS version 9.1 software.
Results
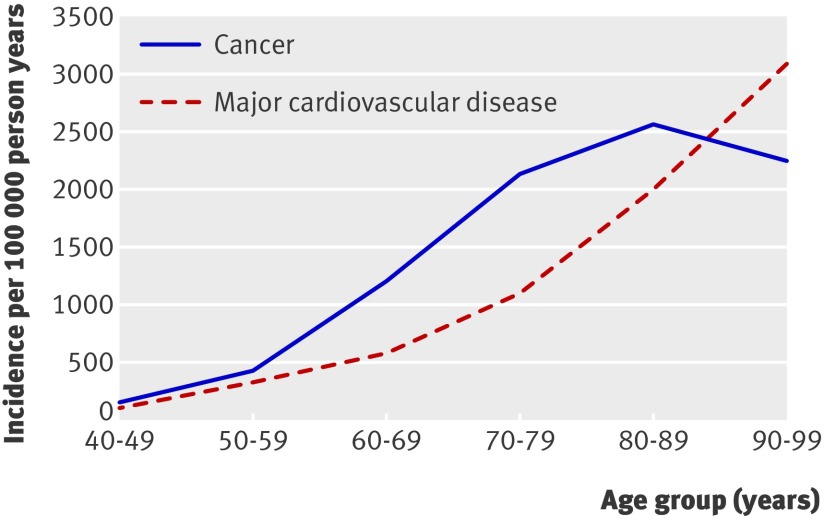
Table 1 shows the baseline characteristics of the participants by age at entry to the study. A total of 3051 participants were aged 65 or older at study baseline. After 23 years of follow-up (478 692 person years), 76.5% of the cohort was still alive. Overall, 32 142 person years had accrued in men aged 80-89 and 3312 person years in those aged 90-99. During follow-up, 3252 cases of major cardiovascular disease and 5400 incident cancers were confirmed. The incidence of major cardiovascular disease continued to rise through the 10th decade, with a rate of 3110 per 100 000 person years (fig 1). In contrast, the age specific incidence of overall cancer increased steadily from 160 per 100 000 person years in those aged 40-49 to 2555 per 100 000 person years in those aged 80-89. It then declined to 2264 per 100 000 person years in those aged 90-99.
Table 1.
Baseline characteristics of participants in Physicians’ Health Study. Values are percentages of participants
| Variables | <55 years (n=13 003) | 55-64 years (n=5994) | ≥65 years (n=3051) |
|---|---|---|---|
| Smoking status: | |||
| Former | 35.3 | 44.5 | 47.2 |
| Current | 10.9 | 11.9 | 9.4 |
| Daily alcohol use | 20.6 | 28.5 | 36.1 |
| Exercise to sweat 1-3 times or less monthly | 26.2 | 29.1 | 31.5 |
| Body mass index: | |||
| 25-29 kg/m2 | 36.9 | 41.3 | 37.8 |
| ≥30 kg/m2 | 4.4 | 4.1 | 3.6 |
| Diabetes | 1.1 | 2.8 | 5.3 |
| Hypertension | 16.2 | 29.8 | 44.6 |
| High cholesterol level | 10.0 | 14.4 | 15.1 |
Fig 1 Crude incidence of overall cancer and major cardiovascular disease by age
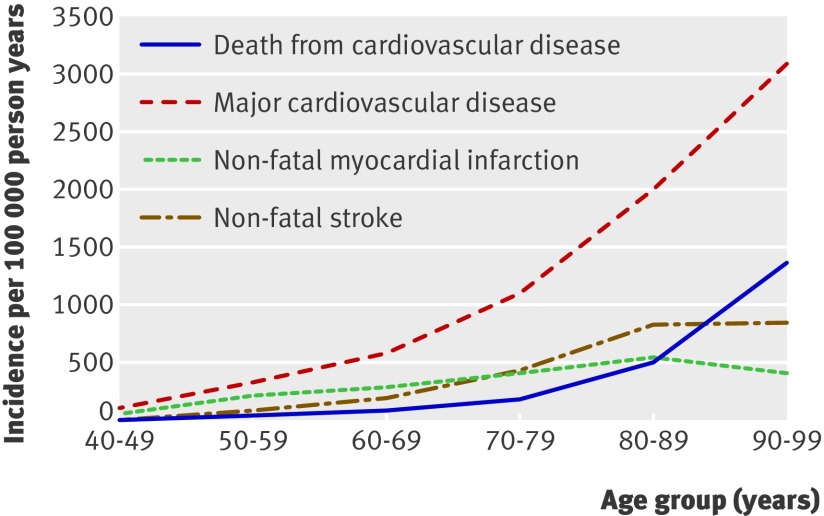
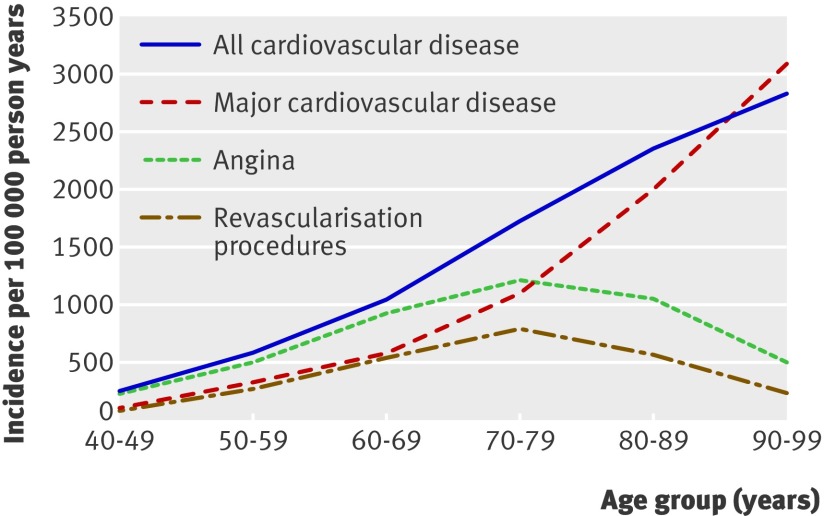
Whereas cardiovascular disease diagnosed at death increased dramatically with age, the incidence of non-fatal myocardial infarction declined, and the incidence of non-fatal stroke increased only slightly after age 89 (fig 2). Revascularisation procedures and angina declined noticeably with age as the first manifestation of cardiovascular disease, whereas the rate of confirmed major cardiovascular disease events continued to increase with age (fig 3). Table 2 shows the age specific incidence of cardiovascular disease by subtypes.
Fig 2 Age specific crude incidence of confirmed major cardiovascular disease by type of first event (non-fatal myocardial infarction, non-fatal stroke, and death from cardiovascular disease)
Fig 3 Age specific crude incidence of major cardiovascular disease compared with angina and revascularisation procedures as first cardiovascular disease events. Curve for all cardiovascular disease includes angina and revascularisation in addition to major end points. Participants were considered to have cardiovascular disease at first end point reported
Table 2.
Age specific incidence of cardiovascular disease by subtype in participants of Physicians’ Health Study
| Variables | No of events | Incidence per 100 000 person years (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| 40-49 years | 50-59 years | 60-69 years | 70-79 years | 80-89 years | 90-99 years | Overall | ||
| Person years of follow-up | — | 46 901 | 125 436 | 160 772 | 99 028 | 32 142 | 3312 | 467 591 |
| Confirmed end points: | ||||||||
| Major cardiovascular disease* | 3252 | 104.5 (77.29 to 138.12 | 333.2 (302.05 to 366.76) | 579.7 (543.08 to 618.16) | 1107.8 (1043.21 to 1175.31) | 2031.6 (1878.80 to 2193.63) | 3110.4 (2538.72 to 3772.3) | 695.5 (671.76 to 719.82) |
| Non-fatal myocardial infarction | 1430 | 68.2 (46.64 to 96.27) | 209.9 (185.28 to 236.73) | 311.4 (284.81 to 339.71) | 414.7 (376.16 to 456.11) | 545.4 (470.24 to 629.22) | 434.5 (248.38 to 705.69) | 300.6 (285.26 to 316.63) |
| Non-fatal stroke | 1210 | 27.7 (14.74 to 47.33) | 84.5 (69.25 to 102.11) | 184.6 (164.44 to 206.49) | 454.8 (414.77 to 497.63) | 807.1 (715.31 to 907.36) | 849 (576.84 to 1205.03) | 251.3 (237.39 to 265.92) |
| Death from cardiovascular disease | 612 | 8.5 (2.31 to 21.79) | 37.0 (27.18 to 49.19) | 72.4 (60.34 to 86.52) | 184.9 (160.08 to 212.43) | 497.3 (428.24 to 574.39) | 1,373.6 (1037.55 to 1783.72) | 124.9 (115.18 to 135.17) |
| Self reported end points: | ||||||||
| Angina | 3521 | 227.2 (185.99 to 274.76) | 496.9 (502.58 to 589.81) | 906.0 (858.95 to 954.87) | 1,207.8 (1137.51 to 1281.40) | 1,020.9 (907.90 to 1144.08) | 513.1 (293.26 to 833.21) | 788.5 (762.66 to 814.98) |
| CABG or PTCA | 2197 | 81.0 (57.32 to 111.18) | 277.7 (249.25 to 308.41) | 536.8 (501.54 to 574.00) | 773.9 (719.87 to 830.80) | 552.0 (474.71 to 638.25) | 242.6 (110.91 to 460.45) | 470.2 (450.78 to 490.31) |
| All cardiovascular disease† | 4517 | 246.9 (203.88 to 296.41) | 571.1 (529.47 to 615.10) | 1038.7 (987.65 to 1091.65) | 1719.7 (1633.04 to 1809.86) | 2338.9 2156.09 to 2533.13) | 2827.1 (2219.97 to 3549.20) | 1044.8 (1014.58 to 1075.75) |
CABG=coronary artery bypass grafting; PTCA=percutaneous coronary angioplasty.
*Confirmed end points of myocardial infarction, stroke, or death from cardiovascular disease.
†Self reported angina or revascularisation procedures are included as cardiovascular disease defining events as well as confirmed end points of myocardial infarction, stroke, or death from cardiovascular disease.
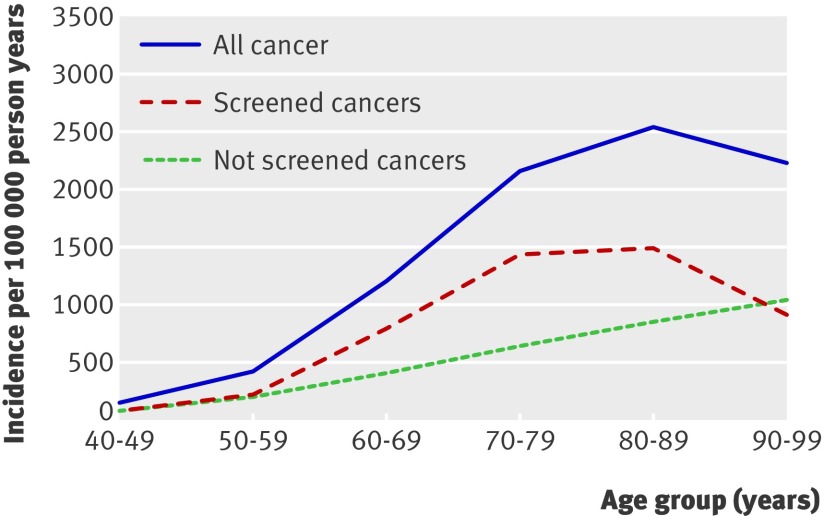
Table 3 displays the age specific incidence of cancer by subtypes. The most common cancers were prostate (47.2%), colorectal (10.3%), lymphoma (6.6%), lung (6.6%), and melanoma (5.7%). Most of the cancers that declined before age 100 were those detected by screening, whereas the incidence of cancers for which there was no routine screening continued to increase up to age 99 (fig 4). The cancer rate among ever smokers peaked in those aged 80-89, at 2883 per 100 000 person years, and then declined, whereas the rate among never smokers peaked at 2205 per 100 000 person years in the ninth decade and then remained stable. In contrast, the incidence of major cardiovascular disease increased through the 10th decade in both smokers and non-smokers (data not shown).
Table 3.
Age specific incidence of overall and specific cancers in participants of Physicians’ Health Study
| Variables | No (%) of cancers | Incidence per 100 000 person years (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| 40-49 years | 50-59 years | 60-69 years | 70-79 years | 80-89 years | 90-99 years | Overall | ||
| Person years of follow-up | — | 46 838 | 125 132 | 157 849 | 92 701 | 29 076 | 2960 | 454 557 |
| Cancer subtype: | ||||||||
| All cancers | 5400 (100.0) | 160.1 (125.95 to 200.72) | 431.5 (395.90 to 469.51) | 1242.3 (1187.96 to 1298.53) | 2172.6 (2078.73 to 2269.59) | 2555.3 (2374.86 to 2745.82) | 2263.5 (1754.20 to 2874.57) | 1188.0 (1156.53 to 1220.05) |
| Prostate | 2548 (47.2) | 4.3 (0.52 to 15.44) | 133.7 (114.15 to 155.53) | 641.2 (602.21 to 681.97) | 1152.6 (1084.33 to 1224.04) | 1008.7 (896.08 to 1131.47) | 545.2 (311.64 to 885.45) | 562.3 (540.70 to 584.57) |
| Colorectal | 554 (10.3) | 15.0 (6.01 to 30.81) | 52.9 (40.87 to 67.23) | 114.0 (97.95 to 132.03) | 209.2 (180.64 to 240.95) | 351.2 (286.08 to 426.78) | 307.1 (140.41 to 582.90) | 122.5 (112.54 to 133.18) |
| Lymphoma | 354 (6.6) | 17.1 (7.38 to 33.68) | 35.2 (25.60 to 47.30) | 75.2 (62.24 to 90.05) | 126.4 (104.47 to 151.64) | 226.2 (174.57 to 288.28) | 102.5 (21.13 to 299.43) | 78.3 (70.37 to 86.91) |
| Lung | 340 (6.3) | 2.1 (0.05 to 11.90) | 20.0 (12.96 to 29.55) | 72.0 (59.35 to 86.58) | 154.7 (130.34 to 182.39) | 181.0 (135.17 to 237.35) | 239.0 (96.06 to 492.23) | 75.2 (67.43 to 83.65) |
| Melanoma | 305 (5.7) | 49.1 (31.15 to 73.72) | 38.4 (28.34 to 50.95) | 66.9 (54.73 to 81.00) | 95.9 (76.94 to 118.19) | 135.8 (96.55 to 185.60) | 68.3 (8.27 to 246.83) | 67.5 (60.12 to 75.49) |
| Urinary tract* | 300 (5.6 ) | 15.0 (6.01 to 30.81) | 23.2 (15.55 to 33.35) | 68.18 (55.88 to 82.40) | 123.16 (101.50 to 148.07) | 142.73 (102.43 to 193.63) | 102.46 (21.13 to 299.43) | 66.37 (59.07 to 74.32) |
| Other gastrointestinal† | 286 (5.3) | 8.6 (2.33 to 21.88) | 23.2 (15.55 to 33.35) | 49.1 (38.73 to 61.33) | 119.9 (98.54 to 114.51) | 205.3 (156.30 to 264.86) | 238.9 (96.06 to 492.23) | 63.3 (56.15 to 71.05) |
| Leukaemia | 181 (3.4) | 2.1 (0.05 to 11.91) | 23.2 (15.55 to 33.35) | 42.1 (32.53 to 53.52) | 56.7 (42.34 to 74.35) | 101.0 (67.63 to 145.02) | 136.6 (37.22 to 349.71) | 40.1 (34.43 to 46.33) |
| Head and neck | 116 (2.2) | 8.6 (2.33 to 21.88) | 13.6 (7.83 to 21.80) | 31.2 (23.11 to 41.29) | 33.8 (22.97 to 47.98) | 45.3 (24.11 to 77.43) | 68.3 (8.27 to 246.79) | 25.7 (21.21 to 30.79) |
| Unknown | 101 (1.9) | 2.1 (0.05 to 11.91) | 8.8 (4.40 to 15.76) | 18.5 (12.38 to 26.55) | 36.0 (24.76 to 50.53) | 73.1 (45.27 to 111.79) | 204.8 (75.16 to 445.80) | 22.4 (18.20 to 27.16) |
| Brain | 97 (1.8) | 2.1 (0.05 to 11.91) | 18.4 (11.68 to 27.34) | 23.6 (16.61 to 32.51) | 22.9 (14.18 to 35.00) | 45.3 (24.11 to 77.43) | 68.3 (8.27 to 246.79) | 21.5 (17.41 to 26.19) |
| Other | 218 (4.0) | 34.2 (19.54 to 55.51) | 41.6 (31.10 to 54.60) | 45.9 (35.90 to 57.79) | 57.8 (43.28 to 75.58) | 66.2 (39.84 to 103.33) | 204.8 (75.16 to 445.80) | 48.23 (42.04 to 55.08) |
*Includes urinary tract and genital tumours.
†Includes oesophagus, stomach, pancreas, and hepatobiliary cancers.
Fig 4 Age specific crude incidence of overall cancer compared with subgroups of cancers detected by screening (prostate, colorectal, melanoma) and cancers for which there is no routine screening
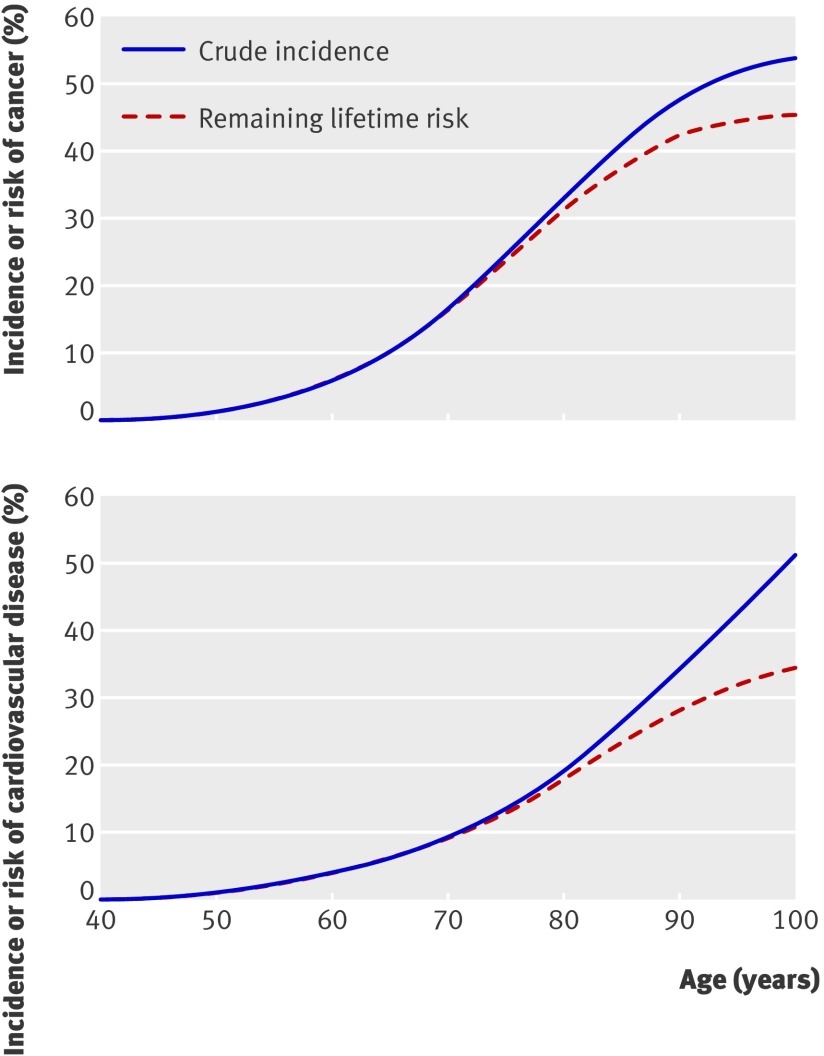
Adjustment for competing risks of death clearly attenuated the estimate of cumulative incidence for both major cardiovascular disease and cancer (fig 5). Whereas cumulative incidence continued to increase among the oldest participants, mortality adjusted curves tended to flatten out in the 10th decade. Table 4 shows the remaining lifetime risk of major diseases for men who reached various index ages free of the disease of interest. The risk of both cardiovascular disease and cancer decreased as remaining lifetime diminished. The risk of major cardiovascular disease in 40 year olds was 34.8% (33.1% to 36.5%) and in 90 year olds was 16.7% (12.9% to 20.6%).The risk of any cancer in 40 year olds was 45.1% (95% confidence interval 43.8% to 46.3%) and in 90 year olds was 9.6% (7.2% to 11.9%).
Fig 5 Effect of adjustment for competing risks of mortality on cumulative risk of cancer and of major cardiovascular disease. Estimates of cumulative incidence and mortality adjusted lifetime risk are conditional on disease free survival to age 40
Table 4.
Remaining lifetime risk of first cancer or major cardiovascular disease event by age reached free of events in participants of Physicians’ Health Study
| Age (years) | Remaining lifetime risk (%) (95% CI) | ||
|---|---|---|---|
| All cancer | Major cardiovascular disease | All cardiovascular disease | |
| 40 | 45.1 (43.8 to 46.3) | 34.8 (33.1 to 36.5) | 41.3 (39.9 to 42.7) |
| 50 | 44.5 (43.3 to 45.7) | 34.5 (32.8 to 36.2) | 40.5 (39.1 to 41.9) |
| 60 | 42.9 (41.6 to 44.2) | 33.0 (31.2 to 34.8) | 38.0 (36.5 to 39.5) |
| 70 | 36.6 (35.2 to 38.0) | 30.5 (28.5 to 32.4) | 33.2 (31.5 to 34.9) |
| 80 | 24.3 (22.5 to 26.1) | 25.7 (23.3 to 29.1) | 24.9 (22.7 to 27.1) |
| 90 | 9.6 (7.2 to 11.9) | 16.7 (12.9 to 20.6) | 13.7 (10.3 to 17.2) |
Remaining lifetime risk=mortality adjusted cumulative risk conditional on disease-free survival to age specified.
Discussion
In this large prospective cohort of US male doctors without serious diseases at entry to the study, the incidence of major cardiovascular disease increased exponentially through the 10th decade. In contrast, the incidence of cancer peaked in men aged 80-90 and then declined. In those aged more than 80, new cases of cardiovascular disease were most often diagnosed at death. The decrease in incidence of cancer late in life seemed largely due to a decline in cancers detected by screening. The cumulative incidence of both cardiovascular disease and cancer were clearly attenuated by adjustment for risk of mortality. The remaining lifetime risk of both diseases decreased with advancing age.
Incidence of cardiovascular disease
Previous studies have reported an increasing incidence of cardiovascular disease in the oldest participants, but those aged 85 and older were combined in a single group.18 19 The rate of major cardiovascular disease in our study continued to increase up to age 99. However, the rate of non-fatal myocardial infarction declined after age 89, whereas that of non-fatal stroke reached a plateau by age 90. The decline in non-fatal cardiovascular events in the face of increased deaths due to cardiovascular disease suggests that people of advanced age may be living with a substantial amount of undiagnosed cardiovascular disease. Self reported revascularisation procedures and angina declined noticeably after age 79. This may be due in part to decreased symptoms or decreased reporting, but may also reflect a decrease in aggressive medical care and diagnostic procedures. When we considered events based on symptoms (angina) or coronary procedures as the first manifestation of cardiovascular disease, age specific incidence approached a plateau in the oldest participants, illustrating the importance of defining disease when doing incidence studies.
Cancer incidence
Many studies have shown a sharp increase in incidence of cancer with age. Our findings of a decline after age 89 agree with those of other large cohorts with sufficient follow-up.4 20 They are similar to results of an analysis using the 1995-7 data from SEER (Surveillance, Epidemiology, and End Results), a US cancer registry programme.21 The incidence of invasive cancer in white men peaked at a similar age range to ours (85-89) and at about the same incidence (2500 per 100 000), and then declined.
However, the decrease in incidence of cancer late in life seemed to be largely driven by cancers detected by screening. The most dramatic decline was in prostate cancer, which accounted for 47% of all cancers diagnosed in the Physicians’ Health Study. Prostate cancer peaked the earliest of all cancers, in those aged 70-79. Melanoma and colorectal cancer declined after age 89. With the exception of lymphoma and tumours of the urinary tract, the incidence of all other cancers continued to increase to age 100. Gastrointestinal malignancies and tumours of unknown origin became particularly prominent with age, as has been shown in other studies.4 20 Our findings suggest that a substantial part of the decline in overall incidence of cancer late in life is accounted for by decreased ascertainment of disease and may not represent a true decrease in risk. It is also possible that our participants were less likely to report new diagnoses of cancer in advanced age. However, the fact that cancers not detected by screening peaked later in our cohort than in other studies possibly reflects the higher level of medical surveillance and reporting of symptoms in this cohort of doctors than would be the case in a general population. Despite the overall decline in its incidence, cancer clearly remains an important cause of disease in advanced age. As life expectancy continues to increase, research on the benefits of individualised screening programmes for people aged more than 80 is warranted.
Lifetime risks
While the cumulative incidence curve shows cardiovascular disease increasing sharply to age 100, adjustment for competing risks of death resulted in a substantial decrease in risk in men aged 80 or more (fig 5). A similar decrease was seen for cancer. Cumulative incidence overestimated the actual risk of cardiovascular disease in our population by 16% and cancer by 8.5%. Our findings underline the importance of taking mortality into account when predicting disease risk in a cohort of elderly people.
The remaining lifetime risk of major cardiovascular disease decreased from 1 in 2 for men aged 40 to 1 in 6 for men aged 90. When we used a broader definition of cardiovascular disease the remaining lifetime risk at age 40 was higher (41.3% v 34.8%), whereas the risk at age 90 was lower (16.7% v 13.7%). This is because some participants reported angina or revascularisation procedures before a major cardiovascular event, thus shifting their date of diagnosis to earlier in life. The age specific and cumulative incidence curves would suggest that cardiovascular disease is intimately related to the normal aging of the host and that its incidence continues to increase indefinitely with age.22 However, lifetime risk curves show that the incidence of cardiovascular disease begins to plateau later in life, as any increased risk is outpaced by competing risks of death. This has been shown in previous studies of coronary heart disease and stroke.5 6 In contrast, the remaining lifetime risk of heart failure, a true age dependent disease, is the same in 80 year olds as in 40 year olds.16 Cancer is often thought of as inextricably linked with aging, but it seems to fit better the pattern of age related diseases that occur in a particular age range and then decline. The remaining lifetime risk of cancer decreased from 1 in 2 in 40 year olds to 1 in 10 in 90 year olds.
A plateau or decline in lifetime risk with age has previously been reported for overall cancer,21 stroke,6 coronary artery disease,5 and Alzheimer’s disease.23 This may be a function of decreased life expectancy in people of advanced age; it may also be due to the selective survival of those who are more resistant to disease. Those who survive disease-free to older ages may have fewer exposures to risk factors or may be “immune” to them. The relation between risk factors and disease may also change. For example, although a high cholesterol level is a well established risk factor for cardiovascular disease and mortality in middle aged people the relation is less clear in elderly people,24 25 and high levels in people aged 85 and older have been associated with longevity.26 Finally, the observed decline in lifetime risk may not represent a true decrease in risk at all but rather decreased reporting of or diagnosis of disease.
It is important to emphasise that estimates of lifetime risk strongly depend on life expectancy and they cannot be compared across populations unless mortality rates are similar. At age 65 our participants had an additional 25 years of life expectancy, exceeding the national average for white men by seven years.27 The goal of our analysis was not to provide estimates that would be readily applicable to men in general, but to investigate the risk of disease in a population of very elderly people. On the one hand a population with a shorter life expectancy might have smaller lifetime risks than our cohort. On the other hand a less health conscious population might have a higher incidence of disease.
Strengths and limitations
Our study has several strengths, including the large number of participants and outcome events, prospective design, and well defined population with a long follow-up. End points were ascertained and confirmed after review of medical records. We adjusted cumulative incidence for competing risks of mortality.
Several limitations must be considered. Firstly, our findings may not be generalisable to a broader population as our cohort consisted almost exclusively of highly educated white men. Our participants might have a lower risk of cancer and cardiovascular disease than a general population for several reasons. They were healthy at baseline and had a lower incidence of smoking and obesity than expected. All members of our cohort had participated in a primary prevention trial of aspirin and β carotene. Although β carotene was found to have no influence on the risk of cardiovascular disease or cancer,11 randomisation to aspirin was associated with a decreased risk of myocardial infarction but not of stroke or death from cardiovascular disease.10 Most participants became regular users of aspirin after completion of the trial.28 Aspirin use was not associated with risk of cancer. Study treatment thus had no effect on the study outcomes, with the exception of lower rates of myocardial infarction. Although the factors mentioned might influence the risk of disease, they would not be expected to affect the shape of the incidence curves. Our participants may also have had increased rates of screening, as reflected in higher rates of cancers detected by screening than in the general population. Nevertheless, the lifetime risk for overall cancer (45.1%) in our study was nearly identical to that of the SEER estimate for white men based on a sample of the US population (44.9%).21 The lifetime risk of stroke in men aged 55 in our cohort (15.4%) was also similar to that of men in the population based Framingham Heart Study (16.9%).5 6 We did not address secular trends in screening and risk factors for disease, which might also explain changes in incidence. Because we chose to focus our analysis only on diseases that were confirmed outcomes of the Physicians’ Health Study, we did not tackle other important conditions such as neurodegenerative and psychiatric disease. Finally, despite the homogeneity of our population for race and education, biological associations are expected to be similar in our study compared with other male populations.
Conclusion
In summary, the lifetime risks of both cardiovascular disease and cancer approached a plateau in the 10th decade. This may be due to several factors, including decreased detection or reporting of disease and increased resistance to disease, and has important implications for predicting the risk of disease in individuals and populations. Accurate estimates of long term risk for disease require adjustment for competing risks of mortality, particularly in an aging population. Finally, our results suggest that people of advanced age may have a substantial amount of undiagnosed disease, which may in turn contribute to geriatric syndromes such as frailty. Additional research is needed to determine if continued screening and detection of these conditions up to and beyond age 80 might help improve health and wellbeing late in life.
What is already known on this topic
Age is the strongest risk factor for cardiovascular disease and cancer but the incidence of these diseases in people aged 80 or more is less clear
Studies of incidence in the ninth and 10th decade of life are sparse
What this study adds
The incidence of cardiovascular disease in a cohort of US male doctors increased to age 100 whereas that of overall cancer decreased after age 89
The decline in cancer incidence was largely driven by a decrease in screening related cancers, whereas cardiovascular disease after age 80 was most commonly diagnosed at death
Definitions17 29 30
Competing risks—study participants who may be removed from follow-up—for example, because of death from causes other than the disease of interest. The effect is minimal over short periods but significant when follow-up is long or participants are older and have an increased risk of death from many causes
Incidence—number of participants who develop a disease divided by the sum of the time contribution (person time) for all participants followed. Accounts for competing risk and loss to follow-up, as participants only contribute actual time they are followed in the study. Usually expressed as an annual incidence—for example, 50 cases per 100 000 persons per year
Cumulative incidence—commonly used to estimate the long term risk of developing a disease. Assumes no loss to follow-up and that those who die would have developed the disease at the same rate as survivors; however, those who die have no risk. Leads to overestimation of risk when the population prevalence of a condition is more than 10% or competing risks of death are high
Remaining lifetime risk—estimates the absolute risk of ever developing a disease before dying of something else. Adjusts cumulative incidence for competing risks of death. Provides one summary measure of absolute risk for the remainder of one’s lifetime—for example, a lifetime risk of 25%, or 1 in 4. Appropriate measure for individual and population risk
We thank the staff of the Physician’s Health Study and the participants.
Contributors: JAD conceived and designed the study, did the data analyses, wrote the first draft of the article, and is guarantor. LD, JMG, GL, and TK gave detailed advice at all stages of the analyses. All authors contributed to the writing of the paper and gave substantial advice and input into the study.
Funding: The authors’ work was independent of the funders. JAD received support for this research from the Hartford Foundation’s Center of Excellence in Geriatric Medicine at Harvard Medical School and the Eleanor and the Miles Shore 50th anniversary fellowship programme for scholars in medicine at Harvard Medical School. The Physicians’ Health Study is supported by grants CA-34944, CA-40360, and CA-097193 from the National Cancer Institute, and grants HL-26490 and HL-34595 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Competing interests: Although the authors believe they have no competing interests that could influence their decisions, work, or writing of the manuscript, they report a full disclosure for the past five years. LD received investigator initiated funding and support as principal investigator from the National Institutes of Health, the Alcoholic Beverage Medical Research Foundation, the Biomedical Research Institute at Brigham and Women’s Hospital, and the Huntington Disease Society of America. GL received investigator initiated research funding from the National Institutes of Health and received honorariums from Pfizer and Lilly Pharmaceutical for speaking engagements in 2003. JMG received investigator initiated research funding and support as principal investigator from the National Institutes of Health, BASF, DSM Pharmaceuticals, Wyeth Pharmaceuticals, McNeil Consumer Products, and Pliva; received honorariums from Bayer and Pfizer for speaking engagements; and is a consultant for Bayer, McNeil Consumer Products, Wyeth Pharmaceuticals, Merck, Nutraquest, and GlaxoSmithKline. TK received investigator initiated research funding as principal investigator or co-investigator from the National Institutes of Health, Bayer AG, McNeil Consumer & Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety, and received honorariums from Organon for contributing to an expert panel and from Genzyme for providing educational lectures.
Ethical approval: This study analysed existing data from the Physicians’ Health Study and was approved by the institutional review board of the Brigham and Women’s Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2008;337:a2467
References
- 1.World Health Organization. The top ten causes of death. WHO fact sheet. Geneva: WHO, 2007.
- 2.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer 2006;6:63-74. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet 1997;349:1498-504. [DOI] [PubMed] [Google Scholar]
- 4.De Rijke JM, Schouten LJ, Hillen HF, Kiemeney LA, Coebergh JW, van den Brandt PA. Cancer in the very elderly Dutch population. Cancer 2000;89:1121-33. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999;353:89-92. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham study. Stroke 2006;37:345-50. [DOI] [PubMed] [Google Scholar]
- 7.United Nations Population Division. World population ageing: 1950-2050. New York: United Nations, 2002.
- 8.Beiser A, D’Agostino RB Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham study. The practical incidence estimators (PIE) macro. Stat Med 2000;19:1495-522. [DOI] [PubMed] [Google Scholar]
- 9.Yates LB, Djousse L, Kurth T, Buring JE, Gaziano JM. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med 2008;168:284-90. [DOI] [PubMed] [Google Scholar]
- 10.Final report on the aspirin component of the ongoing physicians’ health study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-35. [DOI] [PubMed] [Google Scholar]
- 11.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334:1145-9. [DOI] [PubMed] [Google Scholar]
- 12.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol 2000;10:125-34. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Ischemic heart disease registers: report of the Fifth Working Group (including a second revision of the operating protocol), Copenhagen, 26-29 April 1971. Copenhagen, Denmark: Regional Office for Europe, WHO, 1971.
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. [Google Scholar]
- 15.Gaynor J, Feuer E, Tan C, Wu D, Little C, Straus D, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc 1993;88:400-9. [Google Scholar]
- 16.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation 2002;106:3068-72. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham study. Neurology 1997;49:1498-504. [DOI] [PubMed] [Google Scholar]
- 18.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam study. J Neurol Neurosurg Psychiatry 2003;74:317-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psaty BM, Furberg CD, Kuller LH, Bild DE, Rautaharju PM, Polak JF, et al. Traditional risk factors and subclinical disease measures as predictors of first myocardial infarction in older adults: the cardiovascular health study. Arch Intern Med 1999;159:1339-47. [DOI] [PubMed] [Google Scholar]
- 20.Saltzstein SL, Behling CA, Baergen RN. Features of cancer in nonagenarians and centenarians. J Am Geriatr Soc 1998;46:994-8. [DOI] [PubMed] [Google Scholar]
- 21.Merrill RM, Weed DL. Measuring the public health burden of cancer in the United States through lifetime and age-conditional risk estimates. Ann Epidemiol 2001;11:547-53. [DOI] [PubMed] [Google Scholar]
- 22.Brody JA, Schneider EL. Diseases and disorders of aging: an hypothesis. J Chronic Dis 1986;39:871-6. [DOI] [PubMed] [Google Scholar]
- 23.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham study. Lancet Neurol 2007;6:1106-14. [DOI] [PubMed] [Google Scholar]
- 24.Castelli WP, Wilson PW, Levy D, Anderson K. Cardiovascular risk factors in the elderly. Am J Cardiol 1989;63:12H-19H. [DOI] [PubMed] [Google Scholar]
- 25.Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA 1994;272:1335-40. [PubMed] [Google Scholar]
- 26.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet 1997;350:1119-23. [DOI] [PubMed] [Google Scholar]
- 27.Arias E. United States life tables, 2003. Natl Vital Stat Rep 2006;54:1-40. [PubMed] [Google Scholar]
- 28.Cook NR, Hebert PR, Manson JE, Buring JE, Hennekens CH. Self-selected posttrial aspirin use and subsequent cardiovascular disease and mortality in the physicians’ health study. Arch Intern Med 2000;160:921-8. [DOI] [PubMed] [Google Scholar]
- 29.Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health 1994;48:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman KJ. Epidemiology: an introduction. Oxford: Oxford University Press, 2002.