Abstract
Stem cells have a fascinating biology and offer great prospects for therapeutic applications, stimulating intense research on what controls their properties and behavior. Although there have been significant advances in our understanding of how local microenvironments, or niches, control the maintenance and activity of stem cells, it is much less well understood how stem cells sense and respond to variable external, physiological, or tissue environments. This review focuses on the multidirectional interactions among stem cells, niches, tissues, and the systemic environment and on potential ideas for how changes in this network of communication may relate to the aging process.
IN spite of their small numbers and considerable stealth, stem cells have a tremendous impact on the biology of multicellular organisms. Endowed with self-renewal ability and multipotency, stem cells maintain tissues that undergo rapid turnover, regenerate damaged tissue, and ensure optimal tissue and organ function. Many layers of regulation in response to local, systemic, and environmental factors govern stem cell behavior (Drummond-Barbosa 2005; Morrison and Spradling 2008). Evidence from several systems also suggests that stem cell functional output is altered during aging (Rando 2006; Jones 2007; Sharpless and Depinho 2007), although causal relationships and molecular underpinnings are poorly understood. To effectively wield these double-edged swords for therapeutic interventions, it will be essential to further explore their regulation and function using the combined power of multiple model organisms.
STEM CELLS RECEIVE LOCAL NICHE SIGNALS
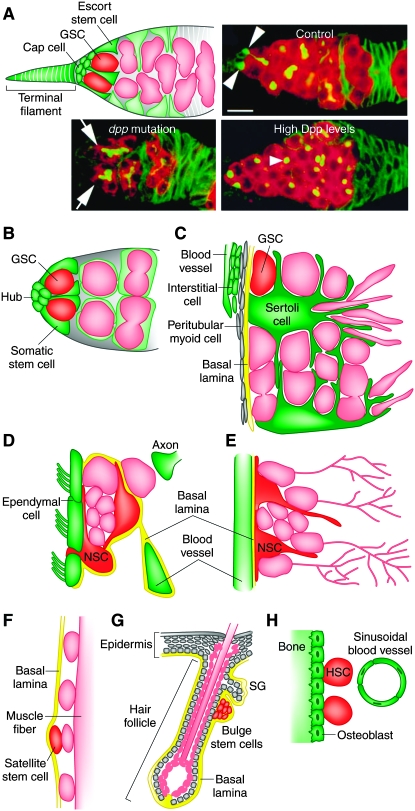
Although the theoretical existence of a specialized microenvironment, or niche, that controls stem cell activity was proposed 3 decades ago (Schofield 1978), a strong experimental basis for the niche concept came from more recent studies of Drosophila melanogaster germline stem cells (GSCs). In the anterior region of the ovary, each germarium houses two to three GSCs anchored via E-cadherin-containing adherens junctions to somatic cap cells and in close proximity to terminal filament cells (Figure 1A). Bone morphogenetic protein (BMP) signals are expressed in the somatic niche and act directly on GSCs to repress differentiation and maintain stem cell fate (Wong et al. 2005). Janus kinase–signal transducer and activator of transcription (JAK–STAT) signaling controls BMP signal production in the niche (Lopez-Onieva et al. 2008; Wang et al. 2008). At the tip of the testis, five to nine GSCs, each surrounded by a pair of somatic cyst stem cells, maintain close contact with a dome-shaped apical hub composed of tightly packed somatic cells (Figure 1B). The hub-produced ligand Unpaired (Upd) stimulates JAK–STAT signaling in both stem cell types, which is required for their maintenance (Wong et al. 2005). Surprisingly, ectopic JAK–STAT activation in somatic but not germ cells is sufficient to induce overproliferation of GSCs and somatic stem cells. Zfh-1, a transcriptional repressor induced by JAK–STAT signaling, is required in somatic stem cells for their maintenance, and its forced somatic expression induces their overproliferation and, non-cell autonomously, that of GSCs (Leatherman and Dinardo 2008). Analogously, the germarium contains a population of escort stem cells that are in close association with GSCs and require JAK–STAT signaling for their proliferation and for GSC maintenance (Decotto and Spradling 2005). BMP signaling is also directly required for GSC maintenance and bam repression in the testis (Wong et al. 2005).
Figure 1.—
Stem cell niches. (A) Drosophila germarium illustrating GSCs in their niche, formed by cap cells, terminal filament cells, and escort stem cells (top left). Escort stem cell and GSC progeny are shown in light green and pink, respectively. Confocal images (top right and bottom) showing germaria from control, decapentaplegic (dpp) mutant, and dpp-overexpressing females. dpp encodes a BMP signal. GSCs (arrowheads) are lost in dpp mutants. Arrows point to differentiating germ-cell cysts. Increased numbers of GSC-like cells (arrowhead) result from high Dpp levels. Bar, 10 μm. Confocal images (top right and bottom) were reproduced from Figure 1D and Figure 4, A and G, in Xie and Spradling (1998). (B) Male Drosophila GSCs in their niche, comprising the hub and somatic stem cells. (C) Seminiferous epithelium in the mammalian testis. GSCs and their progeny (pink) are closely associated with Sertoli cells, and GSCs reside in proximity to the vasculature and interstitial cells. (D) The subventricular zone showing astrocytes that function both as NSCs and as niche components. NSCs are closely associated with ependymal cells, blood vessels, a specialized basal lamina, and axon terminals. (E) The subgranular zone depicting NSCs in close association with blood vessels. In D and E, NSC progeny are shown in pink. (F) Satellite stem cell (red) in the mammalian muscle. Satellite stem cells and committed satellite cells (pink ovals) reside sandwiched between the muscle fiber and the basal lamina. The depicted satellite stem cell has recently divided to produce one stem cell and one committed daughter. (G) Mammalian hair follicle and part of epidermis. Hair follicle stem cells reside in the bulge (bulge stem cells), and separate populations of stem cells reside in the basal layer of the epidermis and in the sebaceous gland (SG). (H) HSC in the bone marrow. HSCs reside in close proximity to the inner bone surface and to specialized blood vessels.
There have also been advances toward defining stem cell niches in other systems (Morrison and Spradling 2008). In Caenorhabditis elegans adult hermaphrodites, germ cells in the distal regions of each gonad arm define a self-renewing population blocked from entry into meiosis via their Notch-mediated interaction with somatic distal tip cells (Wong et al. 2005). Transplantation assays have demonstrated the existence of GSCs (or spermatogonial stem cells) in the mammalian testis (Figure 1C) (Wong et al. 2005). Large somatic Sertoli cells closely associate with GSCs and other undifferentiated spermatogonia next to the basement membrane in the seminiferous tubules. Sertoli cells produce glial cell-line-derived neurotrophic factor (GDNF), which is required for GSC maintenance (Wong et al. 2005). Recent studies revealed that undifferentiated spermatogonia are preferentially localized to regions adjacent to the vascular network and associated interstitial cells underlying the basement membrane (Yoshida et al. 2007), suggesting a vascular niche for GSCs. Neural stem cells (NSCs) reside in the subventricular zone (SVZ; Figure 1D) and in the subgranular zone (SGZ; Figure 1E) of the hippocampus and, in both regions, neural precursors are astrocytes that self-renew and generate neuroblasts (Riquelme et al. 2008). Lineage-tracing studies demonstrated that NSCs in the SVZ are heterogeneous (Merkle et al. 2007). Astrocytes are also key components of the NSC niche, which includes blood vessels, a basal lamina, and axon terminals (Riquelme et al. 2008). Recently, a pinwheel niche architecture of cerebrospinal-fluid-contacting ependymal cells encircling clustered apical surfaces of astrocytes that also extend long basal processes to blood vessels has been described (Mirzadeh et al. 2008).
In other cases, the niche remains undefined. Satellite cells are sandwiched between the basal lamina and the muscle fiber (Figure 1F) and generate myogenic precursor cells (Morrison and Spradling 2008). Notch signaling is required for maintaining satellite stem cells, and lineage tracing and transplantation studies have shown that one-tenth of satellite cells behave as stem cells. Planar division frequently results in two stem cells, while apical–basal division results in one stem cell and one committed daughter (Morrison and Spradling 2008). In the mammalian skin, different types of stem cells reside in different microenvironments (Figure 1G). Epidermal stem cells reside in the basal layer of the epidermis, while hair follicle stem cells reside in the bulge at the base of the permanent portion of the follicle (Morrison and Spradling 2008). Wnt and BMP signaling play critical roles in regulating bulge stem cells during hair follicle growth cycles (Morrison and Spradling 2008). Melanocyte stem cells, whose progeny produce melanin that is transferred to hairs, are located near hair follicle stem cells and respond to Steel/Kit signaling (Nishimura et al. 2002). Most hematopoietic stem cells (HSCs) reside in the bone marrow at or near the inner bone surface (endosteum) (Figure 1H) and although osteoblasts lining the endosteum appear to play a niche role, many HSCs localize adjacent to specialized blood vessels (sinusoids) (Morrison and Spradling 2008).
STEM CELLS RESPOND TO SYSTEMIC SIGNALS
It seems intuitively obvious that stem cells must respond to environmental and systemic signals to adjust cell production to varying demands. Several examples illustrate the responsiveness of stem cells to tissue-extrinsic factors, although the underlying mechanisms remain poorly understood. A well-studied example is the response of ovarian stem cells to diet and insulin signals in Drosophila. On a protein-rich diet, GSCs and follicle stem cells (and their descendants) have high division rates, whereas on a protein-poor diet, these rates are reduced; this process requires insulin signaling (Drummond-Barbosa and Spradling 2001). Brain-derived insulin-like peptides have been shown by genetic mosaic analysis to directly stimulate GSCs to control their proliferation (Lafever and Drummond-Barbosa 2005). In contrast, daf-2/insulin receptor mutations in C. elegans have been reported not to affect proliferation of germ cells in a wild-type background, although this conclusion was based on comparisons of the total number of phosphohistone H3 (a mitotic marker)-positive cells per gonad arm (Pinkston et al. 2006). Intriguingly, insulin signaling has an additional, separate role in controlling GSC maintenance via the niche in Drosophila (H. J. Hsu and D. Drummond-Barbosa, unpublished results). Notch signaling controls cap cell number (Ward et al. 2006; Song et al. 2007). Insulin-like peptides regulate Notch signaling to maintain cap cell numbers, and they also promote cap cell–GSC association at least in part via E-cadherin (H. J. Hsu and D. Drummond-Barbosa, unpublished results). This illustrates the profound impact that the systemic environment can have on both stem cell activity and the niche that controls stem cell fate.
NSCs also sense and respond to injury or physiological changes. For example, in adult rodents, focal cerebral ischemia leads to increased proliferation of neural progenitors in the SVZ and SGZ, including those far from the area of infarction (Jin et al. 2001; Zhang et al. 2001; Arvidsson et al. 2002; Parent et al. 2002). Insulin-like growth factor-1 (IGF-1) is a key diffusible factor mediating this response because anti-IGF-1 antibodies significantly inhibit ischemia-induced proliferation in the SVZ and SGZ in vivo (Yan et al. 2006). Low estradiol levels increase the number of newborn neurons in the dorsal region of the SVZ following stroke injury, and this effect requires the estrogen receptors α and β (Dubal et al. 2006; Suzuki et al. 2007). It is unclear, however, what cells are the direct IGF-1 or estradiol targets. Effects of androgens on normal adult neurogenesis in the dentate gyrus have also been reported, although this is due to increased cell survival (Spritzer and Galea 2007). Moderate estrogen levels stimulate cell proliferation and neurogenesis in the female rat hippocampus, and progesterone antagonizes this effect (Tanapat et al. 1999, 2005). Pregnancy stimulates the proliferation of SVZ NSCs via prolactin (Shingo et al. 2003). Pregnancy and postpartum also influence neurogenesis in the dentate gyrus, although specific effects on NSCs have not been directly examined. Low thyroid hormone levels lead to reduced proliferation of neural precursors in the SVZ (Lemkine et al. 2005). The location of NSCs near blood vessels is conducive to exposure to systemic signals. NSCs are also near axon terminals, and neuronal activity or signals from neuroblasts can influence NSC activity (Riquelme et al. 2008). Nevertheless, dissecting the network of direct and indirect inputs into NSCs and their contributions to neurogenesis will require the manipulation of gene function in specific cell types followed by the analyses of individual steps of neurogenesis.
Pregnancy and lactation in mice influence the hair cycle because estrogens and prolactins inhibit anagen induction in telogen and catagen induction in anagen (Paus et al. 2008), although it is unclear whether stem cells are directly affected. Insulin acts as a major growth factor for human hair follicle by inducing IGF-1. IGF-1 and IGF-2 double knock-out and IGF-1 receptor knock-out mice have epidermal hypoplasia and reduced hair follicle number (Liu et al. 1993), while transgenic mice expressing IGF-1 in the hair follicle have altered follicular proliferation and differentiation and abnormal hair growth cycle (Weger and Schlake 2005). It remains unclear, however, which cells are directly controlled by insulin or IGF-1 or how these signals are integrated with other local factors controlling hair follicle biology.
Other stem cell systems also respond to systemic signals. Follicle-stimulating hormone promotes GDNF expression, which controls GSCs in the testis (Tadokoro et al. 2002). Growth hormone stimulates NSC and mammary stem cell proliferation, and testosterone induces increased satellite cell numbers (Sinha-Hikim et al. 2003). SVZ cell proliferation requires thyroid hormone and its α-receptor (Lemkine et al. 2005). Estrogen controls mammary stem cell proliferation via paracrine signals (Lamarca and Rosen 2008). In many of these examples, it remains to be demonstrated that stem cell numbers or activity are affected (as opposed to those of subsequent progenitors). Nevertheless, they likely represent the “tip of the iceberg” of systemic influences on stem cells.
STEM CELLS RESPOND TO THE SURROUNDING TISSUE
The aforementioned examples show that systemic signals can influence stem cells, but each tissue itself also has the capacity to modulate the activity of its stem cells. As mentioned above, NSCs proliferate at higher rates in response to focal ischemia. Similarly, in mammalian testes depleted of differentiating germ cells, GSC proliferation is vastly increased (Tadokoro et al. 2002). Satellite stem cells are activated in response to injury to generate myoblasts that differentiate into muscle (Luo et al. 2005). Delta is induced within 24 hr of a muscle injury and activates Notch to induce satellite cell proliferation and increased premyoblast numbers. Which specific satellite cell subpopulations require Delta or Notch remains to be determined. Satellite cells respond to exercise-induced physiological stimuli in a similar manner (Adams et al. 2002; Parise et al. 2008).
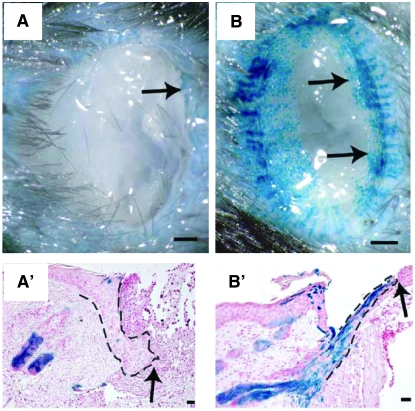
Not only stem cell activity level but also stem cell behavior changes according to the needs of the surrounding tissue. Lineage-tracing studies have shown that hair follicle bulge stem cells do not normally contribute to the epidermis; however, after epidermal injury, bulge stem cells respond rapidly to generate short-lived cells that migrate from the bulge to the epidermis and toward the center of the wound (Figure 2) (Ito et al. 2005). Hair follicles are formed from epidermal cells in the repaired region, and lineage analysis shows that these epidermal cells are not derived from hair follicle stem cells. Wnt signaling regulates this process because inhibiting Wnt abrogates new hair follicle formation, while overexpressing Wnt increases new hair follicle number (Ito et al. 2007). Analogous flexibility of stem cell progeny fate is also apparent in the brain. Normally, SVZ NSCs generate new interneurons that populate the olfactory bulb; however, upon damage to the mouse cerebral cortex, neurogenesis increases and neuroblasts migrate to the site of injury (Magavi et al. 2000; Parent et al. 2002; Goings et al. 2004; Sundholm-Peters et al. 2005). In the Drosophila ovary, each GSC normally divides asymmetrically to self-renew and to form a more differentiated cystoblast; upon GSC loss, however, the remaining GSC can divide symmetrically to generate two GSCs (Xie and Spradling 2000). The plasticity of stem cell behavior hinted at by the findings above is fascinating and holds great therapeutic promise. How is tissue damage sensed by stem cells to modulate cell production? How is the fate of their daughters altered? Do stem cells actively redirect the fate of their progeny? What are the cellular and molecular mechanisms involved? Could we modulate this process for therapeutic purposes? These are some key questions to be addressed.
Figure 2.—
Response of bulge cells to wounding. (A and A′) Bulge-derived cells, labeled in blue, are largely restricted to hair follicles at 2 days after wounding. (B and B′) At 5 days after wounding, blue streaks indicate that bulge-derived cells move centripetally within wounded area. A and B show gross appearance, and A′ and B′ show the histology of the wound. Arrows indicate leading edge of reepithelialization. Dashed lines delineate epidermis. Bars in A and B, 500 μm; in A′ and B′, 25 μm. Images were reproduced from Figure 4, A, B, E, and F, in Ito et al. (2005).
STEM CELLS INFLUENCE THE SURROUNDING TISSUE
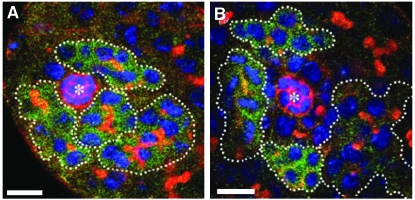
Not only do stem cells respond to changes in the tissue, but also the surrounding tissue may sense stem cells. The absence of GSCs can lead to regeneration of GSCs from dedifferentiation as differentiated cells move into the niche in the Drosophila testis (Figure 3) (Brawley and Matunis 2004). Under certain conditions, dedifferentiation also occurs in females (Kai and Spradling 2004). An empty niche can also affect other cell types within a tissue. Forced differentiation of GSCs in Drosophila females results in gradual changes in the niche surroundings, bringing follicle cell progenitors into the vacant niche. These incoming somatic cells receive BMP signals and divide within the invaded niche (Kai and Spradling 2003). In agametic Drosophila testis, cyst cells, which are normally quiescent, continue to proliferate and adopt the hub cell fate, suggesting that either germ cells restrict cyst cell proliferation and fate or cyst cells become exposed to signals not normally available to them in the absence of germ cells (Gonczy and Dinardo 1996). In mutant mice that lack tail hair follicles (and bulge stem cells), tail wound reepithelialization is delayed, but eventually occurs from the surrounding epidermis (Langton et al. 2008). In mouse testes depleted of germ cells (including GSCs) by busulfan treatment, GDNF expression increases fourfold (Ryu et al. 2006). Finally, somatic stem cells in the Drosophila testis give rise not only to cyst cells, but also to hub cells, showing how a stem cell can directly impact its own niche (Voog et al. 2008).
Figure 3.—
Dedifferentiation of spermatogonial cysts to regenerate GSCs in the Drosophila testis. (A) In stat92EF/stat92E06346 testes at the restrictive temperature for 2 days, Bam-positive cysts surround the hub (anti-armadillo, red, asterisk). (B) After a 2-day recovery at the permissive temperature, Bam-negative GSCs reappear around the hub. Fusomes are also shown in red (1B1), DNA in blue (DAPI), and four- to eight-cell cysts are shown in green (anti-Bam-C). Bars, 10 μm. Images were reproduced from Figure 2, C and D, in Brawley and Matunis (2004).
Are stem cells passive in the communication with the tissue, merely adjusting their activity levels according to tissue demands? Do stem cells influence their tissues strictly by virtue of the progeny that they produce or the signals normally destined for them that become available to other cells upon their absence? Or is it possible that stem cells have a more direct/active role in the tissue where they reside, potentially via secreted or membrane-bound factors? In fact, intestinal stem cells in Drosophila express Delta and, when they divide to generate a stem cell and an enteroblast, Delta is rapidly downregulated in the enteroblast. The intestinal stem cell Delta signal activates Notch in the enteroblast, and stem cell Delta levels determine whether the enteroblast differentiates into an enterocyte (high Delta) or enteroendocrine cell (low Delta) (Ohlstein and Spradling 2007). Transcriptome analyses revealed that bulge stem cells express many secreted or membrane-bound factors, including known signaling molecules such as EphrinB1 and Fgf1 (Morris et al. 2004; Tumbar et al. 2004). In the HSC transcriptome, signaling molecules, including secreted ligands, are conspicuously present (Ivanova et al. 2002). Microarray analysis of Drosophila GSCs show expression of secreted factors (Kai et al. 2005), and Delta has been proposed to function in GSCs (Ward et al. 2006). Moreover, as described below, GSCs negatively modulate life span in C. elegans (Arantes-Oliveira et al. 2002). Thus, perhaps more effort should be put into studying how stem cells influence their surrounding cells, tissues, and organs, not just in terms of cell production for tissue maintenance or repair, but also in terms of active signaling mechanisms that may trigger chain reactions that impact organismal physiology as a whole.
STEM CELL FUNCTION DECLINES WITH AGING
Stem cell number or activity decreases during organismal aging. As discussed below, the cause/consequence relationship between changes in stem cell number or function and aging is unclear. First, however, I will describe the correlation between organismal aging and stem cell aging and review the potential mechanisms involved in stem cell decline. GSCs proliferate more slowly and are gradually lost in aging Drosophila females and males (Jones 2007). The numbers of HSCs, NSCs, or satellite cells do not appear to decline with age, but their activity becomes compromised (Geiger and Van Zant 2002; Conboy et al. 2003; Hattiangady and Shetty 2008). In contrast, impaired melanocyte stem cell self-renewal underlies hair graying (Sharpless and Depinho 2007), while numbers of GSCs are significantly decreased in older mouse testes (Ryu et al. 2006; Zhang et al. 2006). Although the mechanisms involved are poorly understood, it is clear that both intrinsic and extrinsic factors contribute to the changes exhibited by stem cells with aging, as discussed below.
Some evidence suggests that stem cells age intrinsically. Overexpression of superoxide dismutase, an antioxidant enzyme, in Drosophila GSCs extends their life span (Pan et al. 2007). Colonies arising in host testes from GSCs derived from 2-year-old mouse donors with atrophied testes are smaller than those of younger mice, suggesting intrinsic aging of GSCs (Zhang et al. 2006). Another study, however, showed no age-dependent difference in colony size (Ryu et al. 2006). Depending on the strain of mice, HSCs from old individuals are at a significant disadvantage relative to young HSCs in competitive repopulation or serial transplantation assays (Geiger and Van Zant 2002). Several mechanisms have been proposed to explain the intrinsic aging of stem cells, including exhaustion of their proliferative potential, telomere shortening, accumulation of DNA damage, or epigenetic alterations (Rando 2006).
Aging of the niche has also been proposed to contribute to the decline in stem cell function with age. In Drosophila ovaries, the number of cap cells decreases and BMP signaling from the niche to GSCs is impaired with age, while the E-cadherin-mediated association between GSCs and cap cells weakens. Increasing niche expression of BMP signals, strengthening the GSC–niche association, or expressing superoxide dismutase in the niche can prolong GSC life span and increase their proliferation rates (Pan et al. 2007). In older Drosophila testes, hub cells display lower levels of E-cadherin and of Upd, whereas Upd overexpression in the niche maintains GSCs in aging males (Boyle et al. 2007). Similarly, in aging mouse testes, expression of GDNF in Sertoli cells is markedly reduced (Ryu et al. 2006) and GSCs can be consecutively transplanted to the testes of young males for extended periods of time (Ogawa et al. 2003; Ryu et al. 2006), underscoring a major role of aging of the niche in GSC function decline. Old HSCs are also able to reconstitute blood in serial-transplantation experiments, although their effectiveness varies with the mouse strain (Geiger and Van Zant 2002; Warren and Rossi 2008). Finally, in the SGZ, there is a correlation between decreased neurogenesis and a decline in vascular niches (Hattiangady and Shetty 2008), although causality has not been tested.
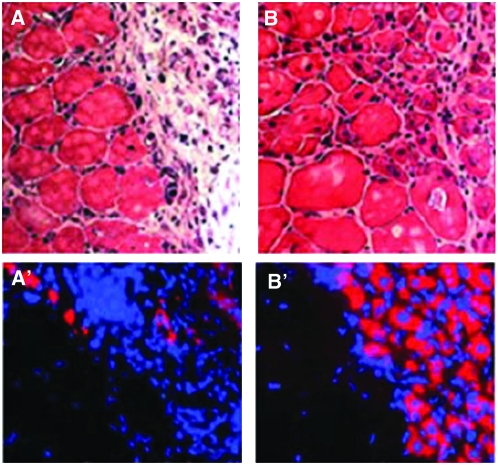
Given the remarkable ability of stem cells to sense and respond to external stimuli, it is not surprising that the physiological changes that result from aging can also impact stem cells and their niches. As discussed above, insulin-like peptides control cap cell number via Notch signaling in the niche, and they also control how effectively cap cells associate with GSCs in Drosophila (H. J. Hsu and D. Drummond-Barbosa, unpublished results). As females age, systemic insulin-signaling levels decline (H. J. Hsu and D. Drummond-Barbosa, unpublished results). Remarkably, excess insulin-like peptides suppress the normal process of GSC loss that occurs with age, while a poor diet enhances GSC loss, demonstrating how changes in systemic signals can potentially impact the GSC niche during the aging process (H. J. Hsu and D. Drummond-Barbosa, unpublished results). Several reports suggest that insulin secretion or sensitivity decline in aging humans (Shimizu et al. 1996; Chang et al. 2006a,b; Szoke et al. 2008); it is possible that this also impacts certain stem cell populations. In old mice, muscle regeneration is impaired due to insufficient levels of Notch signaling in satellite cells, and forced activation of Notch is sufficient to restore efficient regeneration of older muscle (Conboy et al. 2003). Intriguingly, exposure of old muscle to the systemic environment of a young mouse in heterochronic parabiosis is sufficient to restore Notch signaling and efficient satellite cell activation in older muscle (Figure 4) (Conboy et al. 2005), suggesting potential parallels with the Drosophila ovary.
Figure 4.—
Exposure to a young systemic environment restores muscle stem cell activation and regeneration in aged mice. (A and A′) In isochronic parabiosis between two aged mice, muscles do not regenerate well after hind-limb injury, showing prominent fibrosis 5 days later. (B and B′) In contrast, heterochronic parabiosis between young and aged mice significantly enhances the regeneration of muscle in the old partner. Nascent myotubes can be recognized because they maintain centrally located nuclei and express embryonic myosin heavy chain (eMHC). A and B show hematoxylin and eosin staining. A′ and B′ show eMHC (red) and Hoechst dye (blue, nuclei). Images were reproduced from part of Figure 1A in Conboy et al. (2005).
GERMLINE STEM CELLS, INSULIN SIGNALING, AND LONGEVITY ARE CONNECTED
As reviewed above, changes in intrinsic factors, the niche microenvironment, or the systemic environment can lead to an aged phenotype of stem cells. Conversely, changes in stem cell function may also have an impact on the physiological status of an individual, possibly creating positive or negative feedback loops during aging. The best-known example is the reported negative effect that GSCs exert on longevity in C. elegans.
Ablation of the germline leads to life-span extension in C. elegans, and this effect requires DAF-16/FOXO, a transcriptional factor that acts as a negative downstream effector of the insulin pathway, and a nuclear hormone receptor, DAF-12 (Guarente and Kenyon 2000). Although lack of germline due to mutations in the early acting genes tudor, germ cell-less, or oskar does not extend life span in Drosophila (Barnes et al. 2006), another study suggests that the effect of the germline on longevity may be evolutionarily conserved (Flatt et al. 2008). Loss of germ cells due to forced bam expression leads to life-span extension and also to alterations in systemic insulin signaling, suggesting active communication between the germline and other tissues (Flatt et al. 2008).
Caloric restriction also leads to increased longevity in many organisms, possibly due in part to reduced insulin/IGF signaling (Guarente and Kenyon 2000). Mutation of the daf-2/insulin receptor gene or of other insulin pathway components doubles the life span of C. elegans hermaphrodites (Guarente and Kenyon 2000). In Drosophila, mutation of dinr/insulin receptor or chico/insulin receptor substrate or ablation of insulin-producing cells also extends life span (Giannakou and Partridge 2007). Recent evidence suggests that this is also the case in mammals (Bartke 2000; Baba et al. 2005; Taguchi et al. 2007). A prospective follow-up study in humans found that, in women, reduced insulin signaling appears to correlate with lower body height and improved old age survival (van Heemst et al. 2005).
Reproduction is also tightly linked to diet and physiological status (Gong 2002; Loucks 2007; Pinelli and Tagliabue 2007). Despite this apparent intersection, however, several lines of evidence suggest that the extension of life span in insulin pathway mutants is not simply due to effects on reproduction. Ablation of somatic gonad precursors along with the germline does not increase life span in C. elegans (Guarente and Kenyon 2000). There is also a temporal separation of requirements of daf-2/insulin receptor for reproduction and longevity. On the basis of RNA interference (RNAi) treatment, reduction in insulin signaling during adulthood is required and sufficient for extending life span. In contrast, initiating daf-2 RNAi treatment in hatchlings, but not in adults, impairs reproduction (Dillin et al. 2002). One caveat of these experiments, however, is that the effectiveness of the RNAi treatment in the germline (or whatever tissue requires daf-2 for reproduction) may be lower in adults relative to young larvae; therefore, the possibility that daf-2 functions in adults to control reproduction cannot be ruled out. Nevertheless, these results clearly show that it is possible to create conditions that separate extension of life span from impairment of reproductive function. In Drosophila, effects of dFOXO overexpression on longevity could also be uncoupled from reproduction (Hwangbo et al. 2004; Giannakou et al. 2007). Honeybee queens are long lived and fertile, whereas workers are short lived and sterile (Corona et al. 2007). Not only can longevity and reproduction be uncoupled, but also the effect of diet on longevity does not stem solely from what is ingested. In Drosophila, yeast odorants can reverse the life extension afforded by a restricted diet, whereas impairment of olfaction due to the Orb83 mutation results in life-span extension in the absence of dietary restriction (Libert et al. 2007).
Intriguingly, GSCs are responsible for the effects of the germline on longevity in C. elegans because genetically blocking the production of differentiated germ cells does not extend life span, whereas forcing GSCs to differentiate does, and GSC overproliferation shortens life span (Arantes-Oliveira et al. 2002). These findings suggest that GSCs communicate with other cells/tissues to control aging. In fact, a screen for genes required for the extended longevity of animals lacking a germline resulted in the identification of kri-1, which controls DAF-16 nuclear localization in the intestine likely in response to a lipophilic hormone (Berman and Kenyon 2006). Thus, GSCs may control longevity independently of their role in gamete production per se. Moreover, part of the effect of diet and insulin signaling on longevity may occur via the modulation of GSCs/germ cells, which in turn may produce secreted/humoral factors. As described above, insulin signaling controls Drosophila female GSCs, and the GSC loss and decreased activity observed with aging may at least partially be explained by reduced insulin signaling (Lafever and Drummond-Barbosa 2005; H. J. Hsu and D. Drummond-Barbosa, unpublished results). Although this has not been addressed experimentally, it is conceivable that part of the effects of reduced insulin signaling in extending life span in Drosophila result from lower GSC numbers, and that the natural decrease in insulin signaling over time represents a feedback mechanism to prolong life span once the peak period of reproduction is past. To understand the potential role of GSCs on life-span control will require that many questions be addressed. Are the germline effects on life span specific to GSCs in Drosophila? Is a complete absence of GSCs necessary for an impact on longevity or would less drastic decreases in GSC numbers be sufficient for an effect? How well conserved is the role of the germline or GSCs across species, including humans (in which males, but not females, have GSCs)? How are the effects of GSCs integrated with those of other factors controlling longevity?
HOW DO SOMATIC STEM CELLS IMPACT LONGEVITY?
Declines in stem cell function and/or number are clearly associated with aging, but causal relationships are largely unknown. In other words, does aging lead to stem cell decline, or does stem cell decline lead to aging? A less often asked question is, would stem cell decline always promote aging, as our intuitions suggest, or could stem cell decline in some instances counteract the aging process? As discussed above, GSCs in C. elegans, and possibly in Drosophila, exert a negative effect on longevity. However, unlike many other stem cell types, GSCs are required for reproduction, a process not essential for survival of a particular individual. An important question, therefore, is whether the negative effects of GSCs on longevity are a peculiarity of the germline, or whether they could reflect a function shared by some other stem cell types.
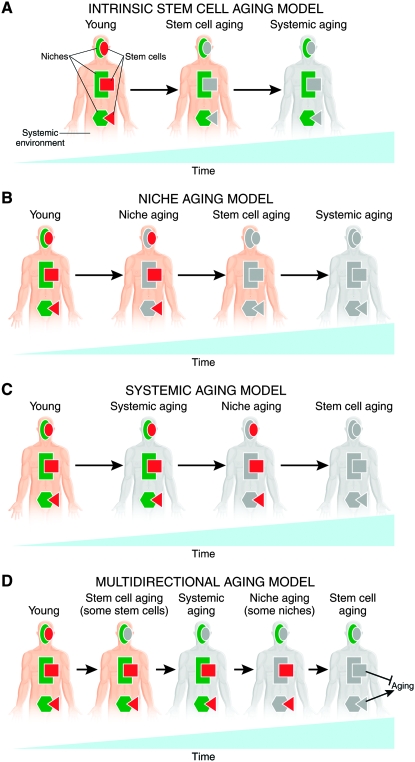
One possibility is that, with the exception of GSCs, decreases in stem cell function lead to reduced longevity. Intrinsic aging of stem cells (Figure 5A), their niches (Figure 5B), or the systemic environment (Figure 5C) would result in decreased stem cell function, impaired tissue function, and further systemic aging. For example, HSC activity is required for blood cell production, and blood cells have immune functions, carry oxygen to tissues, and enable blood clotting; declines in any of these functions would be expected to decrease longevity (Geiger and Van Zant 2002). Another possibility, albeit counterintuitive, is that small decreases in stem cell function may actually promote longevity. Excessive stem cell activity may result in uncontrolled proliferation as tumors, and this would clearly tend to shorten life span. For example, ectopic Wnt signaling induced by overexpression of the Wnt1 ligand or of an activated form of β-catenin, which is highly oncogenic, increases progenitor cell numbers and activity in mouse mammary epithelium (Liu et al. 2004). A variant of this general possibility is that factors that increase stem cell function may have a deleterious effect on longevity. Ames Dwarf mice are long lived and have low levels of circulating growth hormone and IGF-1 (Sun et al. 2005); however, they have a local increase in IGF-1 in the hippocampus and an increase in neurogenesis, suggesting that there could be mechanisms to circumvent this potential problem. But could stem cells perhaps have more subtle or indirect negative effects on longevity? For example, could maintenance of young-equivalent levels of stem cell function in older individuals not only increase the risk of cancer, as has been proposed (Sharpless and Depinho 2007), but also overtax an older physiological environment to sustain a high rate of cell production and elevated tissue performance in multiple tissues? In that case, the decrease in stem cell function in response to an older physiological environment would actually serve as a negative feedback mechanism and slow down the aging of the organism. Alternatively, whether or not the effect of decreased stem cell function would positively or negatively impact longevity may vary with the stem cell type (Figure 5D). Further research will be required to determine the causal relationships between physiological changes and stem cell function during aging and to determine what the impact of those changes on organismal longevity is for different types of stem cells. As discussed above, multiple mechanisms contribute to stem cell aging. Perhaps because different stem cell types have distinct specific requirements for their maintenance and function, they also appear to have different susceptibility to intrinsic or extrinsic aging. It is therefore very likely that not all stem cells age simultaneously or in a similar fashion—some may simply respond to an aged environment, others may intrinsically age and thereby potentially contribute to the age-induced physiological changes, or a combination of these factors (Figure 5D). Given the complexity of the network of interactions (Rando 2006), the large number of stem cell types supporting tissues with diverse functions, very fine, tissue-specific genetic manipulation will be required to address whether or not the more global physiological changes that stem cells respond to during aging (stem cell extrinsic aging) originate from intrinsic changes in subsets of stem cells (intrinsic stem cell aging).
Figure 5.—
Models of how stem cell function affects and is affected during aging. (A) Intrinsic stem cell aging may be a predominant factor leading to aging of the organism. (B) Aging of the niches may significantly contribute to aging by affecting stem cell activity. (C) Aging of the systemic environment may drive aging of stem cells. (D) Complex interactions are likely to drive aging. It is possible that different stem cells age at different rates and via different mechanisms and that the impact that their aging has on the aging of the organism as a whole varies according to their specific function. Different shapes represent distinct types of stem cells and niches. Gray color represents aging of stem cells, niches, and the systemic environment. See text for details.
CLOSING THOUGHTS
Stem cells participate in a web of multidirectional interactions with their niches and tissues of residence, the systemic environment, and external factors. Stem cells not only respond to multiple stimuli, but also have an impact on the organism; it is therefore important to consider each stem cell interaction in both directions. By producing cells, stem cells influence tissue activity and function, which in turn determines both the level of demand for resources and the generation of beneficial cells or factors. But stem cells may also be active beyond their role in the production of cells, perhaps via the secretion of factors. A detailed cellular and molecular map of all the interactions for each type of stem cell, combined with tools for manipulating particular interactions and measuring aging parameters, will help reveal the relationships between various stem cell types and the aging process. Are stem cells major determinants of aging because of their role in maintaining tissue function? If this is the case, organisms age as a consequence of stem cell function decline resulting from changes in intrinsic factors or in the niche. Or is maintenance of high levels of stem cell function over time deleterious because of elevated cancer risk or an increased demand for resources imposed by excessive tissue activity? In this case, reductions in stem cell activity and/or numbers, at least in some tissues (such as the germline and, perhaps, additional tissues), could prolong life. Or is stem cell function not a major determinant of aging, but some of the changes involved in aging also happen to affect niche/stem cell activity, showing a simple correlation instead of a causal relationship? Or, as seems more logical, is there a combination of all possibilities above, with the particular role of stem cells in aging varying according to the particular stem cell type or the function of the tissue they support?
Acknowledgments
I am grateful for anonymous reviewer comments. This work was supported by National Institutes of Health grant no. GM 069875 and by American Cancer Society grant no. RSG-07-182-01-DDC.
References
- Adams, G. R., V. J. Caiozzo, F. Haddad and K. M. Baldwin, 2002. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 283 C1182–C1195. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira, N., J. Apfeld, A. Dillin and C. Kenyon, 2002. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295 502–505. [DOI] [PubMed] [Google Scholar]
- Arvidsson, A., T. Collin, D. Kirik, Z. Kokaia and O. Lindvall, 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8 963–970. [DOI] [PubMed] [Google Scholar]
- Baba, T., T. Shimizu, Y. Suzuki, M. Ogawara, K. Isono et al., 2005. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J. Biol. Chem. 280 16417–16426. [DOI] [PubMed] [Google Scholar]
- Barnes, A. I., J. M. Boone, J. Jacobson, L. Partridge and T. Chapman, 2006. No extension of lifespan by ablation of germ line in Drosophila. Proc. Biol. Sci. 273 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke, A., 2000. Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size. Results Probl. Cell Differ. 29 181–202. [DOI] [PubMed] [Google Scholar]
- Berman, J. R., and C. Kenyon, 2006. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124 1055–1068. [DOI] [PubMed] [Google Scholar]
- Boyle, M., C. Wong, M. Rocha and D. L. Jones, 2007. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1 470–478. [DOI] [PubMed] [Google Scholar]
- Brawley, C., and E. Matunis, 2004. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304 1331–1334. [DOI] [PubMed] [Google Scholar]
- Chang, A. M., M. J. Smith, C. J. Bloem, A. T. Galecki, J. B. Halter et al., 2006. a Limitation of the homeostasis model assessment to predict insulin resistance and beta-cell dysfunction in older people. J. Clin. Endocrinol. Metab. 91 629–634. [DOI] [PubMed] [Google Scholar]
- Chang, A. M., M. J. Smith, A. T. Galecki, C. J. Bloem and J. B. Halter, 2006. b Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistance. J. Clin. Endocrinol. Metab. 91 3303–3309. [DOI] [PubMed] [Google Scholar]
- Conboy, I. M., M. J. Conboy, G. M. Smythe and T. A. Rando, 2003. Notch-mediated restoration of regenerative potential to aged muscle. Science 302 1575–1577. [DOI] [PubMed] [Google Scholar]
- Conboy, I. M., M. J. Conboy, A. J. Wagers, E. R. Girma, I. L. Weissman et al., 2005. a Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433 760–764. [DOI] [PubMed] [Google Scholar]
- Corona, M., R. A. Velarde, S. Remolina, A. Moran-Lauter, Y. Wang et al., 2007. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 104 7128–7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto, E., and A. C. Spradling, 2005. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9 501–510. [DOI] [PubMed] [Google Scholar]
- Dillin, A., D. K. Crawford and C. Kenyon, 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298 830–834. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa, D., 2005. Regulation of stem cell populations, pp. 67–98 in Encyclopedia of Molecular Cell Biology and Molecular Medicine, edited by R. A. Meyers. Wiley-VCH, Weinheim, Germany.
- Drummond-Barbosa, D., and A. C. Spradling, 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231 265–278. [DOI] [PubMed] [Google Scholar]
- Dubal, D. B., S. W. Rau, P. J. Shughrue, H. Zhu, J. Yu et al., 2006. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology 147 3076–3084. [DOI] [PubMed] [Google Scholar]
- Flatt, T., K. J. Min, C. D'Alterio, E. Villa-Cuesta, J. Cumbers et al., 2008. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. USA 105 6368–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, H., and G. Van Zant, 2002. The aging of lympho-hematopoietic stem cells. Nat. Immunol. 3 329–333. [DOI] [PubMed] [Google Scholar]
- Giannakou, M. E., and L. Partridge, 2007. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 32 180–188. [DOI] [PubMed] [Google Scholar]
- Giannakou, M. E., M. Goss, J. Jacobson, G. Vinti, S. J. Leevers et al., 2007. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6 429–438. [DOI] [PubMed] [Google Scholar]
- Goings, G. E., V. Sahni and F. G. Szele, 2004. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996 213–226. [DOI] [PubMed] [Google Scholar]
- Gonczy, P., and S. DiNardo, 1996. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122 2437–2447. [DOI] [PubMed] [Google Scholar]
- Gong, J. G., 2002. Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: practical implications. Domest. Anim. Endocrinol. 23 229–241. [DOI] [PubMed] [Google Scholar]
- Guarente, L., and C. Kenyon, 2000. Genetic pathways that regulate ageing in model organisms. Nature 408 255–262. [DOI] [PubMed] [Google Scholar]
- Hattiangady, B., and A. K. Shetty, 2008. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging 29 129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo, D. S., B. Gershman, M. P. Tu, M. Palmer and M. Tatar, 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429 562–566. [DOI] [PubMed] [Google Scholar]
- Ito, M., Y. Liu, Z. Yang, J. Nguyen, F. Liang et al., 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11 1351–1354. [DOI] [PubMed] [Google Scholar]
- Ito, M., Z. Yang, T. Andl, C. Cui, N. Kim et al., 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447 316–320. [DOI] [PubMed] [Google Scholar]
- Ivanova, N. B., J. T. Dimos, C. Schaniel, J. A. Hackney, K. A. Moore et al., 2002. A stem cell molecular signature. Science 298 601–604. [DOI] [PubMed] [Google Scholar]
- Jin, K., M. Minami, J. Q. Lan, X. O. Mao, S. Batteur et al., 2001. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 98 4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. L., 2007. Aging and the germ line: where mortality and immortality meet. Stem Cell Rev. 3 192–200. [DOI] [PubMed] [Google Scholar]
- Kai, T., and A. Spradling, 2003. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl Acad. Sci. USA 100 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, T., and A. Spradling, 2004. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428 564–569. [DOI] [PubMed] [Google Scholar]
- Kai, T., D. Williams and A. C. Spradling, 2005. The expression profile of purified Drosophila germline stem cells. Dev. Biol. 283 486–502. [DOI] [PubMed] [Google Scholar]
- LaFever, L., and D. Drummond-Barbosa, 2005. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309 1071–1073. [DOI] [PubMed] [Google Scholar]
- Lamarca, H. L., and J. M. Rosen, 2008. Hormones and mammary cell fate: What will I become when I grow up? Endocrinology 149 4317–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton, A. K., S. E. Herrick and D. J. Headon, 2008. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J. Invest. Dermatol. 128 1311–1318. [DOI] [PubMed] [Google Scholar]
- Leatherman, J. L., and S. Dinardo, 2008. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkine, G. F., A. Raj, G. Alfama, N. Turque, Z. Hassani et al., 2005. Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 19 863–865. [DOI] [PubMed] [Google Scholar]
- Libert, S., J. Zwiener, X. Chu, W. Vanvoorhies, G. Roman et al., 2007. Regulation of Drosophila life span by olfaction and food-derived odors. Science 315 1133–1137. [DOI] [PubMed] [Google Scholar]
- Liu, B. Y., S. P. McDermott, S. S. Khwaja and C. M. Alexander, 2004. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl. Acad. Sci. USA 101 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson and A. Efstratiadis, 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75 59–72. [PubMed] [Google Scholar]
- Lopez-Onieva, L., A. Fernandez-Minan and A. Gonzalez-Reyes, 2008. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development 135 533–540. [DOI] [PubMed] [Google Scholar]
- Loucks, A. B., 2007. Energy availability and infertility. Curr. Opin. Endocrinol. Diabetes Obes. 14 470–474. [DOI] [PubMed] [Google Scholar]
- Luo, D., V. M. Renault and T. A. Rando, 2005. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 16 612–622. [DOI] [PubMed] [Google Scholar]
- Magavi, S. S., B. R. Leavitt and J. D. Macklis, 2000. Induction of neurogenesis in the neocortex of adult mice. Nature 405 951–955. [DOI] [PubMed] [Google Scholar]
- Merkle, F. T., Z. Mirzadeh and A. Alvarez-Buylla, 2007. Mosaic organization of neural stem cells in the adult brain. Science 317 381–384. [DOI] [PubMed] [Google Scholar]
- Mirzadeh, Z., F. T. Merkle, M. Soriano-Navarro, J. M. Garcia-Verdugo and A. Alvarez-Buylla, 2008. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R. J., Y. Liu, L. Marles, Z. Yang, C. Trempus et al., 2004. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22 411–417. [DOI] [PubMed] [Google Scholar]
- Morrison, S. J., and A. C. Spradling, 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, E. K., S. A. Jordan, H. Oshima, H. Yoshida, M. Osawa et al., 2002. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416 854–860. [DOI] [PubMed] [Google Scholar]
- Ogawa, T., M. Ohmura, Y. Yumura, H. Sawada and Y. Kubota, 2003. Expansion of murine spermatogonial stem cells through serial transplantation. Biol. Reprod. 68 316–322. [DOI] [PubMed] [Google Scholar]
- Ohlstein, B., and A. Spradling, 2007. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315 988–992. [DOI] [PubMed] [Google Scholar]
- Pan, L., S. Chen, C. Weng, G. Call, D. Zhu et al., 2007. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1 458–469. [DOI] [PubMed] [Google Scholar]
- Parent, J. M., Z. S. Vexler, C. Gong, N. Derugin and D. M. Ferriero, 2002. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 52 802–813. [DOI] [PubMed] [Google Scholar]
- Parise, G., I. W. McKinnell and M. A. Rudnicki, 2008. Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve 37 611–619. [DOI] [PubMed] [Google Scholar]
- Paus, R., P. Arck and S. Tiede, 2008. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol. Cell. Endocrinol. 288 38–51. [DOI] [PubMed] [Google Scholar]
- Pinelli, G., and A. Tagliabue, 2007. Nutrition and fertility. Minerva Gastroenterol. Dietol. 53 375–382. [PubMed] [Google Scholar]
- Pinkston, J. M., D. Garigan, M. Hansen and C. Kenyon, 2006. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313 971–975. [DOI] [PubMed] [Google Scholar]
- Rando, T. A., 2006. Stem cells, ageing and the quest for immortality. Nature 441 1080–1086. [DOI] [PubMed] [Google Scholar]
- Riquelme, P. A., E. Drapeau and F. Doetsch, 2008. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, B. Y., K. E. Orwig, J. M. Oatley, M. R. Avarbock and R. L. Brinster, 2006. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, R., 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4 7–25. [PubMed] [Google Scholar]
- Sharpless, N. E., and R. A. DePinho, 2007. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8 703–713. [DOI] [PubMed] [Google Scholar]
- Shimizu, M., S. Kawazu, S. Tomono, T. Ohno, T. Utsugi et al., 1996. Age-related alteration of pancreatic beta-cell function. Increased proinsulin and proinsulin-to-insulin molar ratio in elderly, but not in obese, subjects without glucose intolerance. Diabetes Care 19 8–11. [DOI] [PubMed] [Google Scholar]
- Shingo, T., C. Gregg, E. Enwere, H. Fujikawa, R. Hassam et al., 2003. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299 117–120. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim, I., S. M. Roth, M. I. Lee and S. Bhasin, 2003. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am. J. Physiol. Endocrinol. Metab. 285 E197–E205. [DOI] [PubMed] [Google Scholar]
- Song, X., G. B. Call, D. Kirilly and T. Xie, 2007. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134 1071–1080. [DOI] [PubMed] [Google Scholar]
- Spritzer, M. D., and L. A. Galea, 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol. 67 1321–1333. [DOI] [PubMed] [Google Scholar]
- Sun, L. Y., M. S. Evans, J. Hsieh, J. Panici and A. Bartke, 2005. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinology 146 1138–1144. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters, N. L., H. K. Yang, G. E. Goings, A. S. Walker and F. G. Szele, 2005. Subventricular zone neuroblasts emigrate toward cortical lesions. J. Neuropathol. Exp. Neurol. 64 1089–1100. [DOI] [PubMed] [Google Scholar]
- Suzuki, S., L. M. Gerhold, M. Bottner, S. W. Rau, C. Dela Cruz et al., 2007. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 500 1064–1075. [DOI] [PubMed] [Google Scholar]
- Szoke, E., M. Z. Shrayyef, S. Messing, H. J. Woerle, T. W. van Haeften et al., 2008. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care 31 539–543. [DOI] [PubMed] [Google Scholar]
- Tadokoro, Y., K. Yomogida, H. Ohta, A. Tohda and Y. Nishimune, 2002. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 113 29–39. [DOI] [PubMed] [Google Scholar]
- Taguchi, A., L. M. Wartschow and M. F. White, 2007. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317 369–372. [DOI] [PubMed] [Google Scholar]
- Tanapat, P., N. B. Hastings, A. J. Reeves and E. Gould, 1999. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19 5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat, P., N. B. Hastings and E. Gould, 2005. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 481 252–265. [DOI] [PubMed] [Google Scholar]
- Tumbar, T., G. Guasch, V. Greco, C. Blanpain, W. E. Lowry et al., 2004. Defining the epithelial stem cell niche in skin. Science 303 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst, D., M. Beekman, S. P. Mooijaart, B. T. Heijmans, B. W. Brandt et al., 2005. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell 4 79–85. [DOI] [PubMed] [Google Scholar]
- Voog, J., C. D'Alterio and D. L. Jones, 2008. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Z. Li and Y. Cai, 2008. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J. Cell Biol. 180 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E. J., H. R. Shcherbata, S. H. Reynolds, K. A. Fischer, S. D. Hatfield et al., 2006. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr. Biol. 16 2352–2358. [DOI] [PubMed] [Google Scholar]
- Warren, L. A., and D. J. Rossi, 2008. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. (in press). [DOI] [PMC free article] [PubMed]
- Weger, N., and T. Schlake, 2005. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol 125 873–882. [DOI] [PubMed] [Google Scholar]
- Wong, M. D., Z. Jin and T. Xie, 2005. Molecular mechanisms of germline stem cell regulation. Annu Rev Genet 39 173–195. [DOI] [PubMed] [Google Scholar]
- Xie, T., and A. C. Spradling, 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94 251–260. [DOI] [PubMed] [Google Scholar]
- Xie, T., and A. C. Spradling, 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290 328–330. [DOI] [PubMed] [Google Scholar]
- Yan, Y. P., K. A. Sailor, R. Vemuganti and R. J. Dempsey, 2006. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur. J. Neurosci. 24 45–54. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., M. Sukeno and Y. Nabeshima, 2007. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317 1722–1726. [DOI] [PubMed] [Google Scholar]
- Zhang, R. L., Z. G. Zhang, L. Zhang and M. Chopp, 2001. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105 33–41. [DOI] [PubMed] [Google Scholar]
- Zhang, X., K. T. Ebata, B. Robaire and M. C. Nagano, 2006. Aging of male germ line stem cells in mice. Biol. Reprod. 74 119–124. [DOI] [PubMed] [Google Scholar]