Abstract
The conserved histone variant H2A.Z fulfills many functions by being an integral part of the nucleosomes placed at specific regions of the genome. Telomeres cap natural ends of chromosomes to prevent their recognition as double-strand breaks. At yeast telomeres, H2A.Z prevents the spreading of silent chromatin into proximal euchromatin. A role for H2A.Z in capping, however, has not been reported in any organism. Here, I uncover such a role for Drosophila H2A.Z. Loss of H2A.Z, through mutations in either its gene or the domino gene for the Swr1 chromatin-remodeling protein, suppressed the fusion of telomeres that lacked the protection of checkpoint proteins: ATM, ATR, and the Mre11–Rad50–NBS complex. Loss of H2A.Z partially restores the loading of the HOAP capping protein, possibly accounting for the partial restoration in capping. I propose that, in the absence of H2A.Z, checkpoint-defective telomeres adopt alternative structures, which are permissive for the loading of the capping machinery at Drosophila telomeres.
THE telomere serves as a structure to cap chromosome ends so that they are not recognized as DNA double-strand breaks (DSBs). Uncapped telomeres, like DSBs, can be subject to promiscuous DNA repair leading to genome instability (reviewed in Longhese 2008). In most eukaryotic organisms studied, telomeres consist of an array of short repeats elongated by telomerase (reviewed in McEachern et al. 2000). In Drosophila melanogaster, chromosome ends are elongated by three classes of non-LTR retrotransposons (reviewed in Mason et al. 2008; Pardue and Debaryshe 2008). Although Drosophila cells use a non-telomerase-based mechanism for telomere elongation, many of the Drosophila proteins with functions in telomere capping are highly conserved. These include the ATM checkpoint kinase and the Mre11–Rad50–NBS (MRN) complex, well-characterized proteins that also participate in the response to DNA damage. In Drosophila cells that lack any of these proteins, telomeres are subject to fusion (reviewed in Rong 2008). These telomeres are hereby referred to as checkpoint-defective telomeres, as they lack the protection of checkpoint proteins. We also showed that ATM and its related ATR kinase control partially redundant pathways for telomere maintenance, a pathway relationship identical to that observed in yeast (Bi et al. 2005a). One of the mechanisms through which ATM and ATR regulate telomere maintenance is by facilitating the loading of capping proteins. The HP1/Orc-associated protein (HOAP) serves to cap Drosophila telomeres so that loss of HOAP in the caravaggio (cav) mutants leads to telomere fusions with the highest frequency among uncapping mutations identified so far (Cenci et al. 2003). HOAP is highly enriched at all wild-type telomeres, but is absent from telomeres in atm atr double mutants (Bi et al. 2005a). ATM and ATR might protect telomere integrity by safeguarding chromatin architecture that favors the loading of HOAP and other telomere-elongating, capping, and silencing proteins.
Mammalian dysfunctional telomeres elicit a DNA damage response, leading to the phosphorylation of the histone variant H2A.X (γ-H2A.X; d'Adda di Fagagna et al. 2003). In yeast, telomeres may be transiently detected as DSBs as evidenced by the presence of γ-H2A.X at telomeres in the absence of DNA damage (Kim et al. 2007). ATM and ATR are responsible for modifying H2A.X (Shroff et al. 2004; Kim et al. 2007). The H2A.X function in Drosophila is encoded by the H2AvD variant. The extreme C terminus of H2AvD contains an SQ signature motif, which is phosphorylated upon DNA damage similar to H2A.X in other organisms (Madigan et al. 2002), and the modification entirely depends on ATM and ATR (M. Gong and Y. S. Rong, unpublished data).
Interestingly, the single H2AvD polypeptide appears to also encode the H2A.Z variant, as most of the protein is highly homologous to H2A.Z in other organisms (Madigan et al. 2002). H2A.Z is involved in transcriptional regulation on a genomewide level (reviewed in Guillemette and Gaudreau 2006). In Saccharomyces cerevisiae, the Swr1 chromatin-remodeling complex is responsible for H2A.Z deposition onto DNA (Mizuguchi et al. 2004). In Drosophila, Swr1 is encoded by the domino gene (Ruhf et al. 2001). At yeast telomeric regions, H2A.Z is important for maintaining a transcriptionally silenced state (Meneghini et al. 2003). However, a role of H2A.Z in regulating telomere capping has not been reported in any organism. In this study, I demonstrate such a role for Drosophila H2A.Z, specifically in regulating the capping of telomeres that are not protected by checkpoint proteins.
MATERIALS AND METHODS
Drosophila mutations and genetics:
The atmstg and mre11Δ35K1 mutations have been described in Gong et al. (2005); nbs1 in Bi et al. (2005a); cav1 in Cenci et al. (2003); mei-4129D and mei-41RT1 (Drosophila atr mutations) in Laurençon et al. (2003); sir22A-7-11 in Xie and Golic (2004); and lig411 in Wei and Rong (2007). The domk08108 mutation is described at FlyBase.net and was obtained from the Bloomington Stock Center (Bloomington, IN). The h2AvD810 mutation and h2AvD wild-type (h2AvD+) and C-terminally truncated (h2AvDΔCT) rescue constructs and corresponding P-element insertions on the X chromosome have been described in Clarkson et al. (1999) and were obtained from Robert Glaser (Wadsworth Center, Albany, NY). The h2AvDEY04892 (h2AvDEY) is described at FlyBase.net and was obtained from the Bloomington Stock Center. It caused lethality during third instar development either as homozygote or as trans-heterozygote over h2AvD810. Lethal mutations on a chromosome 3 were balanced over the TM6B chromosome with the Tubby (Tb) dominant larval marker. Lethal combinations on chromosomes 2 and 3 were balanced over the TSTL chromosome with Tb. Triple mutants of atm, atr, and h2AvD were recovered as Tb+ males with the aid of an attached compound X chromosome C(1)M3. A third chromosome h2avD-gfp transgene was described in Clarkson and Saint (1999) and obtained from Kami Ahmad at Harvard. An h2avD-gfp h2AvD810 double combination, an h2avD-gfp atmstg h2AvD810 triple combination, and a mei-4129D; h2avD-gfp atmstg h2AvD810 quadruple combination were constructed for H2AvD localization by anti-GFP staining. The presence of the 311-bp deletion in h2AvD810 was verified by PCR using primers h2avd-5031d (5′-agagtcacgttcgtaagcag) and h2avd-5698u (5′-cttgagatgacgatggatgc). The wild-type gene gave rise to a 660-bp product. The mutant produced a 350-bp product.
Cytology:
Mitotic chromosome spreads were performed as described (Gatti et al. 1994). To measure radiation-induced DSBs, third instar larvae were irradiated with 210 rad and processed for mitotic spread after 1 hr. HOAP and H2AvD localization experiments were performed as described (Cenci et al. 2003) with a rabbit anti-HOAP from Rebecca Kellum (Shareef et al. 2001) and a rabbit anti-GFP from Invitrogen. Both atm atr h2AvD triple-mutant and atm h2AvD double-mutant brains were processed on the same slides to control for staining variability.
RESULTS
Mutations in H2AvD suppress fusion of checkpoint-defective telomeres:
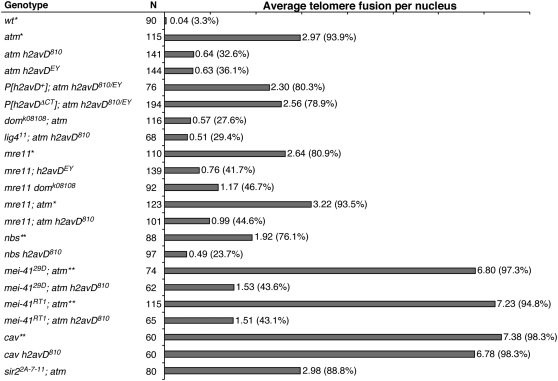
On average, three pairs of telomeres engage in fusion in an atm mutant nucleus (Bi et al. 2004). Remarkably, the null h2AvD810 mutation reduced this frequency to about one in every two nuclei (Figure 1; for fusion classifications, see Figure 4), while alone it did not cause telomere fusion (0 in 52 nuclei). In addition, the percentage of nuclei with at least one fusion was reduced from 94% in the atm single mutant to 33% in the atm h2AvD810 double mutant. To rule out a possible effect of second-site mutations linked to h2AvD810, I confirmed these results using a different allele (h2AvDEY, Figure 1). In addition, a wild-type h2AvD transgene carried in a P element restored fusion frequencies in the double mutant to a level similar to the one in atm single mutants. These results establish that loss of H2AvD suppresses telomere fusion in atm mutants. The residual level of telomere fusion in atm h2AvD810 mutants could not be further suppressed by a DNA ligase IV mutation (Figure 1), similarly to fusions in atm single mutants (Bi et al. 2004).
Figure 1.—
Loss of H2A.Z suppresses telomere fusions. Telomere fusion frequencies are indicated as numbers to the right of the horizontal bars with percentages of nuclei with at least one fusion listed in parentheses. *Data from Bi et al. (2004). **Data from Bi et al. (2005a).
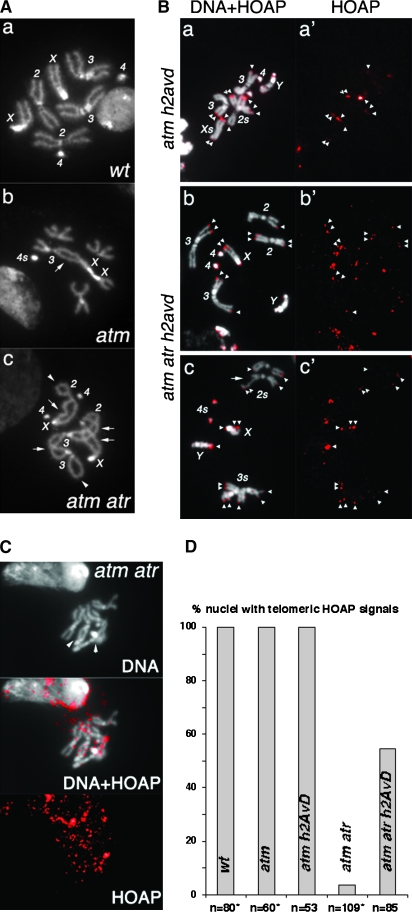
Figure 4.—
Loss of H2A.Z restores HOAP loading to telomeres defective for both ATM and ATR checkpoint functions. (A) DAPI-stained mitotic nuclei from third instar larval neuroblasts. (a) A wild-type (wt) female nucleus with all of the chromosomes labeled. (b) An atm nucleus. A double fusion between chromosomes 3 and X (arrow) is counted as two fusions in Figure 1. The two chromosome 4's are fused, which is counted as one fusion for Figure 1 because one cannot unequivocally define it as either a single or a double fusion. The short arms of two chromosome X's are also fused and counted as one fusion for the same reason. In total, four fusions were identified in this nucleus. (c) An atm atr nucleus. Two single fusions between sisters are indicated by arrowheads. Four double fusions (X–2, X–2, 2–3, 3–3) are indicated by arrows. In total, ten fusions were identified in this nucleus. (B) (a–c) Merged images of DAPI-stained mitotic chromosomes and anti-HOAP signals in red. (a′–c′) Images showing only anti-HOAP signals. Genotypes are indicated at the left. Telomeric HOAP signals are indicated by arrowheads, except those on chromosome 4's. The nucleus in a and a′ had an attached X chromosome so that the X chromosomes are about twice as long as the major autosome arms. The nucleus in c and c′ had a chromatid break (arrow) on one chromosome 2. (C) Lack of HOAP signals on atm atr mutant telomeres. The HOAP signals are overexposed. In the DNA (top), two potential fusion junctions are indicated with arrowheads. (D) The percentage of nuclei with the indicated genotypes that had HOAP signals on at least one telomere. *Data partly from Bi et al. (2004, 2005a).
Mutations in h2AvD also reduced telomere fusion in other checkpoint-defective mutants. As shown in Figure 1, fusion frequencies in mre11 and nbs single mutants or mre11 atm double mutants were similarly reduced by the addition of an h2AvD mutation. In atm atr double mutants, telomere fusion frequency (∼7/nucleus) approaches that caused by the cav mutation, suggesting that the loss of both ATM and ATR functions renders essentially all telomeres susceptible to fusion (Bi et al. 2005a). Strikingly, fusion frequency in h2AvD810 atm atr triple mutants was drastically reduced to ∼1.5 fusions/nucleus, with a concomitant increase in the percentage of cells with no fusion from <5% in the atm atr double mutant to >56% in the triple mutant. I obtained identical results from testing two null alleles of mei-41 (Drosophila ATR) (Figure 1). In summary, loss of H2AvD at checkpoint-defective telomeres suppresses fusion.
Loss of H2A.Z, but not H2A.X, suppresses telomere fusion:
It is possible that the partial reduction in telomere fusion that I observed in the checkpoint and H2AvD double knockouts was simply due to a defect in DNA end joining. To test this hypothesis, Drosophila cells with different mutations were assayed for their ability to repair DSBs induced by X rays. A dose of 210 rad induces similar numbers of DSBs in wild-type (0.81/nucleus) or atm mutant (0.98/nucleus) cells (Bi et al. 2005b). A similarly treated atm h2AvD double-mutant cell displayed 0.91 break on average (n = 60 nuclei), suggesting that the double mutant was as efficient in ligating DSBs as wild-type or atm mutant cells under these conditions. In addition, h2AvD mutants are proficient in cell cycle arrest prior to mitosis in response to higher X-ray doses (X. Bi and Y. S. Rong, unpublished data), suggesting that the loss of H2AvD does not impair the DNA damage response.
The single H2AvD protein encodes both H2A.X and H2A.Z functions. Clarkson et al. (1999) constructed an h2AvD transgene with a C-terminal truncation that lacks H2A.X function. This transgene restores both viability and fertility to a null h2AvD mutation, indicating that the H2A.Z functions are intact. This transgene fully restored telomere fusion to atm h2AvD double mutants (Figure 1), suggesting that it is the loss of H2A.Z functions that suppresses telomere fusion. This is consistent with the finding that a domino mutation, which disrupts the Swr1 complex subunit, behaved similarly to h2AvD mutations in suppressing telomere fusion in both mre11 and atm mutants (Figure 1).
In S. cerevisiae, H2A.Z loading to subtelomeric regions is thought to prevent the spreading of silent chromatin mediated by Sir2 (Meneghini et al. 2003). A Drosophila sir2 mutation was thus tested but did not alter telomere fusion, such that fusion occurred at a similar rate in sir2 atm double mutants as in the atm single mutant (Figure 1) and that sir2 atm h2AvD triple mutants had a similarly reduced fusion frequency as atm h2AvD double mutants (data not shown). These results show that the function of H2AvD in capping regulation is independent of Sir2.
H2AvD distribution on chromosomes:
A possible explanation for the ability of h2AvD mutations to suppress fusion is that H2AvD enrichment at checkpoint-defective telomeres is partly responsible for their uncapping and that the loss of H2A.Z at these telomeres alleviates this defect. To test this, I examined the distribution of H2AvD on wild-type and checkpoint-defective chromosomes by immunostaining. For this purpose, I constructed checkpoint normal and mutant strains in which the only functional h2AvD gene was a gfp-tagged h2avD+ transgene (see materials and methods). This transgene is functional in that it rescued the null mutation and restored telomere fusion to atm h2avD double mutants (data not show). It allowed me to localize H2AvD using anti-GFP antibodies.
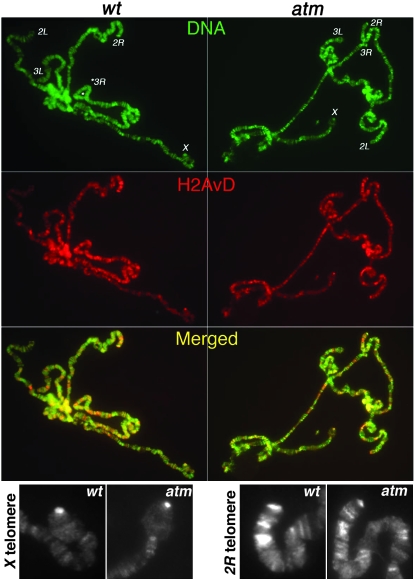
Consistent with a previous report (Leach et al. 2000), a broad distribution of H2AvD was observed on larval polytene chromosomes (Figure 2). H2AvD is enriched at some, but not all telomeric regions. Nonetheless, similar levels were observed at wild-type and atm mutant telomeres (Figure 2). Therefore, loss of ATM protection does not lead to H2AvD enrichment at polytene telomeres.
Figure 2.—
H2AvD distribution on polytene chromosomes. DNA signals (DAPI) are in green. Anti-GFP signals are in red. Enrichments of H2AvD-GFP were detected for telomeric regions of chromosome arms X, 2L, and 2R in both wild-type (wt, left) and atm mutant (right panels) cells. (Bottom) Zoomed images of anti-GFP staining at X and 2R telomeres from wild-type and atm mutant cells.
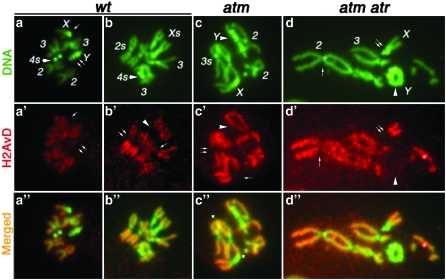
H2AvD distribution was also studied on mitotic chromosomes from larval neuroblasts. Wild-type, atm, and atm atr mutant cells showed similar distribution. In all, H2AvD is underrepresented in regions commonly considered heterochromatic, which include centromeric regions of all chromosomes and the Y chromosome (Figure 3). It appears evenly distributed on euchromatic chromosome arms with some chromosome-to-chromosome variation, and it is neither enriched nor underrepresented in telomeric regions. Therefore, there does not seem to be a detectable enrichment of H2AvD at checkpoint-defective telomeres, and it is unlikely that telomere uncapping in checkpoint mutants is due to H2AvD accumulation.
Figure 3.—
H2AvD distribution on mitotic chromosomes DNA is in green, with major chromosomes labeled (a–d). Anti-GFP signals are in red (a′–d′). Regions in which H2AvD-GFP is underrepresented are selectively labeled as follows: In the male wt nucleus (a, a′, and a″), anti-GFP did not stain X centric heterochromatin (single arrow) or the entire Y chromosome (double arrow). In the female wild-type (wt) nucleus (b, b′, and b″), H2AvD is missing for centric heterochromatin on chromosomes X (arrowhead in b′), 2 (double arrow), and 3 (single arrow). In the male atm nucleus (c, c′ and c″), H2AvD is missing on the Y chromosome (arrowhead), on X centric heterochromatin (single arrow), and on chromosome 3 (double arrow). In the male atm atr nucleus (d, d′, and d″), H2AvD is missing from Y (arrowhead), X centric heterochromatin (double arrow), and chromosome 2 centric heterochromatin (single arrow). In c″, several sites of telomere fusion are marked with an asterisk. In d″, several telomere fusions are present. However, the precise fusion points are difficult to pinpoint due to severe aneuploidy and gross genome rearrangement in atm atr nuclei. Nevertheless, the Y chromosome formed a ring due to telomere fusions.
Loss of H2AvD partially restores the loading of HOAP capping protein to checkpoint-defective telomeres:
Mutations in h2AvD might suppress telomere fusion in checkpoint-defective mutants by enabling normal capping mechanisms. Alternatively, mechanisms that do not involve the normal capping machinery might be in use in these mutants. To distinguish these alternatives, I measured telomere fusion in different combinations of h2AvD, cav, and checkpoint mutants. h2AvD810 did not affect the frequency of fusions in cav (Figure 1), which lacks the HOAP capping protein. In addition, atm h2avD cav triple mutants had a degree of uncapping similar to that of a cav single or a cav h2AvD double mutant (data not shown), suggesting that the effect of h2avD mutations on checkpoint-defective telomeres is dependent on normal capping functions, mediated by HOAP.
To provide additional evidence that h2AvD mutations partially restore normal capping functions, I studied the localization of HOAP in these mutants. HOAP's localization to mitotic telomeres requires either ATM or ATR proteins as HOAP is absent from telomeres of cells that lack both ATM- and ATR-controlled pathways (Bi et al. 2005a). I combined results of HOAP staining from this and our earlier study (Figure 4D). From six atm atr double-mutant animals, HOAP signals were observed on no more than two fusion-free ends in 4 of 109 nuclei. Remarkably, it was detected on multiple telomeres in 47 of 85 nuclei from five atm atr h2AvD triple-mutant animals (Figure 4). In parallel experiments, a robust signal was observed on a majority of the telomeres in all of 53 atm h2AvD double-mutant nuclei (Figure 4). HOAP signal on atm atr h2AvD triple mutants was generally weaker than that on atm h2AvD double mutants. Background signals were also higher in the triple- than in the double-mutant nuclei. These results suggest that the h2AvD mutation partially restored HOAP loading to atm atr mutant telomeres, which is consistent with the partial reduction in telomere fusion frequencies in the triple mutant.
DISCUSSION
In this study, I show that loss of H2AvD in Drosophila suppresses fusion of telomeres that lack the protection of conserved checkpoint proteins: ATM, ATR, or MRN. Drosophila H2AvD encodes the functions for both H2A.X and H2A.Z variants that are translated from separate genes in other organisms. By using transgenes that either have or lack H2A.X function, I establish that H2AvD's role in regulating capping resides in its H2A.Z-homologous region. This conclusion is strengthened by the result from analyzing a domino mutation that behaved similarly to an h2AvD mutation. This represents a novel function of H2A.Z that has not been demonstrated in any other organism.
It is possible that the effect of h2AvD mutations on fusion frequencies is an indirect effect of transcriptional mis-regulation of genes controlling the repair and/or response to DSBs. This, however, is unlikely since cav mutant cells lacking the HOAP capping component are equally prone to telomere fusion with or without H2A.Z, suggesting that H2AvD mutant telomeres are not refractory to being repaired as DSBs. In addition, an h2AvD mutation was unable to suppress fusion in an atm cav h2AvD triple mutant, suggesting that cav is epistatic to h2AvD. In light of the observation that an h2AvD mutation can partially restore HOAP binding to atm atr double-mutant telomeres, I suggest that loss of H2AvD might permit more efficient loading of capping proteins and, therefore, more efficient capping.
Another hypothesis that I considered is that H2AvD accumulates at checkpoint-defective telomeres, interfering with the binding of the capping machinery. However, evidence obtained from immuno-localization of H2AvD did not support this hypothesis. H2AvD has an interesting distribution on mitotic chromosomes in wild-type cells in that it is underrepresented in regions commonly considered heterochromatic. Telomeres are generally considered heterochromatic on the basis of their ability to silence nearby genes. However, recent results suggest that the heterochromatic features of Drosophila telomeres reside in the subtelomeric telomere-associated sequence (TAS) repeats and that the retro-transposon arrays at the extreme of chromosome ends possess certain euchromatic features (Biessmann et al. 2005). This is consistent with the fact that telomeric retro-transposons are actively transcribed to serve as transposition intermediates (Pardue and Debaryshe 2008). Therefore, H2AvD may not be excluded from wild-type telomeric regions, a suggestion supported by a recent genomewide localization study (Mavrich et al. 2008). Nevertheless, I did not observe an elevated level of H2AvD at checkpoint-defective telomeres even though these experiments were set up to favor detection of such enrichment. First, the atm atr double mutant—which has the strongest capping defects and on which the h2AvD mutation had the strongest suppressing effect—was included. Second, H2AvD enrichment would have been prominently detected on telomeres from the Y and fourth chromosomes as well as the short arm of the X chromosome on which H2AvD is normally underrepresented. Therefore, it is unlikely that H2AvD interferes with HOAP loading at checkpoint-defective telomeres and that the loss of such interference partly restores capping in h2AvD mutants.
Finally, the absence of H2A.Z might allow telomeres to adopt an alternative structure that is permissive to the loading of capping proteins. At S. cerevisiae telomeres, H2A.Z may demarcate the euchromatin–heterochromatin boundary (Meneghini et al. 2003). It may serve a similar function in Drosophila. Interestingly, recent results suggest that the heterochromatic features of Drosophila telomeres might reside in the subtelomeric TAS regions (Biessmann et al. 2005). It is possible that H2AvD prevents the spreading of TAS-associated heterochromatin into the transposon arrays. In the absence of H2AvD, Drosophila telomeres might adopt a heterochromatin-like structure, which facilitates the loading of capping proteins. This model is purely speculative due to the fact that the structure of Drosophila telomeres is poorly understood. In particular, the structural elements necessary for the loading of capping machinery remain undetermined. Nevertheless, due to the high degree of conservation in H2A.Z variants from different organisms, its role in regulating telomere capping uncovered in this study may also be conserved.
Acknowledgments
I thank Robert Glaser, Kami Ahmad, and Rebecca Kellum for fly stocks and antibodies and Xiaolin Bi and Xiaofeng Zheng for technical assistance. I thank Michelle Beaucher, Michael Lichten, and Natalia Wesolowska at the National Cancer Institute (NCI) for comments on the manuscript. Research at the Laboratory of Biochemistry and Molecular Biology is supported by the Intramural Program of NCI.
References
- Bi, X., S. C. Wei and Y. S. Rong, 2004. Telomere protection without a telomerase: the role of ATM and Mre11 in Drosophila telomere maintenance. Curr. Biol. 14 1348–1353. [DOI] [PubMed] [Google Scholar]
- Bi, X., D. Srikanta, L. Fanti, S. Pimpinelli, R. Badugu et al., 2005. a Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc. Natl. Acad. Sci. USA 102 15167–15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, X., M. Gong, D. Srikanta and Y. S. Rong, 2005. b Drosophila ATM and Mre11 are essential for the G2/M checkpoint induced by low-dose irradiation. Genetics 171 845–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann, H., S. Prasad, V. F. Semeshin, E. N. Andreyeva, Q. Nguyen et al., 2005. Two distinct domains in Drosophila melanogaster telomeres. Genetics 171 1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci, G., G. Siriaco, G. D. Raffa, R. Kellum and M. Gatti, 2003. The Drosophila HOAP protein is required for telomere capping. Nat. Cell Biol. 5 82–84. [DOI] [PubMed] [Google Scholar]
- Clarkson, M., and R. Saint, 1999. A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol. 18 457–462. [DOI] [PubMed] [Google Scholar]
- Clarkson, M. J., J. R. Wells, F. Gibson, R. Saint and D. J. Tremethick, 1999. Regions of variant histone His2AvD required for Drosophila development. Nature 399 694–697. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna, F.,. P. M. Reaper, L. Clay-Farrace, H. Fiegler, P. Carr et al., 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426 194–198. [DOI] [PubMed] [Google Scholar]
- Gatti, M., S. Bonaccorsi and S. Pimpinelli, 1994. Looking at Drosophila mitotic chromosomes. Methods Cell Biol. 44 371–391. [DOI] [PubMed] [Google Scholar]
- Gong, M., X. Bi and Y. Rong, 2005. Targeted mutagenesis of Drosophila atm and mre11 genes. Drosoph. Inf. Serv. 88 79–83. [Google Scholar]
- Guillemette, B., and L. Gaudreau, 2006. Reuniting the contrasting functions of H2A.Z. Biochem. Cell Biol. 84 528–535. [DOI] [PubMed] [Google Scholar]
- Kim, J. A., M. Kruhlak, F. Dotiwala, A. Nussenzweig and J. E. Haber, 2007. Heterochromatin is refractory to gamma-H2AX modification in yeast and mammals. J. Cell Biol. 178 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurençon, A., A. Purdy, J. Sekelsky, R. S. Hawley and T. T. Su, 2003. Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, T. J., M. Mazzeo, H. L. Chotkowski, J. P. Madigan, M. G. Wotring et al., 2000. Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J. Biol. Chem. 275 23267–23272. [DOI] [PubMed] [Google Scholar]
- Longhese, M. P., 2008. DNA damage response at functional and dysfunctional telomeres. Genes Dev. 22 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan, J. P., H. L. Chotkowski and R. L. Glaser, 2002. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 30 3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, J. M., R. C. Frydrychova and H. Biessmann, 2008. Drosophila telomeres: an exception providing new insights. BioEssays 30 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrich, T. N., C. Jiang, I. P. Ioshikhes, X. Li, B. J. Venters et al., 2008. Nucleosome organization in the Drosophila genome. Nature 453 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern, M. J., A. Krauskopf and E. H. Blackburn, 2000. Telomeres and their control. Annu. Rev. Genet. 34 331–358. [DOI] [PubMed] [Google Scholar]
- Meneghini, M. D., M. Wu and H. D. Madhani, 2003. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112 725–736. [DOI] [PubMed] [Google Scholar]
- Mizuguchi, G., X. Shen, J. Landry, W. H. Wu, S. Sen et al., 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303 343–348. [DOI] [PubMed] [Google Scholar]
- Pardue, M. L., and P. G. DeBaryshe, 2008. Drosophila telomeres: a variation on the telomerase theme. Fly 2 1–10. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., 2008. Telomere capping in Drosophila: dealing with chromosome ends that most resemble DNA breaks. Chromosoma 117 235–242. [DOI] [PubMed] [Google Scholar]
- Ruhf, M. L., A. Braun, O. Papoulas, J. W. Tamkun, N. Randsholt et al., 2001. The domino gene of Drosophila encodes novel members of the SWI2/SNF2 family of DNA-dependent ATPases, which contribute to the silencing of homeotic genes. Development 128 1429–1441. [DOI] [PubMed] [Google Scholar]
- Shareef, M. M., C. King, M. Damaj, R. Badagu, D. W. Huang et al., 2001. Drosophila heterochromatin protein 1 (HP1)/origin recognition complex (ORC) protein is associated with HP1 and ORC and functions in heterochromatin-induced silencing. Mol. Biol. Cell 12 1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff, R., A. Arbel-Eden, D. Pilch, G. Ira, W. M. Bonner et al., 2004. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr. Biol. 14 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, D. S., and Y. S. Rong, 2007. A genetic screen for DNA double-strand break repair mutations in Drosophila. Genetics 177 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, H. B., and K. G. Golic, 2004. Gene deletions by ends-in targeting in Drosophila melanogaster. Genetics 168 1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]