Abstract
Coordination of animal behavior with reproductive status is often achieved through elaboration of hormones by the gonad. In the nematode Caenorhabditis elegans, adult males explore their environment to locate mates. Mate searching is regulated by presence of mates, nutritional status, and a signal from the gonad. Here we show that the gonadal signal acts via the nuclear receptor DAF-12, a protein known to regulate several C. elegans life-history traits. DAF-12 has both activational and organizational functions to stimulate exploratory behavior and acts downstream of the gonadal signal, outside of the gonad. DAF-12 acts upstream of sensory input from mating partners and physiological signals indicating nutritional status. Mate searching was rescued in germ-line ablated animals, but not if both germ line and somatic gonad were ablated, by a precursor of the DAF-12 ligand, dafachronic acid (DA). The results are interpreted to suggest that the germ line produces a DA precursor that is converted to DA outside of the germ line, possibly in the somatic gonad. As it does in other pathways in which it functions, in regulation of male mate searching behavior DAF-12 acts at a choice point between alternatives favoring reproduction (mate searching) vs. survival (remaining on food).
TRANSCRIPTION factors of the nuclear receptor (NR) family serve to coordinate biological pathways in diverse tissues through their responses to lipophilic ligands that circulate through the body. Their roles encompass multiple aspects of organismal biology, including physiology, growth, differentiation, metabolism, and behavior (Mangelsdorf et al. 1995). They are widely expressed in the mammalian brain, but their role in development or function of the nervous system is not well understood (Gofflot et al. 2007). Through elaboration of hormones by the gonad, NRs play an important role in coordinating reproductive behavior with sexual phenotype, both by promoting development of sex-specific neuronal structures and circuits (organizational effects) and by activating expression of behavioral pathways in the mature nervous system (activational effects) (Meisel and Sachs 1994; Pfaus 1999; Nef and Parada 2000; Baum 2002; Carter 2002; Morris et al. 2004).
Caenorhabditis elegans, with its small nervous system composed of identifiable neurons, provides a powerful model system in which to study regulation of animal behavior, including sexual behavior (White et al. 1986; Chalfie and White 1988; De Bono and Maricq 2005). In C. elegans, male-specific sexual behavior consists of copulation and exploratory behavior (Lipton et al. 2004; Barr and Garcia 2006; Emmons 2006). Exploratory behavior serves to bring males into the vicinity of hermaphrodite mates, where they respond to short-range secreted cues (Simon and Sternberg 2002; Chasnov et al. 2007; White et al. 2007). During copulation, the male slides its tail along the hermaphrodite body to find the vulva, inserts its spicules, and transfers sperm. In support of these sex-specific behaviors, the male nervous system contains a complement of 89 male-specific neurons, along with 294 neurons that make up the core nervous system shared with the hermaphrodite (Sulston et al. 1980; Portman 2007).
C. elegans male-specific exploratory behavior has been studied as a model of a motivated sexual behavior (Lipton et al. 2004). If mating partners are not present, males explore their environment and will leave a source of food to do so (Lipton et al. 2004). Once mating partners are located on a food source, males remain there. Since exploratory behavior ceases when a mating partner on food is located, their exploratory behavior causes males to accumulate with hermaphrodites on food and therefore functions as a mate-searching strategy.
We have exploited the C. elegans male's exploratory behavior to study regulation of a male-specific reproductive behavior. Immature males remain on food while mature males lacking germ cells have a diminished tendency for exploration, showing that exploratory behavior is regulated both by developmental stage and by presence of the germ line (Lipton et al. 2004). In view of the role in other animals of hormones in coordinating sexual behavior with maturity and gonadal status, we wondered whether control of behavior by the gonad in C. elegans also involved regulation by a hormone. As the NR gene daf-12 had previously been shown to mediate gonadal effects in regulation of aging (Hsin and Kenyon 1999), the DAF-12 NR was a candidate for a steroid receptor to mediate a hormonal effect of the gonad on mate-searching behavior.
daf-12, which encodes a NR of the vitamin D family, is ubiquitously expressed in C. elegans tissues and has effects on diverse life-history traits, including choice of a reproductive vs. a survival (dauer diapause) pathway during larval development, developmental timing of cell lineages, and aging (Hsin and Kenyon 1999; Antebi et al. 2000; Broue et al. 2007; Gerisch et al. 2007). Two related steroid derivatives termed dafachronic acids (DA) act as DAF-12 ligands (Motola et al. 2006). A cytochrome P450, product of the daf-9 gene, is proposed to catalyze final oxidative steps of the DA biosynthetic pathway, while a Rieske-like oxygenase, product of the daf-36 gene, acts on cholesterol at an early biosynthetic step (Motola et al. 2006; Rottiers et al. 2006).
Like other NRs, DAF-12 acts as a switch in conjunction with a corepressor protein, DIN-1. In the absence of ligand binding, a DAF-12/DIN-1 complex promotes life-history traits associated with enhanced survival, including longevity, diapause, and fat storage. In the presence of ligand, DIN-1 is displaced and DAF-12 with bound ligand promotes reproductive development and a normal life span (Ludewig et al. 2004; Gerisch et al. 2007).
Here we show that the daf-12 pathway has both organizational and activational effects on the C. elegans male nervous system and regulates male-specific exploratory behavior. As in the other pathways in which it acts, the liganded form of DAF-12 acts both during development and in adulthood to promote a behavior that favors reproduction—male exploration for mates—while the unliganded form, in conjunction with DIN-1, promotes an alternative strategy—remaining on food in the absence of mates. We show that the daf-12 pathway acts downstream of the gonad to regulate behavior and provide evidence that the germ line may be one source of a DA precursor.
MATERIALS AND METHODS
The leaving assay:
We quantitatively measure male exploratory behavior by a behavioral assay termed the leaving assay, which exploits the male's tendency to leave a food source lacking mating partners (Lipton et al. 2004). In the leaving assay, the fraction of animals (of 20 in a typical assay) that have not yet traveled a certain distance (3 cm) away from a food source (a lawn of Escherichia coli) is determined at various time points. From these data the population average rate of leaving is determined. We have shown previously that the probability of leaving food per hour, PL, is a constant, characteristic of a particular genotype under given conditions (Lipton et al. 2004). In the leaving assay under the conditions used here, wild-type adult males leave a food patch with a probability PL = 0.097 ± 0.002 (SEM) (Figure 1A; Table 1). For hermaphrodites and juvenile males, PL is essentially 0. In the context of this assay, male exploratory behavior is sometimes referred to as leaving behavior.
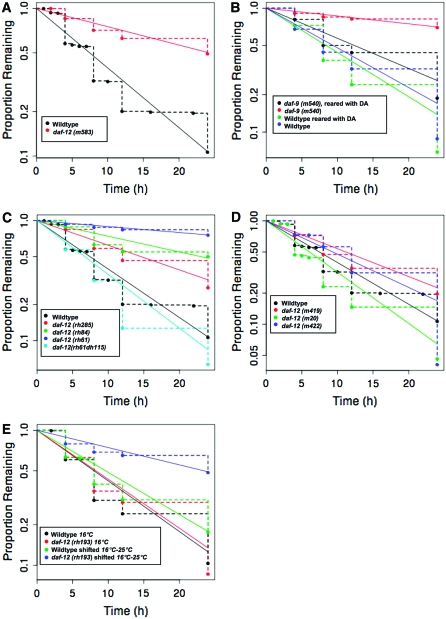
Figure 1.—
The DAF-12 pathway regulates male exploratory behavior. The rate of male exploratory behavior is determined by the leaving assay. For various genotypes and treatments, the fraction of (usually 20) males that have not yet traveled 3 cm away from a 1-cm patch of E. coli food (nonleavers) is scored at various times after the start of the assay. The points on the graphs show the individual observations. The leaving rate, taken as a measure of the tendency for exploratory behavior, is calculated as the probability of leaving per hour, PL. The data are analyzed using the R survival package (http://cran.r-project.org/web/packages/survival/index.html) to fit the censored data to an exponential parametric survival model, using maximum likelihood. The slopes of the straight lines through the data illustrate the constant hazard rate (λ), taken here as PL. The results are compiled in Table 1 and summarized in Figure 2 for the various daf-12 alleles. (A) daf-12 gene function is required for a wild-type rate of exploratory behavior. Males homozygous for a daf-12 strong loss-of-function mutation leave food more slowly than wild-type males. (B) The DAF-12 ligand DA stimulates exploratory behavior. Males homozygous for daf-9(m540), a hypomorphic allele of the daf-9 biosynthetic gene required for synthesis of DA, leave food more slowly than wild-type males. Leaving rate is restored by treatment of daf-9 animals with DA. (C) Evidence a DAF-12/DIN-1 corepressor complex inhibits leaving. The structures of the daf-12 alleles shown are illustrated in Figure 2. Successive deletion of the DAF-12 C-terminal LBD results in decreased leaving rate. Introduction of a point mutation that prevents DIN-1 binding [daf-12(rh61dh115)] restores wild-type leaving rate. Wild-type data are the same as in A. (D) The DAF-12 N-terminal DBD stimulates exploratory behavior. The structure of the daf-12 alleles shown is illustrated in Figure 2. Alleles of daf-12 with deletions covering the entire LBD including the DIN-1 binding region have a high rate of leaving. Wild-type data are the same as in A. (E) daf-12 has an adult function to stimulate exploratory behavior. Males homozygous for a temperature-sensitive allele of daf-12 have reduced leaving rate when shifted to nonpermissive temperature as adults. Quantitative analysis of the data in A–D is given in Table 1. Data for the series of daf-12 alleles shown in A, C, and D were compared to pooled data for wild type.
TABLE 1.
Involvement of the gonad and the dafachronic acid pathway in the modulation of exploratory behavior in C. elegans males
| Leaving
|
P-valuea
|
|||||
|---|---|---|---|---|---|---|
| Strain | PL | 95% C.I. | vs. wild type | vs. daf-12(rh61) | vs. daf-12(m583) | Reps (n) |
| daf-12 mutants | ||||||
| Wild type | 0.097 | 0.092–0.102 | NA | 72 (1461) | ||
| daf-12(m583)X | 0.029 | 0.026–0.032 | <0.0001 | <0.0001 | NA | 35 (619) |
| daf-12(rh285)X | 0.047 | 0.035–0.064 | <0.0001 | <0.0001 | <0.01 | 4 (58) |
| daf-12(rh84)X | 0.030 | 0.021–0.043 | <0.0001 | <0.001 | 0.8 | 4 (62) |
| daf-12(rh61)X | 0.011 | 0.008–0.016 | <0.0001 | NA | <0.0001 | 6 (131) |
| daf-12(rh61dh115)X | 0.10 | 0.08–0.13 | 0.38 | <0.0001 | <0.0001 | 2 (63) |
| daf-12(m419)X | 0.062 | 0.055–0.070 | <0.0001 | <0.0001 | <0.0001 | 9 (296) |
| daf-12(m20)X | 0.114 | 0.106–0.122 | <0.0001 | <0.0001 | <0.0001 | 47 (889) |
| daf-12(m422)X | 0.074 | 0.062–0.089 | <0.01 | <0.0001 | <0.0001 | 6 (121) |
| daf-9(m540)X | 0.053 | 0.042–0.067 | <0.0001 | 6 (114) | ||
|
P-value
|
||||||
| vs. daf-9 | vs. daf-12(m20) | |||||
| daf-12(m20), daf-9(e1406)X | 0.24 | 0.20–0.29 | <0.0001 | <0.0001 | <0.015 | 7 (118) |
| vs. wild type, same temperature | ||||||
| daf-12 temperature shift | ||||||
| Wild type at permissive temperature | 0.086 | 0.072–0.104 | 7 (129) | |||
| daf-12(rh193ts)X at permissive temperature | 0.084 | 0.068–0.103 | 0.081 | 6 (113) | ||
| Wild type shifted as adult | 0.072 | 0.059–0.087 | 7 (128) | |||
| daf-12(rh193ts)X shifted as adult | 0.030 | 0.023–0.039 | <0.0001 | 6 (106) | ||
|
P-value
|
||||||
| vs. wild type | vs. daf-9 | |||||
| DA rescue experiments | ||||||
| Wild type | 0.08 | 0.06–0.09 | NA | <0.0001 | 4 (73) | |
| Wild type + DA from egg | 0.08 | 0.06–0.12 | 0.69 | 2 (29) | ||
| Wild type + DA as adult | 0.08 | 0.06–0.11 | 0.51 | 2 (41) | ||
| daf-9(m540)X | 0.03 | 0.02–0.04 | <0.0001 | NA | 4 (69) | |
| daf-9(m540)X + DA from egg | 0.06 | 0.04–0.08 | 0.31 | <0.05 | 2 (32) | |
| daf-9(m540)X + DA as adult | 0.04 | 0.02–0.06 | <0.01 | 0.38 | 2 (37) | |
| vs. alone, same strain | ||||||
| With hermaphrodites | ||||||
| Wild-type male alone | 0.10 | 0.08–0.13 | 5 (80) | |||
| Wild type + hermaphrodites | 0.002 | 0.001–0.007 | <0.0001 | 4 (60) | ||
| daf-12(m20)X male alone | 0.136 | 0.089–0.209 | 1 (21) | |||
| daf-12(m20)X + hermaphrodites | 0.009 | 0.003–0.024 | <0.0001 | 1 (21) | ||
| Starved animals (censored after 8 hr) | ||||||
| Wild type | 0.059 | 0.041–0.086 | 4 (70) | |||
| Wild type, starved | 0.015 | 0.007–0.032 | <0.0005 | 4 (59) | ||
| daf-12(m20)X | 0.103 | 0.067–0.160 | 2 (31) | |||
| daf-12(m20)X, starved | 0.021 | 0.009–0.050 | <0.0005 | 2 (31) | ||
|
P-value
|
||||||
| vs. wild-type mock | vs. intact, same strain | vs. daf-12 (m583) gonad (−) | ||||
| daf-12 and gonad ablation | ||||||
| Wild-type mock | 0.11 | 0.08–0.16 | 5 (30) | |||
| Wild-type gonad ablated | 0.023 | 0.01–0.04 | <0.0001 | 6 (26) | ||
| daf-12(m583)X mock | 0.041 | 0.03–0.06 | <0.001 | 4 (31) | ||
| daf-12(m583)X gonad ablated | 0.033 | 0.02–0.06 | <0.0005 | 0.57 | 4 (26) | |
| daf-12(m20)X mock | 0.12 | 0.09–0.16 | 0.72 | 3 (47) | ||
| daf-12(m20)X gonad ablated | 0.076 | 0.05–0.12 | 0.19 | 0.07 | <0.05 | 2 (25) |
|
P-value
|
||||||
| vs. wild type, no steroid | vs. no preDA, same ablation | |||||
| Steroid replacement of gonad and germ line | ||||||
| Wild-type mock, no steroid | 0.09 | 0.07–0.11 | 11 (80) | |||
| Germ-line ablated, no steroid | 0.04 | 0.02–0.08 | <0.05 | 4 (17) | ||
| Gonad ablated, no steroid | 0.03 | 0.01–0.67 | <0.01 | 5 (19) | ||
| Germ-line ablated with DA precursor | 0.09 | 0.05–0.15 | 0.9 | 0.1 | 4 (17) | |
| Gonad ablated with DA precursor | 0.04 | 0.02–0.09 | <0.05 | 0.41 | 5 (19) | |
|
P-value
|
||||||
| vs. mes-1 sperm (+) | vs. sperm (−) no preDA | |||||
| mes-1(bn7)X, sperm (+), no steroid | 0.11 | 0.08–0.16 | 1 (39) | |||
| mes-1(bn7)X, sperm (−), no steroid | 0.06 | 0.04–0.10 | <0.05 | 1 (27) | ||
| mes-1(bn7)X, sperm (−) with preDA | 0.15 | 0.10–0.23 | 0.35 | <0.01 | 1 (25) | |
|
P-value
|
||||||
| vs. wild type | vs. no preDA, same strain | |||||
| DA precursor addition | ||||||
| Wild type | 0.11 | 0.08–0.15 | 2 (43) | |||
| daf-9(m540)X | 0.030 | 0.02–0.05 | <0.0001 | 2 (33) | ||
| daf-12(m583)X | 0.035 | 0.02–0.05 | <0.0001 | 2 (46) | ||
| daf-12(m583)X + preDA as adult | 0.054 | 0.04–0.08 | <0.005 | 0.1 | 2 (42) | |
| daf-9(m540)X + preDA from egg | 0.042 | 0.02–0.08 | <0.0005 | 0.74 | 1 (15) | |
| daf-9(m540)X + preDA as adult | 0.034 | 0.02–0.06 | <0.005 | 0.41 | 1 (20) | |
preDA refers to the dafachronic acid precursor 4-cholesten-3-one. Reps indicates the number of independent leaving assays. n refers to total number of animals examined across all trials.
Unless otherwise specified, the first P-value is for a comparison to the control group, which is in the first row of each section. P-values for second and third comparisons are listed when appropriate. The specific comparison is indicated above each section.
Statistical analysis of leaving assays:
Male leaving behavior was modeled with the exponential model N(t)/N(0) = exp(−λt). N(0) is the number of worms at time zero, and N(t) is the number of worms at time t (in hours). λ is the PL value or the probability that each individual will become a “leaver” per hour. To estimate the parameters of the exponential model, including mean PL value, SEM, and the 95% confidence intervals, data were pooled across experiments and right censored after 24 hr. The R survival package (http://cran.r-project.org/web/packages/survival/index.html) was then used to fit the censored data to an exponential parametric survival model, using maximum likelihood. A constant hazard rate (λ) was estimated using the data and used as the PL value. PL values are represented as straight lines through the survival curves in the figures shown.
Strains:
Strains used include the following: CB4088 him-5(e1490)V; SS149 mes-1(bn7)X; EM318 unc-51(e369)him-5(e1490)V; EM408 daf-12(m20)X; him-5(e1490)V; EM918 daf-12(rh193)X; bxIs14, him-5(e1490)V; EM920 daf-12(m25)X; bxIs14, him-5(e1490)V; EM923 daf-12(m421)X; bxIs14, him-5(e1490)V; EM924 daf-12(m420)X; him-5(e1490)V; EM926 daf-12(m422)X; bxIs14, him-5(e1490)V; EM927 daf-12(rh61)X; him-5(e1490)V; EM930 daf-9(m540)X; bxIs14, him-5(e1490)V; EM932 mgEx661; dpy-7(sc27), daf-9(e1406)X; him-5(e1490)V; EM933 daf-12(rh84)X; bxIs14, him-5(e1490)V; EM934 daf-12(rh257)X; bxIs14, him-5(e1490)V; EM935 daf-12(rh285)X; bxIs14, him-5(e1490)V; EM936 daf-12(m583)X; him-5(e1490)V; him-5(e1490)V; EM956 daf-9(e1406), daf-12(m20)X; him-5(e1490)V; EM964 Ex[sdf-9p∷gfp]; him-5(e1490)V; EM1038 daf-12(m419)X; him-5V; AA317 daf-12(dh115, rh61)X; and DR2207 daf-9(e1406), daf-12(m20)X. Worm strains daf-12(dh115, rh61)X from A. Antebi, pKOG9 [sdf-9p∷gfp] from I. Katsura, and DR2207 daf-9(e1406), daf-12(m20)X from D. Riddle were generously provided by these individuals. The remaining strains were ordered from the Caenorhabditis Genetics Center or constructed in this laboratory.
The daf-9∷GFP strain (EM932) was derived by crossing him-5(e1490) into mgEx661 [genotype [daf-9p∷daf-9∷GFP] daf-9(e1406), dpy-7(sc27)X] (Mak and Ruvkun 2004).
Strain construction and worm culture:
In most cases him-5 was added using standard crossing methods. However, many of the daf-12 class 3 alleles do not have a visible phenotype. So we made daf-12; him-5 doubles with class 3 alleles by taking advantage of the fact that daf-12 is on the X chromosome. Males are X/O while hermaphrodites are X/X. him-5(e1490)V males were crossed with daf-12 hermaphrodites. The resulting hemizygous (X/O) males were then crossed back to the parent strain to produce homozygous daf-12 hermaphrodites. The presence of daf-12 was verified by testing their inability to form dauers on crowded starved plates at 25°. A number of the lines used in this article carried the integrated transgenic construct bxIs14, which is a pkd-2∷GFP fusion construct integrated into chromosome V (Jia and Emmons 2006), which allowed both ray axon guidance and leaving behavior to be scored. Neither bxIs14 (L. Jia, personal communication) nor him-5 (J. Lipton, personal communication) affects male leaving behavior.
Worms were cultured according to standard C. elegans propagation methods (Brenner 1974; Sulston and Hodgkin 1988), on nematode growth media (NGM) agar and the OP50 strain of E. coli as a food source. Most strains contained the him-5(e1490) mutation, which spontaneously produces a high percentage of males (Sulston and Hodgkin 1988). Wild type refers to him-5(e1490) mutants (on the N2 background) unless mentioned otherwise. In the cases of daf-12(rh61, dh115)X and mes-1(bn7)X, an alternative method was used to generate males. Since the gene of interest was on the X chromosome, hemizygous (XO) mutant males were generated and tested after the first-generation cross with wild-type him-5 fathers and homozygous mutant mothers.
Starvation assays:
Starvation assays were performed as before (Lipton et al. 2004). Briefly, newly matured adult males were washed three times in M9, placed on a clean agar plate (no bacteria), and allowed to starve for 12 hr prior to the initiation of the leaving assay. Because males often left the plate and died on the walls while being starved, sterile Sephadex beads in M9 were pipetted onto the center of the plate. Male worms were often found swimming over and around the beads and the presence of the beads decreases the number of males that died on the wall of the starvation plate.
Male retention by hermaphrodites:
The retention assays were performed as in Lipton et al. (2004). Briefly, newly matured adult males were placed on a leaving assay plate with four mature paralyzed (unc-51, him-5V) hermaphrodites and the leaving assay was performed as usual.
Ablations:
Gonad and germ-line ablations were performed using genetic methods and laser ablation. For the genetic approach, mes-1(bn7)X was used since it has a variable defect in the germ line such that animals with and without germ lines grow and can be seen among siblings. The penetrance of the defect varies according to rearing temperature (Capowski et al. 1991); we reared animals at 20°, producing ∼50% defective males. When viewed under the compound microscope (Zeiss Axioimager A1, AX10) the germ lines of mes-1(bn7)X males were either full of sperm or empty. At the conclusion of leaving assays worms were anesthetized (25 μm levamisole) and mounted on 5% agarose pads and classified as germ-line defective or germ-line containing.
Germ-line and gonad laser ablations were performed using the method of Bargmann and Horvitz (1991). Briefly, in the larval stage 1 (L1) the worm gonad consists of the four-cell gonad primordium. Whole gonad ablation was conducted by killing all four cells (Z1–Z4) of the gonad precursor, and selective germ-line ablation was conducted by killing only the two germ-line precursors (Z2 and Z3). Mock animals were mounted and anesthetized (10 μm levamisole) alongside ablated animals. Successful germ-line and gonad ablation was confirmed after leaving assays were complete by mounting and viewing the gonad under the compound microscope (Zeiss Axioimager A1, AX10).
XXX cell ablations were performed using the same method, except a fluorescent reporter was used to recognize XXX cells and verify their loss at the conclusion of experiments. To track XXX cells we used a him-5(e1490) line containing a plasmid with sdf-9p (3.5 kb) fused to GFP (a generous gift of Isao Katsura). This plasmid drives GFP expression in XXX cells (Ohkura et al. 2003).
Steroid rescue experiments:
Worms were treated with 250 nm Δ4-dafachronic acid (3-keto-4-cholestenoic 25S-26 acid, the generous gift of D. Mangelsdorf), 25 μm of dafachronic acid precursor (4-cholesten-3-one, steraloids reference no. 135620, catalog no. C6250-000; http://www.steraloids.com), or solvent (ethanol) without hormone. Final steroid concentrations were calculated with respect to the volume of agar on which it was plated (Motola et al. 2006). The appropriate steroid/ethanol solution was then spread on an agar plate together with a 5× concentrate of an overnight bacterial culture (E. coli OP50) at a ratio of 9:1 (v/v) bacteria to steroid. The lid of the plate was left ajar and solutions were allowed to soak into the culture plate for at least 2 hr prior to plating the worms. For exposure during development and adulthood, hermaphrodite worms were allowed to lay eggs on steroid or control plates and the progeny were reared from egg to L4 on the treatment plate. The L4 progeny were then separated to fresh steroid or control plates and tested in a leaving assay after 12 hr exposure to the steroid as adults. For adult exposure, worms were transferred to steroid or control plates on the day that they matured for 12 hr and then tested for mate searching behavior. During the assay steroids were not present. To test the ability of steroid to rescue the behavior of laser ablated animals, each worm was tested twice, first with no steroid exposure then with steroid exposure. To reduce the difference in age of worms between the first and the second experiment, leaving assays were run for 12 hr instead of 24 and worms were treated with steroids in the 12 hr between the two experiments.
RESULTS
The DAF-12 NR pathway regulates male exploratory behavior:
To examine the role of the daf-12 pathway in the regulation of male exploratory behavior, we tested the effect of altering known components of this pathway. First, we examined exploratory behavior in males homozygous for the mutation daf-12(m583), which encodes a severely truncated form of the DAF-12 protein. We measured exploratory behavior with the leaving assay, which determines the rate at which isolated males leave a source of food (materials and methods). daf-12(m583) males left food significantly slower than wild-type males [wild type: him-5(e1490)] (Figure 1A; Table 1). Therefore wild-type daf-12 is required for normal male leaving behavior. daf-12(m583) hermaphrodites remained on food similar to wild type. daf-12(m583) males were able to copulate and produce progeny (data not shown).
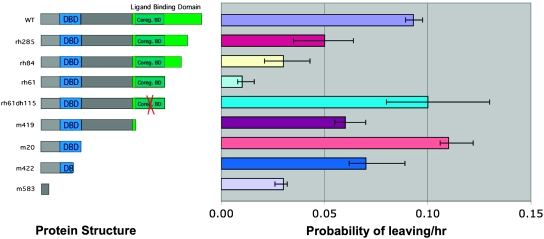
Four DAF-12 protein isoforms have been reported (Antebi et al. 2000; Snow and Larsen 2000). Two (A1 and A3) encompass all 17 DAF-12 exons and differ by 16 amino acids in exon 12. These two isoforms are truncated within exon 3 before the Zn-finger DNA-binding domain (DBD) by the daf-12(m583) mutation and are unlikely to have activity in this mutant. A third reported isoform (A2), which includes all of the DAF-12 protein sequence, including the DBD, Hinge, and the ligand-binding domain (LBD), but excluding the N-terminal hypervariable region, begins within exon 3 and is unaffected by the m583 mutation (Snow and Larsen 2000). Since daf-12(m583) is dauer defective as well as male leaving defective, the A2 isoform alone apparently cannot supply daf-12 activity for either of these phenotypes. A fourth isoform (B) begins with exon 13 and includes only the LBD; it is of unknown function. Considering the mutations used in this work, m583, m422, and m20 affect A isoforms only, while m419, rh61dh115, rh61, rh84, and rh285 affect both A and B isoforms.
To determine whether it was the liganded or the unliganded form of DAF-12 that stimulated exploratory behavior, we examined males mutant for the ligand biosynthetic gene daf-9. Males homozygous for the hypomorphic allele daf-9(m540) had a slow rate of leaving like the daf-12 loss-of-function phenotype (Figure 1B; Table 1). Since a reduced level of ligand slowed the rate of leaving, it appeared to be the liganded form of daf-12 that stimulated exploratory behavior. Animals null for daf-9 could not be tested because they constitutively form dauers or sterile adults (Gerisch et al. 2001; Jia et al. 2002). This result therefore left open the question of the phenotype in the complete absence of ligand.
We rescued the effect of the daf-9 mutation by exogenously supplying DA, consistent with the conclusion that DA is the steroid hormone in the daf-12 pathway for male behavior as it is in other pathways (Motola et al. 2006). daf-9(m540) animals were raised to adulthood from eggs laid on agar containing 250 nm Δ4-dafachronic acid, one of the two dafachronic acids identified in worms (we refer to this compound as DA). Upon maturing, they were cultured on DA for a further 12 hr and then tested in the leaving assay in the absence of hormone. Growth on DA-supplemented medium restored the leaving rate of adult males to the wild-type level (Figure 1B; Table 1). The effect was specific to males, since adult hermaphrodites raised on DA remained on food like untreated hermaphrodites (data not shown). However, when worms were first matured on plates without drug and then cultured only as adults on drug for 12 hr and tested for leaving without drug, leaving by daf-9(m540) males was the same as without drug treatment (Table 1). Thus there appeared to be a requirement for ligand-stimulated daf-12 function during male development for rescue of exploratory behavior.
To further examine whether the DAF-12 protein had a function in the absence of ligand binding, we examined animals carrying a series of mutations that deleted increasing segments of the protein beginning at the C terminus and successively deleting the LBD, the DIN-1 corepressor binding domain, and the DBD (Figure 1, C and D). The measured rates are given in Table 1 and summarized in Figure 2. Deletion of increasing amounts of the LBD [daf-12(rh285) and daf-12(rh84)] gradually decreased the rate of exploratory behavior. When the LBD was deleted up to the DIN-1 binding region, but leaving the DIN-1 binding region intact [daf-12(rh61)], the rate of leaving was slower than in the presumptive null background. This form of the protein therefore appeared to inhibit exploratory behavior. This inhibition appeared to require DIN-1 binding since introduction of a point mutation into the DIN-1 binding domain that abolishes DIN-1 binding [daf-12(rh61dh115)] (Ludewig et al. 2004) restored a wild-type rate of leaving. A similar high rate of leaving was obtained when the entire DIN-1 binding domain was deleted but the DBD remained intact [daf-12(m419) and (daf-12(m20)]. Thus it appears that the DAF-12 N-terminal region containing the DBD is capable of activating exploratory behavior. This effect was specific to males, since daf-12(m419) and daf-12(m20) hermaphrodites (PL = 0.011 and 0.010, respectively) remained on food like wild-type hermaphrodites (PL = 0.007).
Figure 2.—
Correspondence of DAF-12 protein structure with exploratory behavior for a series of C-terminal deletion alleles. Deletion of the LBD excluding the corepressor binding region decreases exploratory behavior. Introduction of a point mutation that abolishes DIN-1 binding (red “x”) restores exploratory behavior, as does deletion of the LBD together with the DIN-1 binding region. Error bars are 95% confidence intervals.
As expected for a ligand-independent form of the protein, daf-12(m20) suppressed the slow leaving rate of ligand-defective daf-9 males. The double mutant daf-12(m20);daf-9(e1406) left food with a rate as high as or even higher than either daf-12(+) or daf-12(m20) (Table 1). daf-9(e1406) is a null allele of daf-9 that can be used in double combination with daf-12(m20) because daf-12(m20) suppresses the dauer-constitutive phenotype of daf-9(e1406). A higher rate of leaving in the double mutant over that in daf-12(m20) could be because of upregulation of daf-12 expression in the absence of ligand (Gerisch and Antebi 2004; Mak and Ruvkun 2004). Restoration of a high leaving rate to daf-9(e1406) by daf-12(m20) indicated that DAF-9, and by implication DA, acts solely through the DAF-12 receptor to affect behavior.
daf-12 functions in the adult as well as during development to stimulate exploratory behavior:
Rescue of exploratory behavior to daf-9 males by administering DA throughout postembryonic larval development and for 12 hr during adulthood, but not for 12 hr during adulthood alone, indicated that liganded DAF-12 had an organizational function in development of a male nervous system capable of supporting exploratory behavior. To determine whether daf-12 also had an activational function to stimulate exploratory behavior, we carried out a temperature-shift experiment with a temperature-sensitive allele of daf-12. Males homozygous for the temperature-sensitive mutation daf-12(rh193ts), when raised at permissive temperature and then shifted to restrictive temperature as adults, had a lower rate of exploratory behavior than similarly treated wild-type males (Figure 1E). Hence daf-12 has an adult function in stimulating exploratory behavior as well as a role during development.
daf-12 is required for differentiation of male-specific neurons:
One possible organizational function of daf-12 is in development of male-specific neural structures. Incompletely penetrant defects in male development in daf-12 mutants have been reported previously, including delayed cell division during L3 lineages leading to sex-specific ray neurons and incomplete tail spike retraction and fan morphogenesis during L4 (Antebi et al. 1998). The 18 bilateral pairs of ray sensory neurons stimulate exploratory behavior when mates are absent and detect mates when they are present (Barrios and Emmons 2008). We therefore examined development of ray neurons in daf-12 mutants and observed abnormalities in axonal outgrowth. Ray neurons are born and differentiate during the L4 larval stage, at which time they send out axonal processes through commissures from the lumbar ganglia into the pre-anal ganglion (Sulston et al. 1980; Jia and Emmons 2006). In 37% of daf-12(m20) and 21% of daf-12(m583) mutants ray neuron axons showed guidance defects while following commissures to the pre-anal ganglion (data not shown). Thus daf-12 plays a role in differentiation of the male nervous system. However, since alleles of daf-12 having opposite effects on exploratory behavior had a similar proportion of abnormal ray axons, the extent to which ray neuron defects contribute to the behavioral abnormality in daf-12 mutants remains unclear.
Regulation of exploratory behavior by starvation and hermaphrodite signals is independent of daf-12:
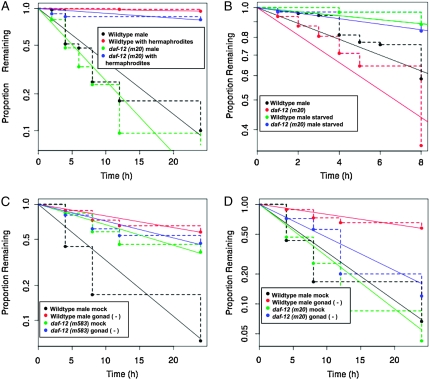
In addition to maturity and presence of the gonad, male exploratory behavior is regulated by presence of mates and nutritional status (Lipton et al. 2004). To determine the relationship of these pathways to daf-12 signaling, we studied the regulation of mate searching in daf-12 mutants. Homozygous males were fully retained by hermaphrodites for all daf-12 alleles tested, including an allele missing the C-terminal portion of the LBD, daf-12(rh84), an allele missing the entire LBD including the DIN-1 binding region, daf-12(m20), and the presumptive null allele daf-12(m583) (Figure 3A and data not shown). Retention of ligand-independent daf-12(m20) males indicated that hermaphrodite signals blocked male exploratory activity downstream of daf-12 activity. Likewise, retention of the daf-12(m583) presumptive null mutant males indicated that the hermaphrodite signal did not block exploratory behavior via the daf-12 inhibitory activity.
Figure 3.—
Relationship between stimulation of exploratory behavior by daf-12 and that of other signaling pathways that regulate male exploratory behavior. (A) Hermaphrodites inhibit exploratory behavior of males homozygous for ligand-independent daf-12(m20). The effect of five paralyzed (unc-51) hermaphrodites on mate searching is shown. (B) A signal indicating nutritional status inhibits exploratory behavior of males homozygous for ligand-independent daf-12(m20). The effect of 12-hr food deprivation on wild-type vs. daf-12(m20) males is shown. Data are given for the first 8 hr of the leaving assay and censored thereafter since after this time the leaving rate returns to the fed rate. (C) The gonad requires daf-12 function to stimulate exploratory behavior. In males homozygous for the daf-12 presumptive null allele m583, ablation of the gonad has little or no effect on leaving rate. (D) Ligand-independent daf-12(m20) stimulates exploratory behavior independently of the gonad. Wild-type mock ablated and gonad ablated data are the same in C and D.
After a period of food deprivation, the leaving rate of daf-12(m20) males was decreased in a manner similar to wild type (Figure 3B). Thus, like the hermaphrodite signal, the signal indicating starvation also appears to act downstream of daf-12 activity.
daf-12 acts downstream of the gonad to stimulate exploratory behavior:
To determine the relationship between the gonad signal that stimulates mate searching and daf-12 activity, we examined the leaving behavior of daf-12 mutants after ablation of the gonad (germ line plus somatic gonad). Stimulation of mate searching by daf-12 and the gonad might be because both signals acted in a single regulatory pathway. Alternatively, each might act in a separate, parallel pathway. We examined the effect of gonad ablation in a daf-12 null background and found that this caused no further decrease in leaving rate over that in the unoperated daf-12 null (Figure 3C). Since the effects of ablation and mutation were not additive, this suggested that both signals acted in the same pathway.
To order the actions of gonad signaling and daf-12 activity in this pathway, we examined the effect of gonad ablation on males carrying the ligand-independent daf-12(m20) allele. If daf-12(m20) acted upstream, possibly during gonad maturation, or to stimulate signaling by the mature gonad, then gonad-ablated daf-12(m20) males were expected to have a slow leaving rate like gonad-ablated wild-type males. However, if daf-12(m20) acted downstream of gonad signaling, then gonad-ablated daf-12(m20) males should leave at a high rate similar to unoperated daf-12(m20) males. We found that, unlike wild-type males, the leaving rate of daf-12(m20) males was not decreased by ablation of the gonad (Figure 3D). We conclude that daf-12 is expressed outside of the gonad to stimulate mate searching and this expression is unaffected by gonad ablation. Further, the targets of daf-12 signaling to stimulate exploratory behavior are outside the gonad and function independently of the gonad signal.
A DA precursor rescues the effect of germ-line but not whole gonad ablation:
To determine whether the gonad signal that stimulated exploratory behavior could be DA, we examined the ability of the DA precursor 4-cholestene-3-one to rescue exploratory behavior after germ-line or whole gonad ablation. DA itself was not available to us in sufficient quantity to directly test its ability to rescue gonad-ablated males. However, 4-cholestene-3-one is converted to DA by DAF-9 (Motola et al. 2006; Rottiers et al. 2006). Hence, a tissue that secretes DA is expected to express DAF-9. We considered two possibilities: either the gonad expressed daf-9 and synthesized DA or the gonad signal acted by stimulating another tissue to secrete DA, by stimulating synthesis of the DA precursor, by inducing expression of daf-9, or both. If the gonad was the site of daf-9 expression required for synthesis of DA, then it would not be possible to rescue gonad-ablated animals with the DAF-9 substrate. However, if the gonad signal acted via another tissue, then gonad-ablated animals might be rescued.
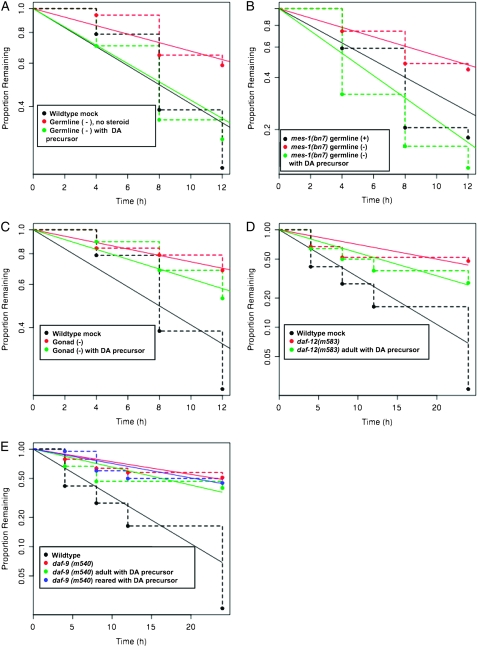
We found that 4-cholestene-3-one could rescue exploratory behavior of germ-line ablated males. Ablated adult males were tested for leaving behavior and then were placed on plates containing 25 μm 4-cholestene-3-one for 12 hr and retested on plates without drug. Leaving rate was reduced by ablation of the germ line and restored to that of unoperated animals after treatment (Figure 4A). We also tested the germ-line defective strain mes-1(bn7), in which the germ line fails to proliferate in ∼50% of animals (Capowski et al. 1991). Leaving rate was reduced in those adult mes-1(bn7) males that lacked germ cells and was restored by 12 hr treatment with 4-cholestene-3-one (Figure 4B). Exploratory behavior of males mutant for daf-12 or daf-9 was not rescued by 12 hr 4-cholestene-3-one treatment, consistent with the conclusion that 4-cholestene-3-one did not act through a daf-12-independent pathway (Figure 4, D and E). The behavior of wild-type adult hermaphrodites was also not affected by 12 hr 4-cholestene-3-one treatment (data not shown). Thus it appears that the effect of 4-cholestene-3-one is specific to the daf-12 pathway in males. Since germ-line ablated or defective males were rescued by 12 hr treatment with 4-cholestene-3-one, it appears that in germ-line defective animals 4-cholestene-3-one is rate limiting for exploratory behavior. Since the presumptive conversion of 4-cholestene-3-one to DA does not require the germ line, the germ line is not the site of DAF-9 activity and hence the germ line is unlikely to be the normal source of DA that stimulates exploratory behavior. However, it remained possible that 4-cholestene-3-one treatment acted by providing an ectopic source of DA not normally involved in regulating exploratory behavior.
Figure 4.—
Stimulation of male exploratory behavior by the DA precursor after germ-line but not whole gonad ablation. (A) The DA precursor can rescue germ-line ablated adult males. (B) The DA precursor can rescue germ-line defective mes-1(bn7) males. (C) In gonad ablated males the DA precursor cannot rescue the mate searching defect. Wild-type mock ablated data are the same as in A. (D) The DA precursor fails to rescue mate searching in the daf-12 null animals. (E) The DA precursor fails to rescue daf-9(m540) adult males. Wild-type data are the same as in D.
A different result was obtained when the entire gonad, germ line plus somatic cells, was ablated. Treatment of whole gonad ablated adult males with 4-cholestene-3-one as described above did not restore the normal rate of exploratory behavior (Figure 4C). We conclude that the somatic gonad may be necessary for expression of daf-9 in the adult, either because daf-9 is expressed within this tissue itself or because expression of daf-9 elsewhere is stimulated by a signal from the somatic gonad. Because rescue by 4-cholestene-3-one treatment requires the somatic gonad, activation of an ectopic, gonad-independent source of DA by 4-cholestene-3-one treatment appeared unlikely. Alternatively, the somatic gonad might be necessary during larval development to promote the organizational effects of the daf-12 pathway, such that the adult is no longer responsive to DA.
A pair of putative neuroendocrine cells in the head, known as XXX cells, express a daf-9∷GFP reporter gene (Gerisch and Antebi 2004). We ablated the XXX cells in adult males and in L3 males and observed no effect on leaving behavior (data not shown). Expression of daf-9∷GFP has also been reported in the hermaphrodite spermatheca (Gerisch and Antebi 2004). We examined the daf-9∷GFP expression pattern in males and observed expression within the pharynx, gut, and hypodermis, as well as in the XXX cells, but did not observe expression in the male gonad. Thus whether daf-9 is expressed in the somatic gonad of the male or elsewhere to stimulate exploratory behavior remains unresolved.
DISCUSSION
Coordination of animal behavior with the needs of the organism and its physiology is essential for survival and reproduction. Synchronization of reproductive behavior with gonadal status is achieved in many cases through elaboration of hormones by the gonad. Here we show that in C. elegans, a gonadal signal functions through the daf-12 NR to stimulate a male-specific exploratory behavior that serves to bring males together with mates. daf-12 functions in nongonadal tissues for full expression of exploratory behavior, while the gonad is required for generation of the DAF-12 ligand, DA, either directly, by synthesizing DA itself, or indirectly through secondary signals.
Among the 284 predicted NR genes in the C. elegans genome, daf-12 has been the most extensively studied (Antebi 2006). Initially identified by its function at a larval choice point between a rapid, reproductive developmental pathway and a diapause (dauer) pathway favoring survival and dispersal, daf-12 is expressed widely in the tissues and acts to coordinate diverse life-history traits, including developmental timing and adult longevity (Larsen et al. 1995; Riddle and Albert 1997; Antebi et al. 1998; Gems et al. 1998). Like other NRs, both liganded and unliganded forms of DAF-12 have biological function. In general, the liganded form of DAF-12 promotes the reproductive choice and its proper execution, while the unliganded form in conjunction with the corepressor protein DIN-1 functions to promote the survival pathway. However, ligand-defective mutants form abnormal dauer larvae, so this distinction is not absolute (Gerisch et al. 2001; Jia et al. 2002). Our results demonstrate for the first time an effect of daf-12 on behavior and are consistent with the generalization that liganded DAF-12 promotes reproduction and unliganded DAF-12 promotes survival. With respect to behavior, DAF-12 in the presence of ligand promotes the reproductive choice—male exploration for mates—while DAF-12 in the absence of ligand binding, together with DIN-1, promotes survival behavior—remaining on food.
While daf-12 function is necessary for full expression of exploratory behavior by males, it is not sufficient to induce this behavior in hermaphrodites. Whereas males homozygous for a ligand-independent mutant form of DAF-12, DAF-12(m20), expressed a wild-type rate of exploratory behavior, hermaphrodites homozygous for this allele did not express exploratory behavior, nor did they after treatment throughout development with DA. Thus expression of the DAF-12 ligand only in males does not appear to be a sufficient explanation to account for a male-specific behavioral pattern. Additional sexual dimorphism in the nervous system not requiring function of the daf-12 pathway presumably accounts for the difference in response of the two sexes (Portman 2007).
daf-12 functions independently of the gonad somewhere in nongonadal tissues. Ultimately, the activity of daf-12 must converge on the nervous system to influence behavior. However, daf-12 is expressed broadly and is important in the coordination of major life-history events across anatomical loci (Antebi et al. 2000). For example, daf-12 mutants uncouple developmental timing events across diverse tissues such as the hypodermis, the intestine, and the gonad (Antebi et al. 1998, 2000). While the site of daf-12 action in the aging pathway is not yet known, daf-12 appears to act directly or indirectly through the intestine to effect changes in longevity after germ-cell ablation (Hsin and Kenyon 1999; Libina et al. 2003; Berman and Kenyon 2006). Since the intestine is an important endocrine tissue (Libina et al. 2003), daf-12 might act through the intestine to affect changes in male exploratory behavior by changing downstream endocrine signals that interact with the nervous system.
Likewise, the source or sources of DA required for either the organizational or the activational effects of daf-12 on behavior are unknown. In view of the possibility raised by our results that DA is generated by the somatic cells of the gonad, expression of a daf-9∷GFP reporter in the spermatheca of the hermaphrodite is suggestive. Possibly lack of observed expression of this reporter in the male gonad is a reporter gene artifact. However, a source of DA outside the gonad is not excluded by our data. We tested two likely candidates, the paired XXX putative neuroendocrine cells in the head, and found they seemed not to be required. The source of DA that stimulates reproductive growth has not been established and could be distributed over multiple tissues (Rottiers et al. 2006).
Our data suggest the germ line and somatic portions of the gonad produce different signals affecting behavior. The germ-line signal is unlikely to be DA itself, since a precursor to DA, 4-cholestene-3-one, requiring the function of DAF-9 for conversion to DA, could rescue exploratory behavior in germ-line defective males. Thus, daf-9 must be expressed outside of the germ line. Since the effect of whole gonad ablation could not be similarly rescued, the signal from the somatic cells must be different from the germ-line signal. Similarly, the somatic gonad and the germ line have been shown to function in different ways to regulate the rate of aging (Hsin and Kenyon 1999; Alcedo and Kenyon 2004; Yamawaki et al. 2008). The simplest hypothesis to account for our observations is that the germ cells generate 4-cholestene-3-one, which is converted to DA by daf-9 expressed in the gonadal somatic cells. However, it is also possible that the germ-line and somatic gonad signals are unrelated molecules that stimulate DA production by other tissues. Resolution of these issues awaits mosaic analysis of daf-9 gene function.
Acknowledgments
We thank G. Ruvkun, K. Nehrke, A. Antebi, D. Mangelsdorf, and I. Katsura for strains and materials. We thank D. Portman, R. Azevedo, R. Garcia, A. Barrios, R. Ghosh, J. Lenz, M. Charron, A. Etgen, H. Buelow, D. Hall, J. Y. Sze, J. Landis, and J. Lipton for valuable discussions and advice. C. Smith and J. Di Mele provided expert technical assistance. Some nematode strains used were provided by the C. elegans Genetics Center. This work was supported by National Institutes of Health grants to S.W.E. S.W.E. is the Siegfried Ullmann Professor of Genetics.
References
- Alcedo, J., and C. Kenyon, 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41 45–55. [DOI] [PubMed] [Google Scholar]
- Antebi, A., 2006. Nuclear hormone receptors in C. elegans, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Antebi, A., J. G. Culotti and E. M. Hedgecock, 1998. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125 1191–1205. [DOI] [PubMed] [Google Scholar]
- Antebi, A., W.-H. Yeh, D. Tait, E. M. Hedgecock and D. L. Riddle, 2000. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 14 1512–1527. [PMC free article] [PubMed] [Google Scholar]
- Bargmann, C. I., and H. R. Horvitz, 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7 729–742. [DOI] [PubMed] [Google Scholar]
- Barr, M. M., and L. R. Garcia, 2006. Male mating behavior, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Barrios, A., S. Nurrish and S. W. Emmons, 2008. Sensory regulation of C. elegans male mate-searching behavior. Curr. Biol. (in press). [DOI] [PMC free article] [PubMed]
- Baum, M. J., 2002. Neuroendocrinology of sexual behavior in the male, pp. 153–203 in Behavioral Endocrinology, edited by J. B. Becker, S. M. Breedlove, D. Crews and M. M. McCarthy. MIT Press, Cambridge, MA.
- Berman, J., and C. Kenyon, 2006. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124 1055–1068. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broue, F., P. Liere, C. Kenyon and E. E. Baulieu, 2007. A steroid hormone that extends the lifespan of Caenorhabditis elegans. Aging Cell 6 87–94. [DOI] [PubMed] [Google Scholar]
- Capowski, E. E., P. Martin, C. Garvin and S. Strome, 1991. Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics 129 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, S. C., 2002. Hormonal influences on human sexual behavior, pp. 205–222 in Behavioral Endocrinology, edited by J. B. Becker, S. M. Breedlove, D. Crews and M. M. McCarthy. MIT Press, Cambridge, MA.
- Chalfie, M., and J. G. White, 1988. The nervous system, pp. 337–391 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Chasnov, J. R., W. K. So, C. M. Chan and K. L. Chow, 2007. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc. Natl. Acad. Sci. USA 104 6730–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono, M., and A. V. Maricq, 2005. Neuronal substrates of complex behaviors in C. elegans. Annu. Rev. Neurosci. 28 451–501. [DOI] [PubMed] [Google Scholar]
- Emmons, S. W., 2006. Sexual behavior of the Caenorhabditis elegans male. Int. Rev. Neurobiol. 69 99–123. [DOI] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch, B., and A. Antebi, 2004. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development 131 1765–1776. [DOI] [PubMed] [Google Scholar]
- Gerisch, B., C. Weitzel, C. Kober-Eisermann, V. Rottiers and A. Antebi, 2001. A hormonal signaling pathway influencing C. elegans matabolism, reproductive development, and life span. Dev. Cell 1 841–851. [DOI] [PubMed] [Google Scholar]
- Gerisch, B., V. Rottiers, D. Li, D. L. Motola, C. L. Cummins et al., 2007. A bile acid-like steroid modulates Caenorhabidits elegans lifespan through nuclear receptor signaling. Proc. Natl. Acad. Sci. USA 104 5014–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofflot, F., N. Chartoire, L. Vasseur, S. Heikkinen, D. Dembele et al., 2007. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell 131 405–418. [DOI] [PubMed] [Google Scholar]
- Hsin, H., and C. Kenyon, 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399 362–366. [DOI] [PubMed] [Google Scholar]
- Jia, L., and S. W. Emmons, 2006. Genes that control ray sensory neuron axon development in the Caenorhabditis elegans male. Genetics 173 1241–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, K., P. S. Albert and D. L. Riddle, 2002. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129 221–231. [DOI] [PubMed] [Google Scholar]
- Larsen, P. L., P. S. Albert and D. L. Riddle, 1995. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina, H., J. Berman and C. Kenyon, 2003. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489–502. [DOI] [PubMed] [Google Scholar]
- Lipton, J., G. Kleemann, R. Ghosh, R. Lints and S. W. Emmons, 2004. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J. Neurosci. 24 7427–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig, A. H., C. Kober-Eisermann, C. Weitzel, A. Bethke, K. Neubert et al., 2004. A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes Dev. 18 2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, H. Y., and G. Ruvkun, 2004. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development 131 1777–1786. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz et al., 1995. The nuclear receptor superfamily: the second decade. Cell 83 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel, R. L., and B. D. Sachs, 1994. The physiology of male sexual behavior, pp. 3–105 in The Physiology of Reproduction, edited by E. Knobil and J. D. Neill. Raven Press, New York.
- Morris, J. A., C. L. Jordan and S. M. Breedlove, 2004. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 7 1034–1039. [DOI] [PubMed] [Google Scholar]
- Motola, D. L., C. L. Cummins, V. Rottiers, K. K. Sharma, T. Li et al., 2006. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 124 1–15. [DOI] [PubMed] [Google Scholar]
- Nef, S., and L. F. Parada, 2000. Hormones in male sexual development. Genes Dev. 14 3075–3086. [DOI] [PubMed] [Google Scholar]
- Ohkura, K., N. Suzuki, T. Ishihara and I. Katsura, 2003. SDF-9, a protein tyrosine phosphatase-like molecule, regulates the L3/dauer developmental decision through hormonal signaling in C. elegans. Development 130 3237–3248. [DOI] [PubMed] [Google Scholar]
- Pfaus, J. G., 1999. Neurobiology of sexual behavior. Curr. Opin. Neurobiol. 9 751–758. [DOI] [PubMed] [Google Scholar]
- Portman, D. S., 2007. Genetic control of sex differences in C. elegans neurobiology and behavior. Adv. Genet. 59 1–37. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L., and P. S. Albert, 1997. Genetic and environmental regulation of dauer larva development, pp. 739–768 in C. elegans II, edited by D. L. Riddle, B. J. Meyer, J. Priess and T. Blumenthal. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Rottiers, V., D. L. Motola, B. Gerisch, C. L. Cummins, K. Nishiwaki et al., 2006. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev. Cell 10 1–10. [DOI] [PubMed] [Google Scholar]
- Simon, J. M., and P. W. Sternberg, 2002. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99 1598–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow, M. I., and P. L. Larsen, 2000. Structure and expression of daf12: a nuclear hormone receptor with three isoforms that are involved in development and aging in Caenorhabditis elegans. Biochim Biophys Acta 1494 104–116. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., and J. Hodgkin, 1988. Methods, pp. 587–606 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sulston, J. E., D. G. Albertson and J. N. Thomson, 1980. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev. Biol. 78 542–576. [DOI] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Ser. B Biol. Sci. 314 1–340. [DOI] [PubMed] [Google Scholar]
- White, J. Q., T. J. Nicholas, J. Gritton, L. Truong, E. R. Davidson et al., 2007. The sensory circuitry for sexual attraction in C. elegans males. Curr. Biol. 17 1847–1857. [DOI] [PubMed] [Google Scholar]
- Yamawaki, T. M., N. Arantes-Oliveira, J. R. Berman, P. Zhang and C. Kenyon, 2008. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans' longevity. Genetics 178 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]