Abstract
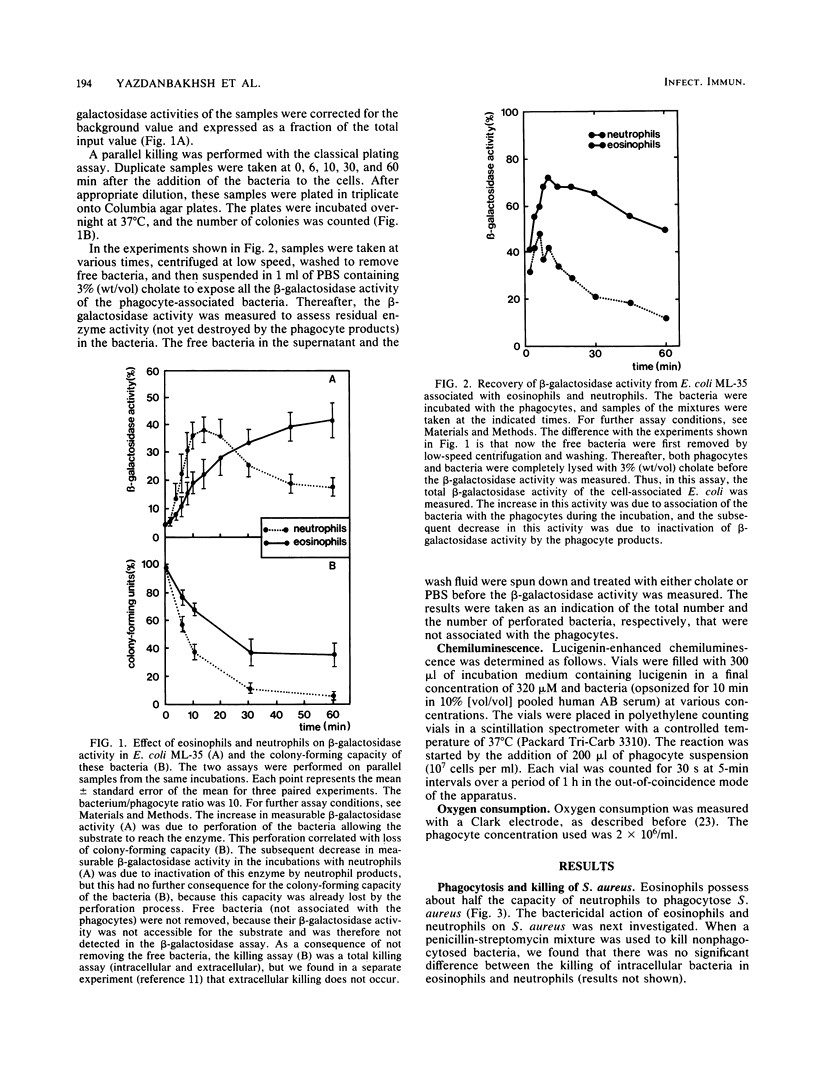
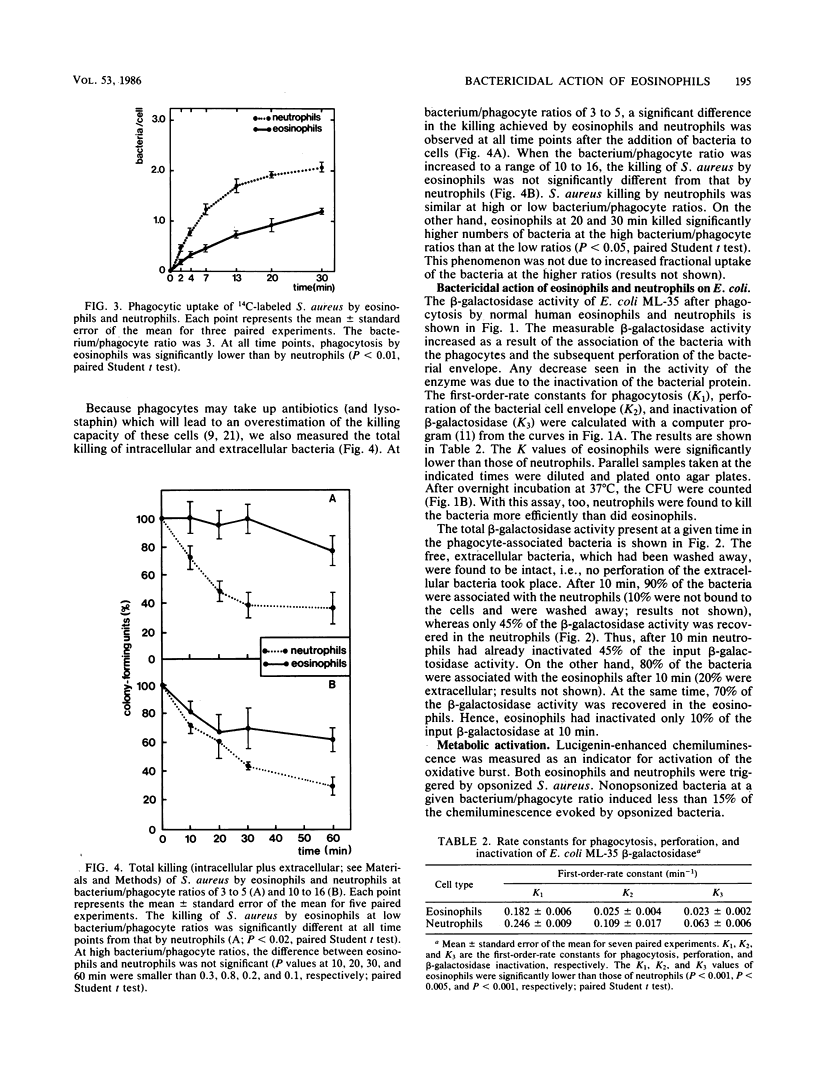
The ability of normal human eosinophils to ingest and kill Staphylococcus aureus and Escherichia coli was investigated and compared with the reactions shown by neutrophils from the same donors. The rate of phagocytosis of S. aureus by eosinophils was 50% of that shown by neutrophils. Unlike neutrophils, eosinophils were not able to kill ingested S. aureus at low bacterium/phagocyte ratios. The degree of S. aureus killing increased with increasing ratios, being equal to that of neutrophils when bacterium/phagocyte ratios of about 15 were used. This was probably due to a better triggering of the eosinophil oxidase system at high bacterium/phagocyte ratios. The early kinetics of the association of bacteria with eosinophils, the perforation of the bacterial envelope and the inactivation of bacterial proteins, was monitored in the ML-35 mutant strain of E. coli. The association of E. coli with eosinophils was 70% of that with neutrophils. Eosinophils had only 25% of the capacity of neutrophils to perforate the E. coli envelope. E. coli loses its colony-forming ability when the bacterial envelope has been perforated, indicating that eosinophils also kill E. coli more slowly than do neutrophils. This was confirmed with a plating assay for colony formation. The perforation of E. coli is independent of peroxidase-mediated reactions. Hence, the defective bactericidal action of eosinophils is probably not related to the differences between myeloperoxidase and eosinophil peroxidase. On the other hand, the inactivation of bacterial proteins is peroxidase dependent and was also seen to occur to a lesser extent in eosinophils compared with neutrophils. We conclude that eosinophils ingest E. coli but only slowly perforate (kill) these bacteria and barely inactivate the bacterial enzymes. In contrast, neutrophils quickly ingest and perforate (kill) E. coli and quickly inactivate the bacterial enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Johnston R. B., Jr Metabolic and bactericidal activities of human eosinophils. Br J Haematol. 1971 Mar;20(3):277–285. doi: 10.1111/j.1365-2141.1971.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Grover W. H., Lewis J. C., Szejda P., DeChatelet L. R., McCall C. E. Comparison of human eosinophils from normals and patients with eosinophilia. J Clin Invest. 1980 Dec;66(6):1265–1273. doi: 10.1172/JCI109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolscher B. G., Plat H., Wever R. Some properties of human eosinophil peroxidase, a comparison with other peroxidases. Biochim Biophys Acta. 1984 Jan 31;784(2-3):177–186. doi: 10.1016/0167-4838(84)90125-0. [DOI] [PubMed] [Google Scholar]
- Bos A. J., Wever R., Hamers M. N., Roos D. Some enzymatic characteristics of eosinophil peroxidase from patients with eosinophilia and from healthy donors. Infect Immun. 1981 May;32(2):427–431. doi: 10.1128/iai.32.2.427-431.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Buys J., Wever R., Ruitenberg E. J. Myeloperoxidase is more efficient than eosinophil peroxidase in the in vitro killing of newborn larvae of Trichinella spiralis. Immunology. 1984 Mar;51(3):601–607. [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Migler R. A., Shirley P. S., Muss H. B., Szejda P., Bass D. A. Comparison of intracellular bactericidal activities of human neutrophils and eosinophils. Blood. 1978 Sep;52(3):609–617. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Huntley C. C., Muss H. B., Bass D. A. Oxidative metabolism of the human eosinophil. Blood. 1977 Sep;50(3):525–535. [PubMed] [Google Scholar]
- Easmon C. S. The effect of antibiotics on the intracellular survival of Staphylococcus aureus in vitro. Br J Exp Pathol. 1979 Feb;60(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Butterworth A. E., Sturrock R. F., Houba V. The mechansim of antibody-dependent, eosinophil-mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase-contrast and electron microscopy. J Cell Sci. 1978 Dec;34:173–192. doi: 10.1242/jcs.34.1.173. [DOI] [PubMed] [Google Scholar]
- Hamers M. N., Bot A. A., Weening R. S., Sips H. J., Roos D. Kinetics and mechanism of the bactericidal action of human neutrophils against Escherichia coli. Blood. 1984 Sep;64(3):635–641. [PubMed] [Google Scholar]
- Kazura J. W., Grove D. I. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978 Aug 10;274(5671):588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Bactericidal and metabolic properties of human eosinophils. Blood. 1972 Jan;39(1):67–80. [PubMed] [Google Scholar]
- Pincus S. H., Schooley W. R., DiNapoli A. M., Broder S. Metabolic heterogeneity of eosinophils from normal and hypereosinophilic patients. Blood. 1981 Dec;58(6):1175–1181. [PubMed] [Google Scholar]
- Prin L., Capron M., Tonnel A. B., Bletry O., Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72(4):336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Sips H. J., Hamers M. N. Mechanism of the bactericidal action of myeloperoxidase: increased permeability of the Escherichia coli cell envelope. Infect Immun. 1981 Jan;31(1):11–16. doi: 10.1128/iai.31.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of inflammatory cells in Chagas' disease. I. Uptake and mechanism of destruction of intracellular (amastigote) forms of Trypanosoma cruzi by human eosinophils. J Immunol. 1984 Apr;132(4):2053–2058. [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Yazdanbakhsh M., Eckmann C. M., Roos D. Characterization of the interaction of human eosinophils and neutrophils with opsonized particles. J Immunol. 1985 Aug;135(2):1378–1384. [PubMed] [Google Scholar]
- van den Broek P. J., Dehue F. A., Leijh P. C., van den Barselaar M. T., van Furth R. The use of lysostaphin in in vitro assays of phagocyte function: adherence to and penetration into granulocytes. Scand J Immunol. 1981 May;15(5):467–473. doi: 10.1111/j.1365-3083.1982.tb00672.x. [DOI] [PubMed] [Google Scholar]


