Abstract
Mitosis is triggered by activation of Cdk1, a cyclin-dependent kinase. Conserved checkpoint mechanisms normally inhibit Cdk1 by inhibitory phosphorylation during interphase, ensuring that DNA replication and repair is completed before cells begin mitosis. In metazoans, this regulatory mechanism is also used to coordinate cell division with critical developmental processes, such as cell invagination. Two types of Cdk1 inhibitory kinases have been found in metazoans. They differ in subcellular localization and Cdk1 target-site specificity: one (Wee1) being nuclear and the other (Myt1), membrane-associated and cytoplasmic. Drosophila has one representative of each: dMyt1 and dWee1. Although dWee1 and dMyt1 are not essential for zygotic viability, loss of both resulted in synthetic lethality, indicating that they are partially functionally redundant. Bristle defects in myt1 mutant adult flies prompted a phenotypic analysis that revealed cell-cycle defects, ectopic apoptosis, and abnormal responses to ionizing radiation in the myt1 mutant imaginal wing discs that give rise to these mechanosensory organs. Cdk1 inhibitory phosphorylation was also aberrant in these myt1 mutant imaginal wing discs, indicating that dMyt1 serves Cdk1 regulatory functions that are important both for normal cell-cycle progression and for coordinating mitosis with critical developmental processes.
Cdk1 is a conserved cyclin-dependent kinase, whose activity is responsible for promoting the dramatic cellular rearrangements associated with mitosis (Nigg et al. 1991; Masui 1992). During interphase, Cdk1 must be maintained in an inactive state by Wee1-related Cdk1 inhibitory kinases, otherwise premature initiation of mitotic events would disrupt essential cellular processes and cause cell lethality by mitotic catastrophe (Lundgren et al. 1991; Grosshans and Wieschaus 2000; Mata et al. 2000; Seher and Leptin 2000). Cell division must also be coordinated with critical developmental processes, such as cell movements and cell shape changes. This is accomplished during most Drosophila somatic cell cycles by regulating the expression of a Cdc25-related phosphatase that releases Cdk1 from inhibitory phosphorylation at the G2/M transition (Edgar and O'Farrell 1989, 1990; Lehman et al. 1999). Much less is known about specific developmental roles of the two types of Cdk1 inhibitory kinases, however, a question further complicated in many organisms by the presence of more than one Wee1 homolog (Wilson et al. 1999; Nakanishi et al. 2000; Leise and Mueller 2002; Okamoto et al. 2002). To address this issue, we have undertaken a genetic analysis of the Drosophila Cdk1 inhibitory kinases.
Drosophila has only one representative of each type of metazoan Cdk1 inhibitory kinase: designated dWee1 and dMyt1. We showed previously that dWee1 regulation of Cdk1 is essential for a premitotic checkpoint mechanism that prevents mitotic catastrophe during the rapid S/M nuclear cycles of early embryogenesis (Price et al. 2000; Stumpff et al. 2004). Zygotic wee1 mutants are viable with no obvious developmental defects although they are sensitive to the DNA replication inhibitor hydroxyurea, suggesting they are impaired for a DNA replication checkpoint (Price et al. 2000). Loss-of-function studies of a mouse Wee1 homolog showed similar defects in rapidly cycling embryonic cells, indicating that this is a conserved developmental role for Wee1-like kinases (Price et al. 2000; Tominaga et al. 2006).
Myt1 was originally discovered in Xenopus as a membrane-associated Cdk1 inhibitory kinase capable of catalyzing Cdk1 inhibitory phosphorylation on both the Y15 and T14 residues (Kornbluth et al. 1994; Mueller et al. 1995). Myt1 kinases also physically interact with Cdk1 complexes through a protein motif that binds to B-type mitotic cyclins (Liu et al. 1999; Wells et al. 1999). This interaction is thought to be responsible for tethering inhibited Cdk1 complexes in the cytoplasm as they accumulate during G2 phase. Thus, Myt1 can potentially regulate Cdk1 by two distinct mechanisms, one of which is kinase independent.
Myt1 activity is negatively regulated during oocyte maturation in many organisms, consistent with a role for Myt1 in inhibiting Cdk1 during meiotic G2 phase (Palmer et al. 1998; Lamitina and L'Hernault 2002; Leise and Mueller 2002; Okumura et al. 2002; Peter et al. 2002; Inoue and Sagata 2005; Burrows et al. 2006). Whether Myt1 has a role in Drosophila oocyte maturation remains unclear (Ivanovska et al. 2004), however, Drosophila myt1 mutants exhibit pleiotropic cell-cycle defects during male and female gametogenesis, which suggest that dMyt1 has a role in developmentally regulated G2 phase arrest and in cell-cycle exit mechanisms that are normally coupled with terminal differentiation (Jin et al. 2005).
Studies in cultured mammalian cells have also recently implicated Myt1 in a novel Cdk1 regulatory mechanism that is important for proper assembly of the Golgi network and endoplasmic reticulum during mitotic exit (Nakajima et al. 2008). The generality of this mechanism and its possible relevance to specialized developmental functions of Myt1 kinases has not yet been established, however.
In this study, we characterized mutant phenotypes as well as Cdk1 inhibitory phosphorylation associated with loss of dMyt1 and dWee1 activity in larval imaginal wing discs and in adult structures derived from this tissue. The results identified dMyt1 as the major Cdk1 inhibitory kinase operating at these stages of development. In comparison, loss of dWee1 activity caused relatively minor cellular and developmental effects, unless dMyt1 functions were also compromised. We also found evidence that dMyt1 is required for normal cellular responses to ionizing radiation. These observations must be incorporated into models for understanding the role of dMyt1 in coordinating cell-cycle progression with critical developmental events.
MATERIALS AND METHODS
Genetic strains:
In a previously published study of myt1 mutants, we analyzed myt11/Df(3L)64D-F hemizygote and myt11/myt12 transheterozygote genotypes (Garcia-Bellido et al. 1994; Jin et al. 2005). These genotypes exhibited identical gametogenesis and macrochaetae defects, indicating that myt11 and myt12 were null alleles. Both of these alleles were later found to have identical mutations in the myt1 coding region, indicating they were likely preexisting mutations that were isolated on chromosomes that had subsequently acquired different second-site lethal alleles during EMS mutagenesis (Jin et al. 2005). To make genetic manipulations easier for subsequent analysis of myt1 mutant phenotypes, we used meiotic recombination to remove secondary lethal mutations from the original myt11 mutant chromosome, and thereby reisolated a homozygous viable myt11 allele. The phenotype of homozygous viable myt11/myt11 mutants was identical to what was observed for myt11/Df(3L)64D-F and myt11/Df(3L)CH39 hemizygotes, two different chromosomal deletions that uncover the myt1 locus (Extavour and Garcia-Bellido 2001). Genetic interactions with wee1 were analyzed with a null allele (weeES1), a hypomorphic allele (wee1DS1), and a deletion, Df(2L)wee1W05, all of which were previously described (Price et al. 2000).
Immunofluorescent analysis of larval wing discs:
For the larval checkpoint assays, wandering third instar larvae were transferred into fresh vials and then irradiated by a Co60 γ-ray source, calibrated to administer a dosage of 40 Gy. In these experiments, myt1 mutants were identified as nonbalancer larvae, by the Tubby marker on the TM6B balancer chromosome, whereas wee1 mutants were identified by the actin-GFP transgene inserted on the SM6 balancer chromosome. An otherwise wild-type yw stock was used as the control genotype for these experiments. Wing imaginal discs were dissected from the larvae for fixation in 3.7% formaldehyde (buffered with 1× PBS) at room temperature for 20 min, washed twice with 1× PBS, and then permeabilized in 1× PBS containing 0.5% Triton X-100 for 30 min. The discs were then processed for immunofluorescent labeling by standard procedures. Primary antibodies and concentrations were: rabbit anti-phospho-S10-histone 3 (1/4000; Upstate), rabbit anti-cleaved caspase-3 (1/500, Cell Signaling Technology), mouse MAb 2B10 anti-Cut (1/200, Developmental Studies Hybridoma Bank). Secondary antibodies conjugated with Alexa Fluor-488 or Alexa Fluor-568 were used at a 1/1000 working dilution (Molecular Probes). Microscopy images were acquired with either a Zeiss Axioskop or a Leica TCS-SP2 multiphoton confocal laser scanning microscope (TCS-MP). The imaginal discs shown in Figures 2 and 3 were composed of more than one overlapping image to include the whole disc and these images were deconvolved using iterative restoration by Volocity software. All of the figures were compiled using Adobe Photoshop software; identical image manipulations were applied to control and experimental panels to prepare them for printing. PH3-positive nuclei of the widefield images used to compare yw, wee1, myt1, and wee1; myt1, as shown in Figure 2, were counted manually. The particle analysis function of ImageJ software (NIH) was used to count PH3-positive nuclei of the confocal images to generate the data shown in Figure 5. The area of activated caspase-3 staining in the wing disc was also determined using ImageJ software. All quantification was performed on a 200–273 μm2 area centered on the wing pouch of 3–7 imaginal discs.
Figure 2.—
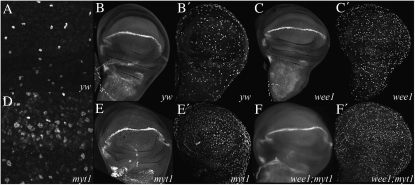
myt1 mutants have increased incidence of mitosis in imaginal wing discs. Wing discs from wandering third instar larvae were fixed and stained with rabbit anti-phospho-histone 3 (PH3) antibodies (A, B′, C′, D, E′, and F′) and mouse anti-Cut antibodies (B, C, E, and F). Sections A and D show differences in PH3 staining between wild-type yw controls (A) and homozygous viable myt11 mutants (D), using confocal microscopy. In wide-field images, Cut and PH3 labeling were shown for yw controls (B and B′), for wee1ES1/Df(2L)weeW05 mutants (C and C′), for homozygous viable myt11/myt11 mutants (E and E′), and for wee1ES1/wee1DS1; myt11/myt11 mutants (F and F′).
Figure 3.—
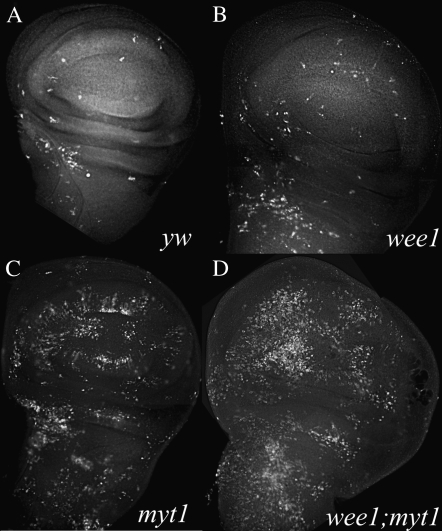
myt1 mutants exhibit increased incidence of apoptosis in imaginal wing discs. Shown are wing discs from wandering third instar larvae that were immunolabeled with antibodies against activated caspase-3 as a marker for apoptosis (Yu et al. 2002). Relatively few apoptotic cells were observed in yw controls (A) or in wee1ES1/Df(2L)weeWO5 (B) mutants. There was a noticeable increase in apoptotic cells in myt11/myt11 (C) and wee1ES1/wee1DS1; myt11/myt11 double mutants (D).
Figure 5.—
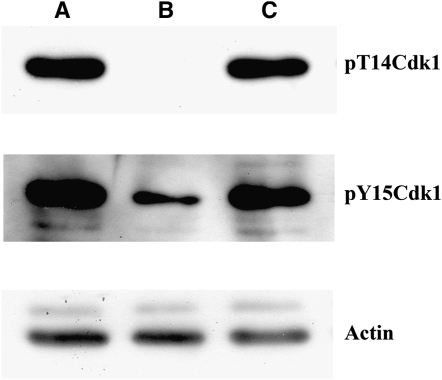
Cdk1 inhibitory phosphorylation defects in myt1 mutant wing discs. Protein extract samples were each prepared from 10 dissected wing discs and then separated by SDS–PAGE, then probed serially with antibodies specific to the pT14-Cdk1 isoform, Actin (as a loading control), and the pY15-Cdk1 isoform (after stripping). (A) The yw wild-type control extract was immunolabeled for both the pT14-Cdk1 and the pY15-Cdk1 isoforms, as expected. (B) In a myt11/myt11 mutant extract, the pT14-Cdk1 isoform was completely absent and the pY15-Cdk1 isoform was markedly reduced. (C) In a wee1ES1/Df(2L)weeWO5 mutant extract, Cdk1 inhibitory phosphorylation appeared normal for both isoforms.
Scanning electron microscopy of adult structures:
Adult flies were fixed for 2 hr in 1% glutaraldehyde:1% formaldehyde in 1 m sodium cacodylate, pH 7.2, with a drop of 0.2% Tween-20 to reduce the surface tension. Following fixation, samples were rinsed with distilled water and dehydrated by passage through a graded ethanol series (once each with 25, 50, and 75%, twice with absolute ethanol). The samples were mounted, gold coated, and then imaged using a Philips/FEI LaB6 environmental scanning electron microscope (ESEM).
Biochemical analysis of Cdk1 inhibitory phosphorylation:
Third instar imaginal wing discs were dissected from the appropriate genotypes and placed in 1× PBS on ice. For each sample, 10 wing discs were homogenized in SDS–PAGE sample buffer and boiled for 5 min. The proteins were separated by electrophoresis on a 10% acrylamide gel containing 2 mm vanadate and 10 mm NaF as phosphatase inhibitors, then transferred to a Hybond P membrane blot (Amersham). The blot was probed with a 1:1000 dilution of rabbit antibodies directed against pT14-Cdk1 (Cell Signaling Technology) overnight at 4°. As a loading control, the blot was reprobed with a 1/1000 dilution of antibodies against actin (Mab1501, Chemicon). The blot was stripped according to manufacturer's instructions and then reprobed with a 1:1000 dilution of rabbit antibodies directed against pY15-CdK1 (Cell Signaling Technology) overnight at 4°. Proteins were detected using anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase diluted 1:10,000 (Amersham) and a GE Healthcare ECL Plus chemiluminescence kit.
RESULTS
Drosophila Myt1 is required for head and thoracic macrochaetae development:
In a previous study of myt11/Df(3L)64D-F hemizygous mutants that focused on male and female gametogenesis (Jin et al. 2005), we noted additional developmental defects affecting large sensory bristles called macrochaetae, located on the adult thorax and head. Further examination revealed similar bristle phenotypes in Df(3L)CH39/myt11 hemizygotes, as well as recombinant, homozygous viable myt11 mutants that were reisolated for this study (see materials and methods). These myt1 mutant macrochaetae defects were complemented by a P{w+, myt1+} genomic rescue transgene (Jin et al. 2005) and by expressing a P{UASp-dMyt1+} cDNA transgene (Price et al. 2002) with a Neu-Gal4 transgene driver line (not shown), confirming that they were due to loss of dMyt1 activity.
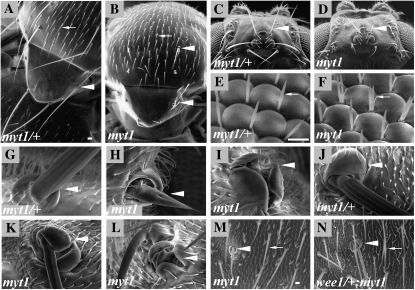
To further investigate the role of dMyt1 in macrochaetae development, we first classified the types and frequencies of defects observed in the thoracic macrochaetae of myt1 mutants. These structures occupy specific locations on the head and thorax of heterozygous control adults (Figure 1, A, C, and G). The pattern and morphology of these macrochaetae was disrupted in myt11/Df(3L)64D-F hemizygotes. Similar macrochaetae defects were also seen, to similar degrees, in myt11 homozygous viable mutants. The observed defects included bristle shafts that were shorter and thinner than normal (Figure 1, B, D, and H), bristle duplications (Figure 1B), missing macrochaetae (Figure 1, B, D, and I), and multiple socket cells (Figure 1, I and K). The myt1 mutant adults also had duplicated eye ommatidial bristles that were not seen in controls (compare Figure 1, E and F). We also observed wing blister defects in myt1 mutant adults (not shown). The macrochaetae located along the anterior wing margin appeared normal, however.
Figure 1.—
Adult macrochaetae defects observed in myt1 mutant adults. Scanning electron micrographs are shown of (A, C, E, G, and J) wild-type controls (myt11/+ heterozygotes), (B, D, F, H, I, and K–M) myt11/Df(3L)64D-F mutant or (N) wee1ES1/CyO; myt1/myt111 mutant adult flies. In (A, B, and G–N), arrowheads denote macrochaetae and arrows denote microchaetae. (A and B) Adult notum. (C and D) Adult head. (E and F) Interommatidial bristles (arrows) in the compound eye, showing duplicated interommatidial bristles in the myt1 mutant. (G–I) Unlike the heterozygous controls (G), the posterior scutellar bristles of myt1 mutants often exhibit bristles with shortened shafts (H) and/or multiple socket cells (I). (J–L) Comparison of macrochaetae on wild-type and mutant adult heads, showing that myt1 mutants exhibit similar macrochaetae defects on the head as were seen on the notum. (M) The notum of a myt1 mutant, shown at higher magnification to show normal appearing microchaetae. (N) The removal of one copy of wee1 in a myt1 mutant background resulted in frequent microchaetae duplications (white arrow). Bar for A–D, 40 μm; for E–L, 10 μm; and for M and N, 20 μm.
Since the arrangement and morphology of adult thoracic macrochaetae is highly stereotyped (Neel 1940), we decided to quantify these morphological defects by comparing homozygous myt11 mutants and heterozygous controls with respect to each of seven different classes of shaft and socket defects, as described in Table 1. Eight thoracic macrochaetae were scored for 25 adult flies of each sex (50 adults), representing a total of 400 macrochaetae analyzed for each genotype (the one indicated exception was due to insufficient numbers of progeny). In heterozygous myt11/TM6 controls, shaft and socket defects were rarely observed (2%, N = 400 macrochaetae). Homozygous viable myt11 mutants exhibited a very high frequency of macrochaetae defects however (91%, N = 400), confirming that dMyt1 activity is important for normal thoracic macrochaetae development.
TABLE 1.
Frequency of macrochaetae defects in myt1, wee1, and double mutants
| Genotype | Short shaft | 2 Sha., 2 Soc. | 0 Sha., 0 Soc. | 0 Sha., 1 Soc. | 2 Sha., 1 Soc. | 1 Sha., 2 Soc. | 0 Sha., 2 Soc. | Total bristles | Total defects | % defective |
|---|---|---|---|---|---|---|---|---|---|---|
| weeESI/CyO | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 400 | 1 | <1 |
| myt11/TM6 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 400 | 6 | 2 |
| weeESI/CyO; myt11/TM6 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 400 | 4 | 1 |
| weeESI/Df(2L)weeWO5 | 8 | 11 | 2 | 9 | 2 | 1 | 0 | 700 | 33 | 8 |
| myt11/myt11 | 251 | 66 | 31 | 12 | 3 | 2 | 0 | 400 | 365 | 91 |
| weeESI/CyO; myt11/myt11 | 278 | 31 | 24 | 16 | 40 | 28 | 2 | 400 | 419 | 105a |
| weeESI/Df; myt11/TM6 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 72 | 5 | 12.5 |
The four dorsocentral bristles on the scutum, the two anterior scutellar bristles, and the two posterior scutellar bristles (eight total) were scored for 25 males and 25 females of each genotype. The only exception was the genotype weeESI/DF(2L)weeW05; myt11/TM6, because only five females and four males of this genotype were recovered. Normal macrochaetae have a single socket (1 Soc.) and a single shaft (1 Sha.), which are consistent in length. Defects included shafts that were <75% of normal length (short shafts), complete bristle duplications (2 Sha., 2 Soc.), absent bristles (0 Sha., 0 Soc.), socket only (0 Sha., 1 Soc.), shaft duplications with single socket (2 Sha., 1 Soc.), socket duplications with a single shaft (1 Sha., 2 Soc.), and two sockets without shafts (0 Sha., 2 Soc.)
Some bristles had multiple defects, resulting in an observed frequency of >100%.
The only known enzymatic activity of Myt1 kinases is inhibitory phosphorylation of Cdk1 (Kornbluth et al. 1994; Mueller et al. 1995; Booher et al. 1997; Liu et al. 1997). If the myt1 mutant bristle phenotype was caused by a defect in Cdk1 inhibitory phosphorylation, we reasoned that a partial loss of dWee1 activity should enhance these defects. To test this hypothesis, we used wee1 alleles that were previously isolated in our laboratory to manipulate dWee1 activity levels (Price et al. 2000). As shown in Table 1, very few macrochaetae defects were observed in wee1ES1/+ heterozygote controls, using a representative null allele of wee1 (<1%, N = 400). We also observed very few bristle or socket defects in wee1ES1/+; myt11/+ double heterozygote controls (1%, N = 400). In wee1ES1/Df(2L)wee1WO5, however, thoracic macrochaetae defects were observed in ∼8% of these flies (N = 400), a developmental defect that had not previously been noticed for this genotype (Price et al. 2000). Removal of one functional copy of wee1 also enhanced the macrochaetae defects observed in myt1 mutants (105%, N = 400). Note that the frequency of macrochaetae defects summed to >100% in this genotype because macrochaetae with more than one type of defect were often observed. Collectively, these results showed that abnormalities in Cdk1 regulation by inhibitory phosphorylation resulted in thoracic macrochaetae defects, consistent with observations from earlier studies (Milan et al. 1996; Lehman et al. 1999; Tio et al. 2001; Fichelson and Gho 2004).
The wing blister defects observed in myt1 mutants were also enhanced by loss of one functional copy of wee1; however, we did not quantify this effect. A new type of defect was also observed in wee1/+; myt1 mutants, involving duplication of small bristles called microchaetae. Although microchaetae duplications were rarely observed in myt1 mutants alone (Figure 1M), they were common in homozygous myt1 mutants that were heterozygous for either wee1ES1 or wee1DS1 (Figure 1N). To quantify this mutant phenotype, we analyzed the microchaetae in a region of the thorax defined by the four dorsocentral macrochaetae. A total of 20 adults of each genotype (10 males and 10 females) were analyzed in this experiment, for four classes of microchaetae defects (Table 2). Unlike myt1 mutants alone, where only ∼1% of the microchaetae were affected, ∼20% of the microchaetae were duplicated in wee1ES1/+; myt11 mutants. The magnitude of phenotypic enhancement seen in this experiment was therefore considerably greater than that observed for the macrochaetae (Table 1). This discrepancy could mean that microchaetae development is more sensitive to lowered Cdk1 inhibitory phosphorylation than macrochaetae development. Another possibility is that the macrochaetae defects were already so severe in myt1 mutants that they could not be made much worse by further loss of dWee1 activity.
TABLE 2.
Frequency of microchaetae defects in myt1 and wee1/+; myt1 mutants
| Genotype | 2 Sha., 1 Soc. | 1 Sha., 2 Soc. | 2 Sha., 2 Soc. | 0 Sha., 1 Soc. | Total bristles | Normal bristles | Total defects | % Defective |
|---|---|---|---|---|---|---|---|---|
| myt11/myt11 | 2 | 1 | 0 | 1 | 367 | 363 | 4 | 1 |
| wee ESI/CyO; myt11/myt11 | 50 | 21 | 10 | 2 | 358 | 275 | 83 | 23 |
Microchaetae are small bristles, found on most of the adult cuticle, with a single shaft and a single socket. These are normal in myt11/myt11 single mutants but exhibit defects in wee1/+; myt11/myt11 mutants. To quantify these defects, all of the microchaetae were scored within the area bounded by the four central thoracic macrochaetae on the scutum of 10 males and 10 females of each of these genotypes. Sha., shaft; Soc., socket.
Drosophila Cdk1 inhibitory kinases are functionally redundant for zygotic viability:
Cdk1 inhibitory phosphorylation is not only essential for normal Drosophila development; it is also essential for viability (Edgar and O'Farrell 1990; Price et al. 2000; Jin et al. 2005; Prokopenko and Chia 2005). Zygotic wee1 and myt1 mutants are both relatively viable, however (Price et al. 2000; Jin et al. 2005), suggesting that dWee1 and dMyt1 may be partially redundant for essential functions. Studies of fission yeast (Schizosaccharomyces pombe) provide a precedent for such a relationship, where loss of both Cdk1 inhibitory kinases (Wee1 and Mik1) causes lethal mitotic catastrophe due to defects in the DNA replication checkpoint (Lundgren et al. 1991). These redundant functions are distinct from the G2 phase Wee1-regulated cell size checkpoint for which this Cdk1 inhibitory kinase was originally named (Nurse and Thuriaux 1980). Partial functional redundancies have also been inferred for other Cdk1 inhibitory kinases, although not directly demonstrated (Wilson et al. 1999; Nakanishi et al. 2000; Lamitina and L'Hernault 2002; Leise and Mueller 2002; Okamoto et al. 2002).
To determine whether dWee1 and dMyt1 serve partially redundant Cdk1 regulatory functions, we quantified adult viability in different mutant genotypes. As expected, most zygotic myt1 and wee1 mutant progeny developed to adulthood, at only slightly lower frequencies than their heterozygous siblings (Table 3). There was a significant reduction in viability observed when myt1 mutants were also heterozygous for wee1, however. These phenotypic interactions were influenced by the relative levels of dWee1 function, as the viability of myt1 mutants that were heterozygous for a hypomorphic wee1 allele (wee1DS1) was lowered by only ∼5-fold, whereas myt1 mutants that were heterozygous for a null allele [either wee1ES1 or a chromosomal deletion, Df(2L)wee1W05] were ∼10-fold less viable. Adult wee1; myt1 double mutants were never recovered from these genetic crosses (Table 3). Dominant genetic markers carried on the balancer chromosomes were used to identify rare wee1; myt1 double mutant homozygotes that survived until the third instar larvae and pupal stages, suggesting that synthetic lethality occurred as maternally provided dWee1 and dMyt1 gene products were progressively depleted during zygotic development. These results indicate that dWee1 and dMyt1 are partially redundant for essential Cdk1 regulatory functions, during zygotic development.
TABLE 3.
Genetic interactions between wee1 and myt1
| Genotypes | Observed (%) | Expected (%) | P-value |
|---|---|---|---|
| myt11/TM6B | 565 (75) | 503 (67) | |
| myt11/myt11 | 189 (25) | 251 (33) | ** |
| 754 (100) | 754 (100) | P < 0.001 | |
| wee1ESI or Df(2L)wee1W05/CyO | 380 (76) | 333 (67) | |
| wee1ESI/Df(2L)wee1W05 | 119 (24) | 166 (33) | ** |
| 449 (100) | 499 (100) | P < 0.001 | |
| wee1DSI/CyO; myt11/TM6B | 375 (63) | 263 (44) | |
| wee1DSI/wee1DSI; myt11/TM6B | 187 (31) | 132 (22) | |
| weelDSI/CyO; myt11/myt11 | 30 (5) | 131 (22) | |
| wee1DSI/wee1DSI; myt11/myt11 | 0 (0) | 66 (11) | ** |
| 592 (100) | 592 (100) | P < 0.001 | |
| wee1ESI or Df(2L)wee1W05/CyO; myt11/TM6B | 549 (67) | 361 (44) | |
| wee1ESI/Df(2L)wee1 W05; myt11/TM6B | 251 (30) | 181 (22) | |
| wee1ESI/CyO; myt11/myt11 | 13 (2) | 181 (22) | |
| wee1ESI/Df(2L)wee1W05; myt11/myt1 1 | 0 (0) | 90 (11) | ** |
| 813 (100) | 813 (100) | P < 0.001 |
Genetic crosses were set up to generate the indicated progeny genotype as a means of investigating genetic interactions between wee1 and myt1. Σ is the sum of individuals from all genotypes in an experiment. The indicated P-values represent the probability that the observed distribution of genotypes is not significantly different from the expected values, according to Pearson's chi-square test. **Highly significant difference.
Novel cell-cycle defects associated with deficient Cdk1 inhibitory phosphorylation:
The cells that eventually develop into the thoracic macrochaetae originate from the wing discs, so we examined this tissue for earlier phenotypic defects in myt1 mutants. We used antibodies against a phosphorylated form of histone H3 (PH3) that labels mitotic cells to examine late third instar wing discs (Hendzel et al. 1997; Brodsky et al. 2000). Using confocal microscopy, we observed normal numbers of PH3-labeled cells in discs from yw control larvae (Figure 2A). We consistently observed more PH3-labeled cells in the myt1 mutant discs (Figure 2D), however. We quantified this observation by counting PH3-labeled cells in predefined areas of third instar wing discs, imaged by wide-field microscopy (see materials and methods). We compared yw controls (Figure 2B′), wee1 mutant (Figure 2C′), myt1 mutant (Figure 2E′), and wee1; myt1 double mutant larvae (Figure 2F′). In the yw controls, the average (Avg.) number and standard deviation of PH3-labeled cells counted per unit area were 68 ± 10 cells/200 μm2; N = 7. In wee1 mutant wing discs we observed slightly more PH3-labeled cells than in the controls (Avg. = 108 ± 23 cells/200 μm2; N = 6). A notable increase in numbers of PH3-labeled cells was observed in myt1 mutants (Avg. = 179 ± 26 cells/200 μm2; N = 5) and wee1; myt1 double mutants (Avg. = 210 ± 31 cells/200 μm2; N = 3). These results suggested that loss of dMyt1 activity caused a novel cell-cycle defect in third instar imaginal wing discs.
We also noticed that the chromatin appeared to be relatively less condensed in many of the myt1 mutant PH3-labeled cells, compared with controls (compare Figure 2, A and D). This observation suggests that loss of dMyt1 activity caused defects in chromatin condensation or decondensation during mitosis, suggesting problems in a mechanism for coupling chromatin condensation with progression through mitosis.
Imaginal wing disc cells proliferate asynchronously during larval development until the late third instar, when the cells that will form the presumptive wing margin withdraw from the cell cycle and begin to differentiate (O'brochta and Bryant 1985). Some of these cells can be identified by expression of the Cut homeodomain protein (Jack and Delotto 1992; Blair 1993). To determine if loss of dMyt1 activity affected these cells, we examined Cut expression in third instar wing discs by immunolabeling. In yw controls, Cut antibody labeling marked the presumptive wing margin, as expected (Figure 2B). We observed similar results in wee1 mutants (Figure 2C), in myt1 mutants (Figure 2E), and in wee1; myt1 double mutants (Figure 2F). Thus, it appears that Cdk1 inhibitory phosphorylation is not required for differentiation of Cut expressing cells in the presumptive wing margin.
Increased levels of apoptosis in myt1 mutant wing discs:
Tissue homeostasis is partly regulated by mechanisms that balance excessive cell proliferation with elevated apoptotic cell death (Neufeld et al. 1998; Abrams 2002). Since our PH3 labeling experiments indicated there was a novel cell-cycle defect in myt1 mutants, we investigated whether apoptosis might also be affected in these mutants. To label apoptotic cells we used antibodies against the active, cleaved form of caspase-3 (Yu et al. 2002), comparing yw controls (Figure 3A), wee1 mutants (Figure 3B), myt1 mutants (Figure 3C), and wee1; myt1 double mutants (Figure 3D). In the yw controls, apoptotic cells were rarely observed. Loss of Myt1 (and to a lesser extent, dWee1) was associated with increased apoptosis, however. We quantified this mutant phenotype by analyzing predefined regions of late third instar imaginal wing discs (materials and methods). The results are presented as the average area labeled by the activated caspase-3 antibodies ± SD, expressed as a percentage of the total area. As expected, very low levels of apoptosis were observed in yw control imaginal wing discs (Avg. = 0.21 ± 0.90% of total area; N = 4). There was a small increase in the area of cells undergoing apoptosis in wee1 mutant wing discs (Avg. = 1.09 ± 0.44% of total area; N = 7), relative to controls. In myt1 wing discs, there were considerably more apoptotic cells, relative to the controls (Avg. = 6.09 ± 2.77% of total area; N = 5). This cellular defect was even more apparent in wee1; myt1 double mutant wing discs (Avg. =12.55 ± 6.02% of total area; N = 5). Thus, we have identified novel cellular defects associated with deficiencies in Cdk1 regulation by inhibitory phosphorylation. Whether these cellular defects are relevant to the morphological defects observed in adults, remains unclear.
dMyt1 activity is required for normal responses to ionizing radiation:
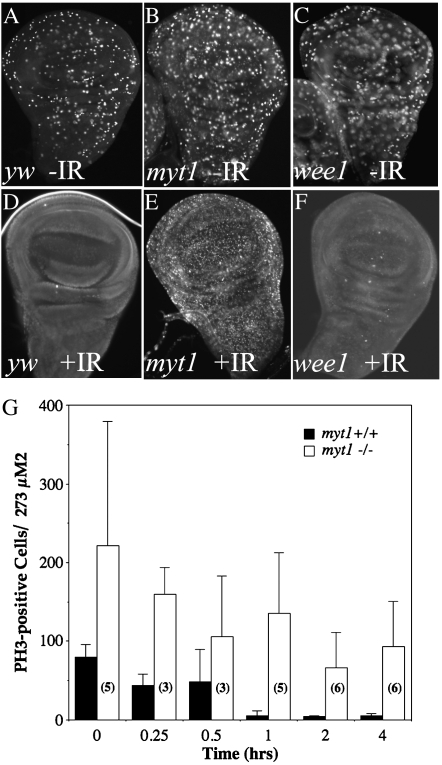
Conserved cell-cycle checkpoint responses promote Cdk1 inhibitory phosphorylation to prevent mitosis when cells sense ongoing DNA replication or repair of damaged DNA (O'connell et al. 1997; Rhind and Russell 2001). Although previous studies had implicated dWee1 in a DNA replication checkpoint response that is required for early embryonic development (Price et al. 2000), a role for dWee1 or dMyt1 in the DNA damage checkpoint had not yet been reported. To address this issue, we used PH3 antibody labeling to assay premitotic checkpoint responses in larvae that had been exposed to ionizing radiation (Brodsky et al. 2000). When yw control larvae were exposed to 40 Gy of ionizing radiation (IR), we observed that PH3-labeled cells had nearly disappeared by 1 hour after exposure (compare Figure 4, A and D). These results demonstrated that premitotic checkpoint responses were active in the controls, as expected. We also observed similar results in wee1 mutant wing discs, indicating that dWee1 activity is not required for the premitotic checkpoint response to ionizing radiation (compare Figure 4, C and F). In contrast, when myt1 mutant wing discs were examined there was only a slight reduction in the numbers of PH3-labeled cells by 1 hour after IR exposure, relative to unirradiated myt1 mutant controls (Figure 4, B and E).
Figure 4.—
myt1 mutants are defective for normal cellular responses to ionizing radiation. Wing imaginal discs were dissected from wandering third instar larvae that had been immunolabeled with antibodies against phospho-histone H3 as a marker for mitotic cells. Comparisons of unirradiated and irradiated wing discs are shown for each of the following genotypes: yw controls (A and D), myt11/myt11 mutants (B and E), and wee1ES1/Df(2L)weeWO5 mutants (C and F). The irradiated (IR) larvae were dissected 60 min after exposure to 40 Gy of ionizing radiation, in D–F. PH3-positive cells were seen in all of the unirradiated controls: yw (A), homozygous viable myt11 mutants (B), and wee1 mutant discs (C). There were almost no PH3-positive cells remaining in yw control discs or in wee1 mutant discs by 60 min after exposure to IR (D and F), indicating the presence of a functional premitotic checkpoint in these genotypes. In myt1 mutant discs (E) the PH3 antibody labeling persisted after IR exposure, suggesting a checkpoint defect. To quantify this defect, we counted the numbers of PH3-positive cells in yw control and myt1 mutant wing discs at intervals after exposure to ionizing radiation (G). The numbers (3–6) shown for each open bar indicate the number of control and mutant discs that were analyzed for each time point, with error bars indicating standard deviation.
To quantify this apparent DNA damage-response defect, we counted PH3-labeled cells to compare myt1 mutant and control imaginal discs at timed intervals after the larvae were exposed to ionizing radiation. There was a marked reduction in PH3-labeled cells in the irradiated yw control wing discs by 15 min after exposure, with a further decline in the numbers of PH3-labeled cells observed over time (Figure 4G). These results indicated that the premitotic checkpoint was fully engaged by 1 hr after IR exposure and remained so for several hours after irradiation, in the control wing discs (Figure 4G).
We observed a variable but greater than twofold average increase in PH3-labeled cells in the unirradiated myt1 mutant wing discs (relative to unirradiated yw control discs), consistent with results presented earlier. Although there was a modest reduction in numbers of PH3 positive cells shortly after exposure to ionizing radiation (Figure 4G), labeled cells persisted in the myt1 mutant wing discs for several hours after irradiation, long after they had disappeared in the yw controls. These results indicate that dMyt1 is important for normal cellular responses to ionizing radiation.
Cdk1 inhibitory phosphorylation is impaired in myt1 mutant wing discs:
Biochemical studies of Myt1 kinases have shown that they are capable of phosphorylating both the T14 and Y15 inhibitory residues of Cdk1 (Mueller et al. 1995; Booher et al. 1997; Liu et al. 1997). In contrast, Wee1 kinases appear to phosphorylate Cdk1 exclusively on the Y15 site (Parker et al. 1995). Drosophila dWee1 also functions as a Y15-specific Cdk1 inhibitory kinase, in vitro (Campbell et al. 1995) and early embryos (Price et al. 2000; Stumpff et al. 2004).
To determine how loss of dWee1 or dMyt1 affected Cdk1 inhibitory phosphorylation in third instar imaginal wing discs, we used phospho-specific antibodies that specifically recognize T14- and Y15-phosphorylated Cdk1 isoforms to assay whole protein extracts on Western blots (see materials and methods). The yw control extracts showed both T14- and Y15-phosphorylated Cdk1 isoforms, demonstrating that we could detect phosphorylation of each of the two Cdk1 inhibitory sites (Figure 5, lane A). In the myt1 mutant wing disc protein extracts (Figure 5, lane B), phosphorylation of Cdk1 on the T14 residue was undetectable in the myt1 mutant samples, even when the protein blots were overloaded and overexposed. This result demonstrated that dMyt1 is the only Cdk1 inhibitory kinase capable of phosphorylating the T14 residue in Drosophila. Phosphorylation of the Cdk1-Y15 site was also substantially reduced in the myt1 mutants relative to the controls (Figure 5, lane B), with the remaining Y15 phospho-isoform that was observed presumably representing dWee1 activity. The simplest interpretation of these results is that dMyt1 functions as a dual specificity Cdk1 inhibitory kinase and is largely responsible for regulating Cdk1 at this stage of development.
In the wee1 mutant wing disc protein extract samples, Cdk1 inhibitory phosphorylation was indistinguishable from the controls (Figure 5, lane C). These biochemical results were therefore consistent with phenotypic data described earlier suggesting that dWee1 was active, but largely dispensable, unless dMyt1 was absent. Collectively, these results indicate that dMyt1 is the major Cdk1 inhibitory kinase in third instar wing discs.
DISCUSSION
Multicellular organisms regulate Cdk1 by inhibitory phosphorylation to prevent mitosis when DNA is being replicated or repaired (Poon et al. 1997) and to ensure that mitosis does not interfere with critical developmental processes that require remodeling of the cytoskeleton (Edgar and O'Farrell 1990; Grosshans and Wieschaus 2000; Mata et al. 2000; Seher and Leptin 2000; Murakami et al. 2004). Previous studies of Drosophila Wee1 and Myt1 revealed that these conserved Cdk1 inhibitory kinases were required during early embryogenesis and gametogenesis, respectively (Price et al. 2000; Stumpff et al. 2004; Jin et al. 2005). We have now characterized imaginal and adult developmental defects caused by loss of dMyt1 activity (and to a much lesser extent, dWee1), that confirm the importance of Cdk1 inhibitory phosphorylation for coordinating cell-cycle events with critical developmental processes.
In Drosophila and other organisms, G2/M delays can be induced by overexpression of Myt1 kinases, suggesting a specific role for Myt1 in regulating this stage of the cell cycle (Booher et al. 1997; Liu et al. 1999; Cornwell et al. 2002; Lamitina and L'Hernault 2002; Price et al. 2002). Further evidence of a role for Myt1 in G2/M regulation comes from studies of oocyte maturation in frogs, starfish, and nematodes (Palmer et al. 1998; Okumura et al. 2002; Peter et al. 2002; Burrows et al. 2006). Not all data indicate that Myt1 is required for G2 phase arrest, however, and there is no evidence that dMyt1 regulates oocyte maturation in Drosophila (Ivanovska et al. 2004; Jin et al. 2005). Nor is there evidence that dMyt1 activity is responsible for the timing of the G2/M meiotic transition that follows a prolonged 4-day-long G2 phase arrest, in Drosophila primary spermatocytes (D. Guha Majumdar, unpublished results). Moreover, a recent study showed that functional depletion of human Myt1 by siRNA did not affect the proportion of cells in G2 phase, but instead affected membrane dynamics during mitotic exit (Nakajima et al. 2008). More clearly needs to be learned about Myt1 mediated regulatory mechanisms before these apparent discrepancies in Myt1 functions are resolved.
Previous work showed that Cdk1 inhibitory phosphorylation is required for proper development of thoracic mechanosensory organs (Milan et al. 1996; Lehman et al. 1999; Tio et al. 2001; Fichelson and Gho 2004). We have now identified dMyt1 as the primary Cdk1 inhibitory kinase for this developmental program. Several molecular mechanisms could explain the role of dMyt1 in mechanosensory bristle development. One obvious possibility is that myt1 mutant sensory organ precursor (SOP) cells and their descendants might divide prematurely due to a defect in G2/M regulation, resulting in aberrant segregation of cell fate determinants. If there was a relatively narrow window for coordinating specific developmental events with the G2/M transition, disrupting this regulatory mechanism could account for the observed loss and duplication of bristles and socket cells in myt1 mutants. Live analysis of mechanosensory organ development could test this possibility (Fichelson and Gho 2004).
Alternatively, myt1 mutant phenotypes could reflect defects in Myt1-mediated regulatory mechanisms that are important for the control of intracellular membrane dynamics during mitosis, particularly the Golgi apparatus and endoplasmic reticulum (Cornwell et al. 2002; Nakajima et al. 2008). The Drosophila Golgi apparatus undergoes significant morphological changes that have been linked to specific developmental states and so the observed myt1 mutant developmental defects might reflect problems in the structure or function of this organelle (Kondylis et al. 2001). Further support for this idea comes from a recent study showing that asymmetrical segregation of mouse Numb (a conserved cell fate determinant) requires the Golgi apparatus, leading the authors to suggest that Golgi fragmentation and reconstitution could represent a mechanism for coupling cell-fate specification and cell-cycle progression (Zhou et al. 2007).
Another possible explanation for myt1 mutant defects concerns the large quantities of actin that are synthesized and packaged to form the large mechanosensory bristle shafts (Wulfkuhle et al. 1998). This process involves extensive reorganization of the endoplasmic reticulum and Golgi apparatus to accommodate increased membrane trafficking (Tilney and Derosier 2005; Lee and Cooley 2007). Defects in the structure or function of the Golgi apparatus and ER caused by loss of dMyt1 activity could therefore account for defects or diminution in these bristles. Resolving which of these potential mechanisms best explain the role of dMyt1 during mechanosensory organ development will be a major challenge of our future research.
We also observed intriguing cell-cycle defects (higher mitotic index, aberrant chromatin condensation, and ectopic apoptosis), as well as defects in responses to ionizing radiation in proliferating cells in myt1 mutant imaginal wing discs. These observations suggest an important role for dMyt1 in conserved cell-cycle checkpoint responses that target Cdk1 by inhibitory phosphorylation (O'connell et al. 1997; Poon et al. 1997; Rhind et al. 1997; Niida and Nakanishi 2006). We had not anticipated that dMyt1 would serve such functions, since Wee1 kinases are generally assumed to be responsible for checkpoint responses that protect the nucleus from premature Cdk1 activity (Heald et al. 1993). It was not clear that myt1 mutants were deficient in conventional premitotic checkpoint responses, however. Indeed, the partial decline in myt1 mutant PH3-labeled cells observed immediately after exposure to ionizing radiation could reflect activation of an otherwise dispensable Wee1-regulated premitotic checkpoint mechanism. The remaining PH3-positive cells that persisted long after irradiation in myt1 mutant discs could be arrested in mitosis by an alternative regulatory mechanism that was responsive to DNA damage (Sanchez et al. 1999; Royou et al. 2005; Kim and Burke 2008; Musaro et al. 2008). Further studies will be needed to clarify the respective roles of dMyt1 and dWee1 in cellular responses to DNA damage.
We also observed profound defects in Cdk1 inhibitory phosphorylation in myt1 mutant imaginal discs. Phosphorylation of the T14 residue of Cdk1 was eliminated, demonstrating that dMyt1 is solely responsible for this regulatory modification, like Myt1 homologs described in other organisms (Mueller et al. 1995; Booher et al. 1997; Liu et al. 1997). We also observed that phosphorylation of the Y15 residue of Cdk1 was markedly reduced in myt1 mutant extracts, demonstrating for the first time that dMyt1 functions as a dual specificity Cdk1 inhibitory kinase, in vivo. Why dWee1 activity is insufficient for maintaining normal levels of phosphorylation of the Y15 residue is not clear, since Cdk1 complexes are thought to shuttle between the nucleus and cytoplasm (Hagting et al. 1998; Wells et al. 1999; Yang et al. 2001). One possible explanation is that the doubly phosphorylated Cdk1 isoform may be more refractory to dephosphorylation by Cdc25 phosphatases, and hence more stably inhibited, than Cdk1 phosphorylated on a single residue (Liu et al. 1997). Another possibility is that the kinase-independent Myt1 mechanism proposed to tether phospho-inhibited Cdk1 complexes in the cytoplasm until cells are ready for mitosis might also protect them from dephosphorylation (Mueller et al. 1995; Liu et al. 1999; Wells et al. 1999). Loss of either of these regulatory mechanisms could therefore underlie the cell-cycle defects observed in myt1 mutants. Testing these hypotheses promises to yield interesting new insights into cell-cycle regulation and the diverse developmental roles of dMyt1 and similar regulatory kinases in other organisms.
Acknowledgments
Thanks go to Don Price, Elizabeth Silva, and Rakesh Bhatnagar of the Biological Sciences Microscopy Unit (University of Alberta) as well as Bill Sullivan (University of California, Santa Cruz) for valuable advice. Gordon Chan (Cross Cancer Institute) provided temporary space for conducting the DNA damage checkpoint assays. The Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank provided fly stocks and antibodies, respectively. This research was supported by an operating grant funded by the Canadian Institutes of Health Research.
References
- Abrams, J. M., 2002. Competition and compensation: coupled to death in development and cancer. Cell 110 403–406. [DOI] [PubMed] [Google Scholar]
- Blair, S. S., 1993. Mechanisms of compartment formation: evidence that non-proliferating cells do not play a critical role in defining the D/V lineage restriction in the developing wing of Drosophila. Development 119 339–351. [DOI] [PubMed] [Google Scholar]
- Booher, R. N., P. S. Holman and A. Fattaey, 1997. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J. Biol. Chem. 272 22300–22306. [DOI] [PubMed] [Google Scholar]
- Brodsky, M. H., J. J. Sekelsky, G. Tsang, R. S. Hawley and G. M. Rubin, 2000. mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14 666–678. [PMC free article] [PubMed] [Google Scholar]
- Burrows, A. E., B. K. Sceurman, M. E. Kosinski, C. T. Richie, P. L. Sadler et al., 2006. The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development 133 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, S. D., F. Sprenger, B. A. Edgar and P. H. O'Farrell, 1995. Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol. Biol. Cell 6 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, W. D., P. J. Kaminski and J. R. Jackson, 2002. Identification of Drosophila Myt1 kinase and its role in Golgi during mitosis. Cell Signal 14 467–476. [DOI] [PubMed] [Google Scholar]
- Edgar, B. A., and P. H. O'Farrell, 1989. Genetic control of cell division patterns in the Drosophila embryo. Cell 57 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., and P. H. O'Farrell, 1990. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell 62 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extavour, C., and A. Garcia-Bellido, 2001. Germ cell selection in genetic mosaics in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98 11341–11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichelson, P., and M. Gho, 2004. Mother-daughter precursor cell fate transformation after Cdc2 down-regulation in the Drosophila bristle lineage. Dev. Biol. 276 367–377. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., F. Cortes and M. Milan, 1994. Cell interactions in the control of size in Drosophila wings. Proc. Natl. Acad. Sci. USA 91 10222–10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans, J., and E. Wieschaus, 2000. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101 523–531. [DOI] [PubMed] [Google Scholar]
- Hagting, A., C. Karlsson, P. Clute, M. Jackman and J. Pines, 1998. MPF localization is controlled by nuclear export. EMBO J. 17 4127–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald, R., M. McLoughlin and F. McKeon, 1993. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell 74 463–474. [DOI] [PubMed] [Google Scholar]
- Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli et al., 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106 348–360. [DOI] [PubMed] [Google Scholar]
- Inoue, D., and N. Sagata, 2005. The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J. 24 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska, I., E. Lee, K. M. Kwan, D. D. Fenger and T. L. Orr-Weaver, 2004. The Drosophila MOS ortholog is not essential for meiosis. Curr. Biol. 14 75–80. [DOI] [PubMed] [Google Scholar]
- Jack, J., and Y. DeLotto, 1992. Effect of wing scalloping mutations on cut expression and sense organ differentiation in the Drosophila wing margin. Genetics 131 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Z., E. M. Homola, P. Goldbach, Y. Choi, J. A. Brill et al., 2005. Drosophila Myt1 is a Cdk1 inhibitory kinase that regulates multiple aspects of cell cycle behavior during gametogenesis. Development 132 4075–4085. [DOI] [PubMed] [Google Scholar]
- Kim, E. M., and D. J. Burke, 2008. DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore. PLoS Genet. 4 e1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis, V., S. E. Goulding, J. C. Dunne and C. Rabouille, 2001. Biogenesis of Golgi stacks in imaginal discs of Drosophila melanogaster. Mol. Biol. Cell 12 2308–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth, S., B. Sebastian, T. Hunter and J. Newport, 1994. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol. Biol. Cell 5 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamitina, S. T., and S. W. L'Hernault, 2002. Dominant mutations in the Caenorhabditis elegans Myt1 ortholog wee-1.3 reveal a novel domain that controls M-phase entry during spermatogenesis. Development 129 5009–5018. [DOI] [PubMed] [Google Scholar]
- Lee, S., and L. Cooley, 2007. Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis. J. Cell Biol. 176 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, D. A., B. Patterson, L. A. Johnston, T. Balzer, J. S. Britton et al., 1999. Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development 126 1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise, W., III, and P. R. Mueller, 2002. Multiple Cdk1 inhibitory kinases regulate the cell cycle during development. Dev. Biol. 249 156–173. [DOI] [PubMed] [Google Scholar]
- Liu, F., C. Rothblum-Oviatt, C. E. Ryan and H. Piwnica-Worms, 1999. Overproduction of human Myt1 kinase induces a G2 cell cycle delay by interfering with the intracellular trafficking of Cdc2-cyclin B1 complexes. Mol. Cell. Biol. 19 5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F., J. J. Stanton, Z. Wu and H. Piwnica-Worms, 1997. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol. Cell. Biol. 17 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, K., N. Walworth, R. Booher, M. Dembski, M. Kirschner et al., 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64 1111–1122. [DOI] [PubMed] [Google Scholar]
- Masui, Y., 1992. Towards understanding the control of the division cycle in animal cells. Biochem. Cell Biol. 70 920–945. [DOI] [PubMed] [Google Scholar]
- Mata, J., S. Curado, A. Ephrussi and P. Rorth, 2000. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101 511–522. [DOI] [PubMed] [Google Scholar]
- Milan, M., S. Campuzano and A. Garcia-Bellido, 1996. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc. Natl. Acad. Sci. USA 93 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, P. R., T. R. Coleman, A. Kumagai and W. G. Dunphy, 1995. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270 86–90. [DOI] [PubMed] [Google Scholar]
- Murakami, M. S., S. A. Moody, I. O. Daar and D. K. Morrison, 2004. Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development 131 571–580. [DOI] [PubMed] [Google Scholar]
- Musaro, M., L. Ciapponi, B. Fasulo, M. Gatti and G. Cenci, 2008. Unprotected Drosophila melanogaster telomeres activate the spindle assembly checkpoint. Nat. Genet. 40 362–366. [DOI] [PubMed] [Google Scholar]
- Nakajima, H., S. Yonemura, M. Murata, N. Nakamura, H. Piwnica-Worms et al., 2008. Myt1 protein kinase is essential for Golgi and ER assembly during mitotic exit. J. Cell Biol. 181 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, M., H. Ando, N. Watanabe, K. Kitamura, K. Ito et al., 2000. Identification and characterization of human Wee1B, a new member of the Wee1 family of Cdk-inhibitory kinases. Genes Cells 5 839–847. [DOI] [PubMed] [Google Scholar]
- Neel, J. V., 1940. The pattern of supernumerary macrochaetae in certain Drosophila mutants. Genetics 25 251–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, T. P., A. F. de la Cruz, L. A. Johnston and B. A. Edgar, 1998. Coordination of growth and cell division in the Drosophila wing. Cell 93 1183–1193. [DOI] [PubMed] [Google Scholar]
- Nigg, E. A., W. Krek and M. Peter, 1991. Vertebrate cdc2 kinase: its regulation by phosphorylation and its mitotic targets. Cold Spring Harbor Symp. Quant. Biol. 56 539–547. [DOI] [PubMed] [Google Scholar]
- Niida, H., and M. Nakanishi, 2006. DNA damage checkpoints in mammals. Mutagenesis 21 3–9. [DOI] [PubMed] [Google Scholar]
- Nurse, P., and P. Thuriaux, 1980. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics 96 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brochta, D. A., and P. J. Bryant, 1985. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature 313 138–141. [DOI] [PubMed] [Google Scholar]
- O'Connell, M. J., J. M. Raleigh, H. M. Verkade and P. Nurse, 1997. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K., N. Nakajo and N. Sagata, 2002. The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle. EMBO J. 21 2472–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura, E., T. Fukuhara, H. Yoshida, S. Hanada Si, R. Kozutsumi et al., 2002. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 4 111–116. [DOI] [PubMed] [Google Scholar]
- Palmer, A., A. C. Gavin and A. R. Nebreda, 1998. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, L. L., P. J. Sylvestre, M. J. Byrnes, 3rd, F. Liu and H. Piwnica-Worms, 1995. Identification of a 95-kDa WEE1-like tyrosine kinase in HeLa cells. Proc. Natl. Acad. Sci. USA 92 9638–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, M., J. C. Labbe, M. Doree and E. Mandart, 2002. A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. Development 129 2129–2139. [DOI] [PubMed] [Google Scholar]
- Poon, R. Y., M. S. Chau, K. Yamashita and T. Hunter, 1997. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 57 5168–5178. [PubMed] [Google Scholar]
- Price, D., S. Rabinovitch, P. H. O'Farrell and S. D. Campbell, 2000. Drosophila wee1 has an essential role in the nuclear divisions of early embryogenesis. Genetics 155 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, D. M., Z. Jin, S. Rabinovitch and S. D. Campbell, 2002. Ectopic expression of the Drosophila Cdk1 inhibitory kinases, Wee1 and Myt1, interferes with the second mitotic wave and disrupts pattern formation during eye development. Genetics 161 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko, S. N., and W. Chia, 2005. When timing is everything: role of cell cycle regulation in asymmetric division. Semin. Cell Dev. Biol. 16 423–437. [DOI] [PubMed] [Google Scholar]
- Rhind, N., B. Furnari and P. Russell, 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11 504–511. [DOI] [PubMed] [Google Scholar]
- Rhind, N., and P. Russell, 2001. Roles of the mitotic inhibitors Wee1 and Mik1 in the G(2) DNA damage and replication checkpoints. Mol. Cell. Biol. 21 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou, A., H. Macias and W. Sullivan, 2005. The Drosophila Grp/Chk1 DNA damage checkpoint controls entry into anaphase. Curr. Biol. 15 334–339. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., J. Bachant, H. Wang, F. Hu, D. Liu et al., 1999. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science 286 1166–1171. [DOI] [PubMed] [Google Scholar]
- Seher, T. C., and M. Leptin, 2000. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 10 623–629. [DOI] [PubMed] [Google Scholar]
- Stumpff, J., T. Duncan, E. M. Homola, S. D. Campbell and T. T. Su, 2004. Drosophila Wee1 kinase regulates Cdk1 and mitotic entry during embryogenesis. Curr. Biol. 14 2143–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L. G., and D. J. DeRosier, 2005. How to make a curved Drosophila bristle using straight actin bundles. Proc. Natl. Acad. Sci. USA 102 18785–18792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio, M., G. Udolph, X. Yang and W. Chia, 2001. cdc2 links the Drosophila cell cycle and asymmetric division machineries. Nature 409 1063–1067. [DOI] [PubMed] [Google Scholar]
- Tominaga, Y., C. Li, R. H. Wang and C. X. Deng, 2006. Murine Wee1 plays a critical role in cell cycle regulation and pre-implantation stages of embryonic development. Int. J. Biol. Sci. 2 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, N. J., N. Watanabe, T. Tokusumi, W. Jiang, M. A. Verdecia et al., 1999. The C-terminal domain of the Cdc2 inhibitory kinase Myt1 interacts with Cdc2 complexes and is required for inhibition of G(2)/M progression. J. Cell Sci. 112(Pt 19): 3361–3371. [DOI] [PubMed] [Google Scholar]
- Wilson, M. A., R. V. Hoch, N. R. Ashcroft, M. E. Kosinski and A. Golden, 1999. A Caenorhabditis elegans wee1 homolog is expressed in a temporally and spatially restricted pattern during embryonic development. Biochim. Biophys. Acta 1445 99–109. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle, J. D., N. S. Petersen and J. J. Otto, 1998. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophila: the role of singed and forked proteins. Cell Motil. Cytoskeleton 40 119–132. [DOI] [PubMed] [Google Scholar]
- Yang, J., H. Song, S. Walsh, E. S. Bardes and S. Kornbluth, 2001. Combinatorial control of cyclin B1 nuclear trafficking through phosphorylation at multiple sites. J. Biol. Chem. 276 3604–3609. [DOI] [PubMed] [Google Scholar]
- Yu, S. Y., S. J. Yoo, L. Yang, C. Zapata, A. Srinivasan et al., 2002. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development 129 3269–3278. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J. B. Atkins, S. B. Rompani, D. L. Bancescu, P. H. Petersen et al., 2007. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasing ACBD3 during mitosis. Cell 129 163–178. [DOI] [PubMed] [Google Scholar]