Abstract
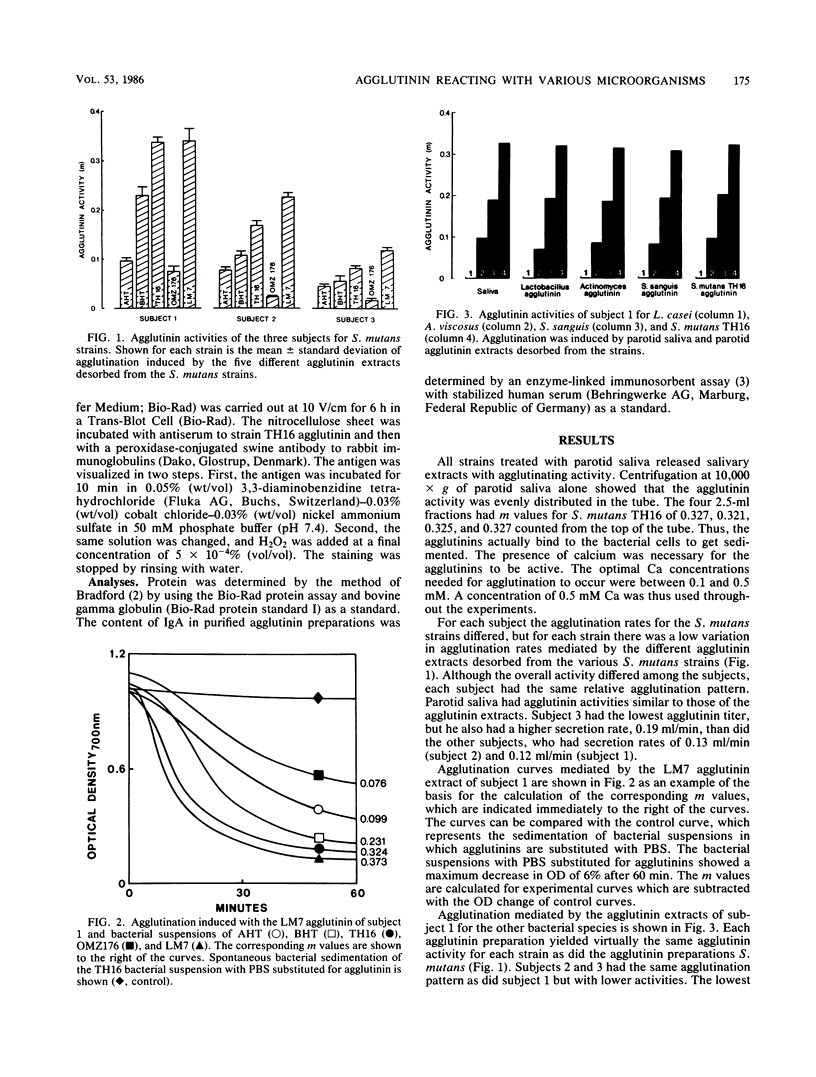
Human parotid agglutinins from three individuals were isolated by adsorption to and desorption from strains of Streptococcus mutans belonging to serotypes a, b, c, d, and e and strains of Lactobacillus casei, Actinomyces viscosus, and Streptococcus sanguis. The desorption was achieved by suspending centrifuged saliva-coated microorganisms in 10 mM phosphate buffer (pH 6.8) containing 0.154 M sodium chloride. After another centrifugation, agglutinin activity was recovered in the supernatants. The L. casei strain was not agglutinated by any of the agglutinin extracts or by saliva, but all the other strains were agglutinated to a variable extent. However, all strains, including the nonagglutinating L. casei strain, adsorbed and desorbed agglutinins active for other strains. The agglutinin extracts from S. mutans serotype c, S. sanguis, and A. viscosus were purified and characterized by electrophoretic and immunological techniques. The purified preparations were positively stained for protein and carbohydrate, and the molecular weights were estimated to be 440,000. All agglutinin extracts needed calcium in the range of 0.1 to 0.5 mM to be active, and for a single strain, all agglutinins gave the same degree of agglutination, indicating that the isolated agglutinins may be of the same molecular species, a hypothesis that was also confirmed by the preliminary characterization of the purified agglutinins. This type of agglutinin, which seems to exert its activity among various bacterial species, could be important in mediating bacterial coaggregation and thus may add to the effect of specific agglutinins in the clearance of bacteria from the human mouth.
Full text
PDF



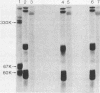
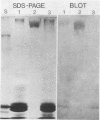
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boat T. F., Cheng P. W. Biochemistry of airway mucus secretions. Fed Proc. 1980 Nov;39(13):3067–3074. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bratthall D., Ellen R. P. Determination of immunoglobulin A in saliva by immunobead enzyme-linked immunosorbent assay: comparison with single radial immunodiffusion. J Clin Microbiol. 1982 Oct;16(4):766–769. doi: 10.1128/jcm.16.4.766-769.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dawes C. The effects of flow rate and duration of stimulation on the condentrations of protein and the main electrolytes in human parotid saliva. Arch Oral Biol. 1969 Mar;14(3):277–294. doi: 10.1016/0003-9969(69)90231-3. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Russell R. R. The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(10):751–757. doi: 10.1016/0003-9969(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. II. Biochemical and immunochemical properties of factors in human saliva and amniotic fluid. Int Arch Allergy Appl Immunol. 1980;61(2):203–212. doi: 10.1159/000232434. [DOI] [PubMed] [Google Scholar]
- Eggert F. M. The nature of secretory agglutinins and aggregating factors. IV. Complexing between non-mucin glycoproteins, immunoglobulins and mucins in human saliva and amniotic fluid. Int Arch Allergy Appl Immunol. 1980;62(1):46–58. [PubMed] [Google Scholar]
- Ericson T., Magnusson I. Affinity for hydroxyapatite of salivary substances inducing aggregation of oral streptococci. Caries Res. 1976;10(1):8–18. doi: 10.1159/000260185. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Mongiello J. R., Burger B. W. Haemagglutination inhibition and aggregation of Fusobacterium nucleatum by human salivary mucinous glycoproteins. Arch Oral Biol. 1979;24(7):483–489. doi: 10.1016/0003-9969(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- Kashket S., Guilmette K. M. Aggregation of oral streptococci in the presence of concanavalin A. Arch Oral Biol. 1975 May-Jun;20(5-6):375–379. doi: 10.1016/0003-9969(75)90030-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagerlöf F., Ekstrand J. The effect of flow rate on the ionized calcium concentration of human parotid saliva. Caries Res. 1982;16(2):123–128. doi: 10.1159/000260588. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Olsson J., Bratthall D., Carlén A. Association between bacterial agglutinins and immunoglobulin A in human saliva. Acta Odontol Scand. 1981;39(2):61–66. doi: 10.3109/00016358109162260. [DOI] [PubMed] [Google Scholar]
- Rundegren J., Ericson T. An evaluation of the specificity of salivary agglutinins. J Oral Pathol. 1981 Aug;10(4):261–268. doi: 10.1111/j.1600-0714.1981.tb01272.x. [DOI] [PubMed] [Google Scholar]
- Rundegren J., Ericson T. Effect of calcium on reactions between a salivary agglutinin and a serotype c strain of Streptococcus mutans. J Oral Pathol. 1981 Aug;10(4):269–275. doi: 10.1111/j.1600-0714.1981.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Rundegren J., Ericson T. Saliva-induced aggregation of micro-organisms from skin, tooth surfaces, oral mucosa, and rectum. J Oral Pathol. 1981 Aug;10(4):248–260. doi: 10.1111/j.1600-0714.1981.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]