Abstract
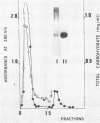
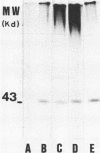
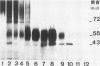
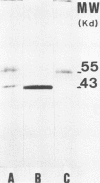
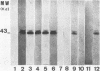
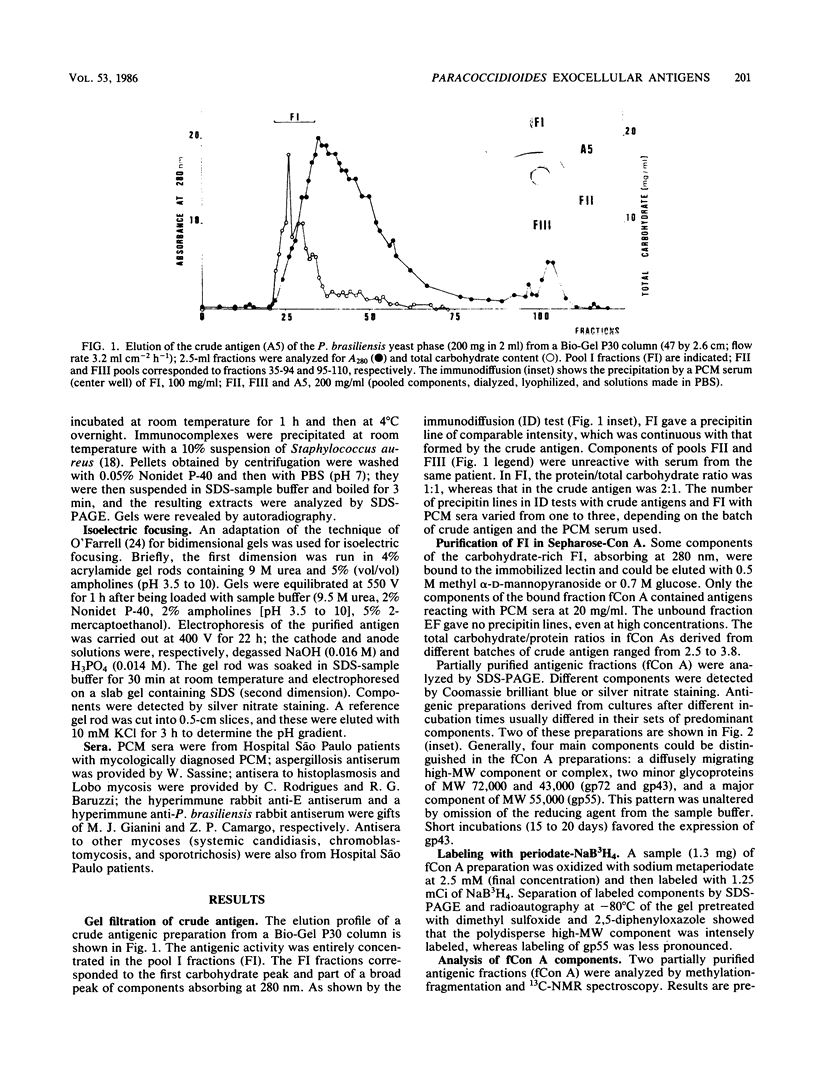
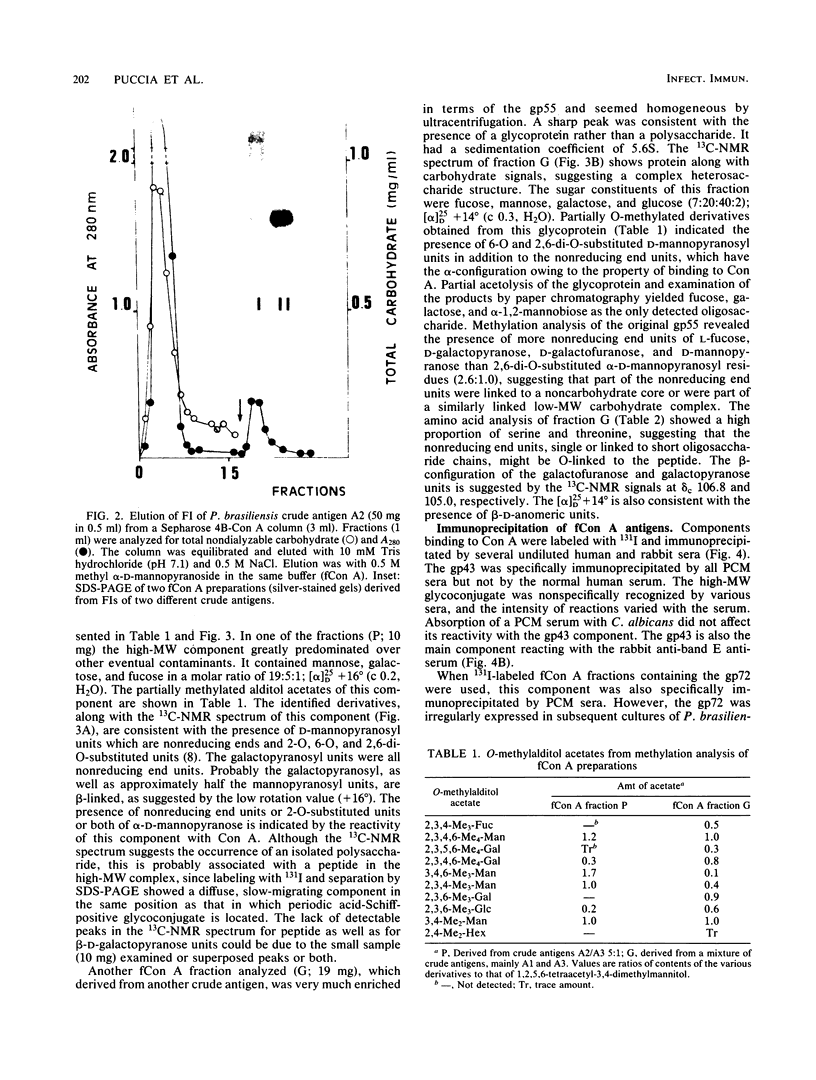
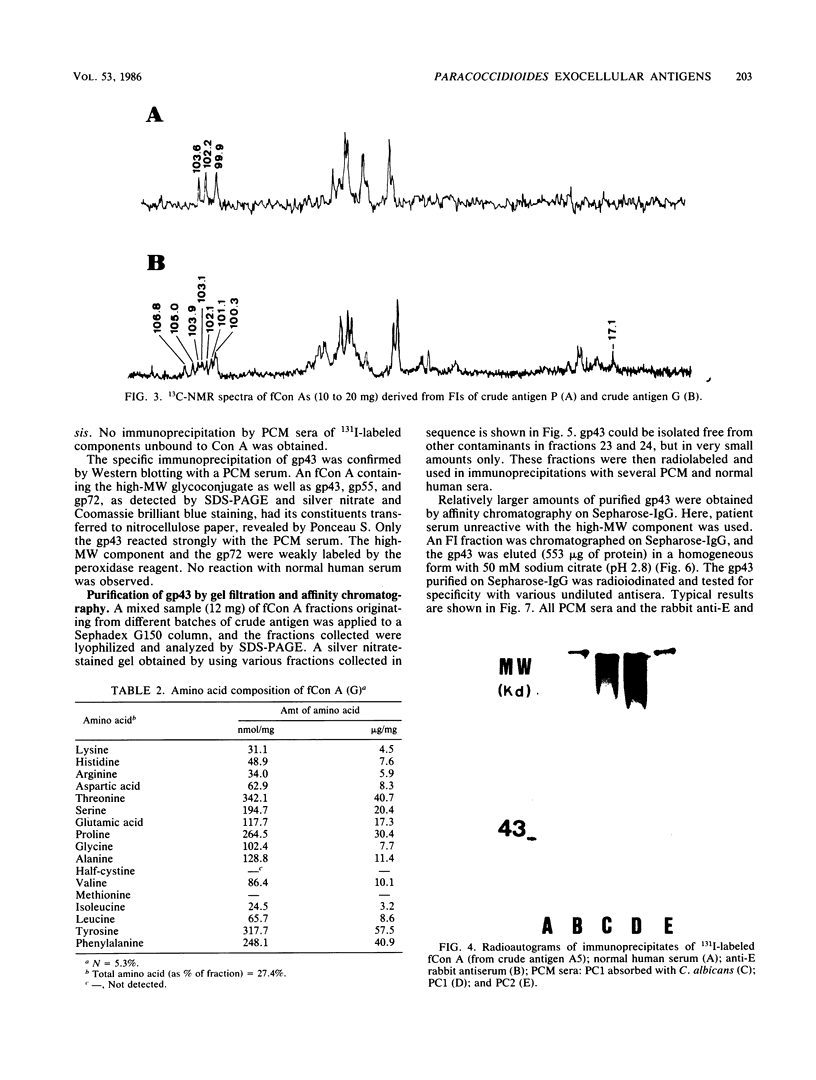
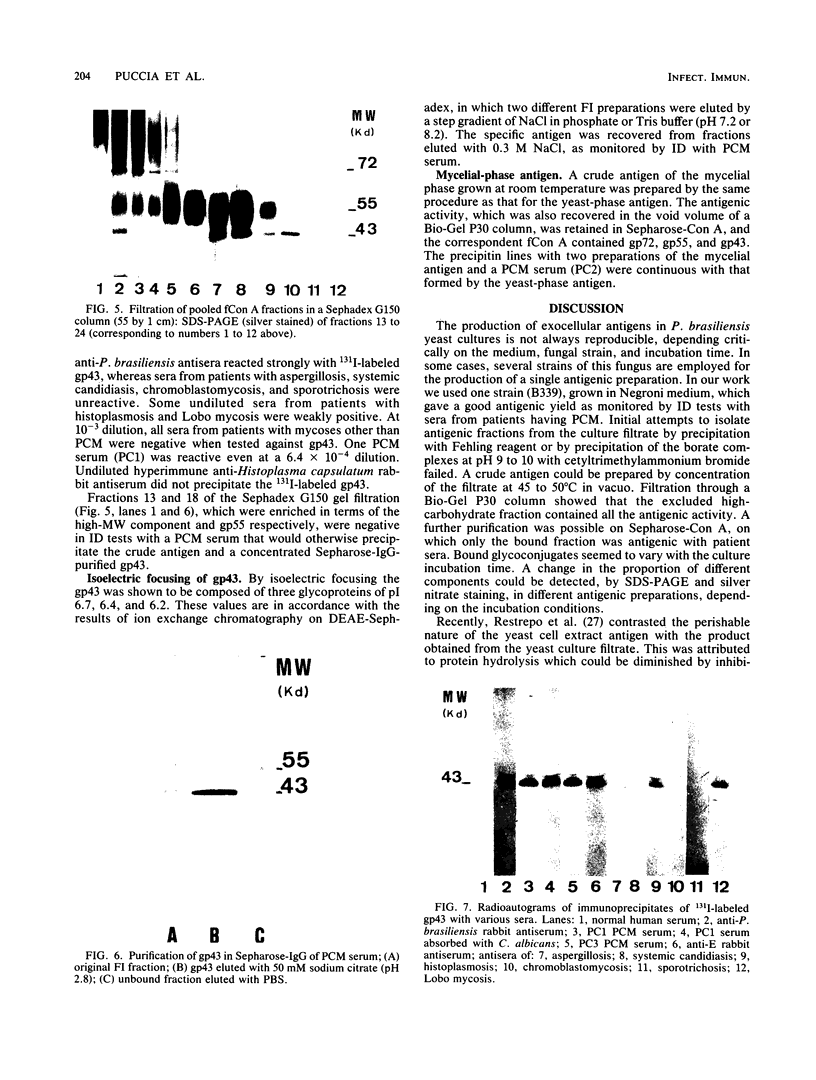
Yeast forms of Paracoccidioides brasiliensis grown in liquid medium produced exocellular components. Immunodiffusion reactions and immunoprecipitations of 131I-radiolabeled antigenic components with sera from patients having paracoccidioidomycosis (PCM) were used to monitor the isolation of specific constituents. Components having the main antigenic activity (fCon A) were isolated by exclusion from a Bio-Gel P30 column, followed by successive binding of eluted material to a Sepharose-concanavalin A column, and elution. The product contained, from sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, a minor 43,000-molecular-weight (MW) component (gp43), a polydisperse high-MW glycoconjugate, and a diffusely migrating 55,000-MW glycoprotein (gp55). Other components, including a 72,000-MW glycoprotein, were irregularly expressed. The high-MW glycoconjugate complex contained, on the basis of methylation and 13C nuclear magnetic resonance data, a branched structure of mainly mannopyranosyl units. These were nonreducing ends, 6-O-, 2-O-, and 2,6-di-O-substituted, and the specific rotation of +16 degrees indicated that the glycosidic configurations of the units were alpha and beta in a ratio of ca. 1:1 (concanavalin A binding indicated that nonreducing ends or 2-O-substituted units or both of alpha-D-mannopyranose were present). A small proportion of nonreducing end units of D-galactopyranose were also present in this polysaccharide. gp55 is a glycoprotein containing a complex carbohydrate moiety with fucose, mannose, galactose, and glucose, either as terminal nonreducing units or substituted in positions indicated by methylation data. Both PCM and normal human sera precipitated the high-MW glycoconjugate from 131I-labeled fCon A preparations, whereas gp55 was unreactive with human sera. gp43 was a specific antigenic component of P. brasiliensis culture filtrates which could be isolated in a pure form by gel filtration column chromatography (Sephadex G150) or by Sepharose-patient immunoglobulin G affinity chromatography. 131I-labeled gp43 reacted equally well with 10 PCM sera and hyperimmune rabbit serum against the band E antigen of Yarzabal at a 10(-3) dilution. At the same dilution, no reaction was detected with sera from normal individuals and from patients with other mycoses. Similarly, only PCM sera and the hyperimmune anti-E serum gave precipitin lines with gp43 in the less sensitive immunodiffusion tests. gp43 consisted of three components, with pI 6.7, 6.4 and 6.2, all of which reacted with PCM serum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Kanetsuna F., Tanaka Y., Yamamura Y., Carbonell L. M. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blasomyces dermatitidis. Mycopathol Mycol Appl. 1974 Oct 15;54(1):111–125. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- Blumer S. O., Jalbert M., Kaufman L. Rapid and reliable method for production of a specific Paracoccidioides brasiliensis immunodiffusion test antigen. J Clin Microbiol. 1984 Mar;19(3):404–407. doi: 10.1128/jcm.19.3.404-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Aasen I., de Cabral N., Yarzábal L. Sub-cellular localization of antigen E/2 of Paracoccidioides brasiliensis. An immunoenzymatic electron microscopy study. Sabouraudia. 1980 Sep;18(3):167–171. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin P. A., Haskins R. H., Travassos L. R., Mendonca-Previato L. Further studies on the rhamnomannans and acidic rhamnomannans of Sporothrix schenckii and Ceratocystis stenoceras. Carbohydr Res. 1977 May;55:21–33. doi: 10.1016/s0008-6215(00)84440-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Ballou C. E. Characterization of a yeast mannan containing N-acetyl-D-glucosamine as an immunochemical determinant. Biochemistry. 1972 Sep 26;11(20):3807–3816. doi: 10.1021/bi00770a021. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Cano L. E., Ochoa M. T. A yeast-derived antigen from Paracoccidioides brasiliensis useful for serologic testing. Sabouraudia. 1985 Feb;23(1):23–29. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Yarzabal L. A., Andrieu S., Bout D., Naquira F. Isolation of a specific antigen with alkaline phosphatase activity from soluble extracts of Paracoccidioides brasiliensis. Sabouraudia. 1976 Nov;14(3):275–280. doi: 10.1080/00362177685190411. [DOI] [PubMed] [Google Scholar]
- Yarzabal L. A. Anticuerpos precipitantes específicos de la blastomicosis sudamericana revelados por inmunoelectroforesis. Rev Inst Med Trop Sao Paulo. 1971 Sep-Oct;13(5):320–327. [PubMed] [Google Scholar]
- Yarzabal L. A., Bout D., Naquira F., Fruit J., Andrieu S. Identification and purification of the specific antigen of Paracoccidioides brasiliensis responsible for immunoelectrophoretic band E. Sabouraudia. 1977 Mar;15(1):79–85. [PubMed] [Google Scholar]
- de Camargo Z. P., Guesdon J. L., Drouhet E., Improvisi L. Magnetic enzyme-linked immunosorbent assay (MELISA) for determination of specific IgG in paracoccidioidomycosis. Sabouraudia. 1984;22(4):291–299. doi: 10.1080/00362178485380491. [DOI] [PubMed] [Google Scholar]