Abstract
Most bone remodeling is thought to occur within the first few years after THA. Loss of bone density later may be associated with stress shielding or normal bone loss of aging. We evaluated remodeling changes over time with a proximally hydroxyapatite-coated tapered titanium stem. We evaluated plain radiographs of 143 hips for cancellous condensation, cortical hypertrophy, cortical porosis, cortical index, and canal fill at early postoperative, 5, 10, and 15 years. Average age was 51 years at THA; 69 patients (77 hips) (53%) were women; and 102 hips (71%) had primary osteoarthrosis. Based on radiographic findings at 15 years, hips were divided into three subgroups: 43 (30%) demonstrated minimal remodeling changes; 53 (37%) demonstrated cortical hypertrophy evident before 5 years; and 47 (33%) demonstrated additional late remodeling and cortical porosis, most often after 10 years. Hips with poorer bone (Dorr Types B or C) and, when including only hips with osteoarthrosis, more female hips had cortical porosis at 15 years. Late radiographic changes in patients with porosis appear more similar to that associated with an extensively rather than proximally coated stem. Whether continued bone adaptation and bone loss of aging will eventually threaten implant stability is unknown, but at 15 years, all 143 implants remained well fixed and clinically asymptomatic.
Level of Evidence: Level III, retrospective study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Long-term fixation in THA requires maintenance of the implant through bone contact. Periprosthetic bone remodeling may enhance this contact, whereas stress shielding and osteolysis may threaten it through bone loss. Mechanical load forces continually expose the bone to a remodeling process according to Wolff’s law. Increased load leads to a gain in bone mass, and reduced load results in a loss. Implantation of a total THA changes the distribution of the mechanical forces around the hip [4]. The stresses occurring in the bone after implantation of a stem are due to a combination of axial, bending, and torsional loads [21, 23]. The location and magnitude of stress transfer from the femoral component to the bone vary depending upon the shape and stiffness of the implant [12–14, 22, 24], the level of coating for fixation and degree of bonding [12, 21, 23], and the stiffness of the bone preoperatively and the resultant hip force loads after implantation [12, 14, 17, 21, 23]. Most reports of cementless implants indicate bone remodeling starts within the first several months after implantation [25, 35, 38, 44, 46], but subsequent changes have also been reported [26, 39, 45]. Radiographic analysis of bone remodeling around proximally hydroxyapatite (HA)-coated femoral implants indicate cancellous condensation and cortical hypertrophy in the area of transition from the coated to uncoated portion of the stem, with these changes typically evident as early as 12 months after implantation [9, 10].
Loss of bone density may be a result of stress shielding secondary to prosthetic design [12, 13, 22, 30], implant stiffness [3, 6, 19, 24], and stress redistribution [4, 16, 42]; however, long-term changes could also be a result of aging [43]. Using CT, one study of 263 cadaveric femurs reported decreases in three subregions (periosteal, midcortical, and endosteal) with advancing age [5], and in another cadaveric study ultimate stress, ultimate strain, and energy absorption capabilities of cortical bone deteriorated by 5%, 9%, and 12% per decade and porosity increased with age [31].
U.S. life expectancy tables in 1990 indicate that a 50-year-old male or female, the average age and time frame of the patients in this study cohort, would live to 76.7 and 81.6 years, respectively [28], with the number of centenarians estimated to double in the next 10 years to more than 130,000 people and those numbers expected to double yet again to 274,000 by the year 2025 [8]. Thus, as life expectancy increases, age-related changes in the structure and composition of bone are becoming increasingly important. Similarly, as the number of people undergoing THA increases, the interaction of the effect of a hip implant on the surrounding bone and the age-related changes in bone tissue must be considered and evaluated.
During a routine annual evaluation of plain radiographs in a cohort of patients enrolled in a prospective study of a proximally HA-coated tapered titanium hip stem we noted long term alterations in bone density. During the first 10 years postimplantation, radiographic review yielded no unexpected results. Early remodeling changes were seen in a number of hips, with more than ½ showing cortical hypertrophy in the area of transition from the coated to uncoated part of the stem within the first few years and progressive cancellous condensation seen in at least one Gruen zone in all hips. After 10 years, two of us (WNC, JAD) began to notice unexpected radiographic changes in a few hips, which then prompted this retrospective radiographic study of all study hips at 15 years postimplantation.
We therefore asked: (1) What percentage of hips demonstrate minimal remodeling, active implant-related remodeling, and a combination of active implant- and age-related remodeling 15 years after primary total hip arthroplasty using a grit-blasted tapered HA-coated titanium femoral component? (2) What demographic or implant-related factors (e.g., age, sex, diagnosis, body mass index, initial bone quality, stem size) are associated with these three remodeling groups? (3) What quantifiable radiographic differences are evident in these three remodeling groups? (4) How do baseline and latest followup bone measurements compare on the contralateral side to the implant side by remodeling group? (5) Finally, only those hips with a diagnosis of osteoarthrosis were culled out for secondary analysis to examine if homogeneity of diagnosis would elucidate potentially important relationships related to demographic and remodeling characteristics.
Materials and Methods
This study is a retrospective examination of serial radiographs in a series of 129 patients (143 hips) with a minimum of 15 years followup after primary THA using a proximally HA-coated tapered titanium femoral component (Omnifit® HA, Stryker Orthopaedics, Mahwah, NJ). After radiographic assessment the 143 hips were divided into three groups based on findings on the operative side. Group 1 included 43 hips with minimal radiographic remodeling changes, ie, evidence of cancellous condensation but no cortical hypertrophy or porosis, over 15 years. The 53 hips in Group 2 demonstrated active remodeling over 15 years, including evidence of cortical hypertrophy and cancellous condensation. Group 3 consisted of 47 hips with radiographic evidence of cortical porosis with or without cortical hypertrophy.
The grit-blasted tapered titanium femoral component is straight and collarless and has normalization steps and a dense 50-μm-thick layer of HA applied circumferentially to the proximal 1/3 of the stem surface. Various cup designs were used in conjunction with this HA-coated stem, including both porous- and HA-coated cups, and all hips had a gamma-in-air-sterilized ultrahigh-molecular-weight polyethylene liner on a cobalt-chrome femoral head bearing surface.
The 143 hips included in this study were initially part of US and European prospective studies aimed at examining the safety and efficacy of this particular stem. After approval by the US Food and Drug Administration in December 1990, study enrollment was closed; however, this particular cohort of patients continues to be followed prospectively to study the long-term efficacy of this stem. Of the original 226 patients (262 hips) in the study, 15-year followup was unavailable on 75 patients (87 hips) for the following reasons: 26 patients (32 hips) died; 12 stems (12 patients) were revised; and 37 patients (43 hips) had either not yet returned for 15-year followup or were lost to followup. Another 32 hips did not have complete radiographs at all pertinent time periods (1-, 5-, 10-, and 15-years) and thus were excluded from this study, leaving the 143 hips in the study cohort. The average age of this cohort at the time of the arthroplasty was 51 years (range, 18–73 years) and the average body mass index was 26.9 (range, 15.4–41.7). 69 patients (77 hips) (54%) were female, and osteoarthrosis was the reason for arthroplasty in 102 (71%) of hips. Other reasons for the arthroplasty were osteonecrosis in 17 hips (12%), inflammatory arthritis in 4 hips (3%), secondary arthrosis in 10 hips (7%), and other diagnoses in 10 hips (7%). There were no differences between groups with regard to age, sex, diagnosis, or body mass index. Clinically, there were no differences in Harris hip scores preoperatively (Table 1).
Table 1.
Demographic characteristics by remodeling group
| Parameter | Group 1 | Group 2 | Group 3 | Total group | p Value |
|---|---|---|---|---|---|
| N | 43 | 53 | 47 | 143 | |
| Age | 51.6 | 49.9 | 50.9 | 50.8 | .5544 |
| Male/female | 24/19 | 23/30 | 19/28 | 66/77 | .3148 |
| 56%/44% | 43%/57% | 40%/60% | 46%/54% | ||
| Osteoarthritis | 34 | 33 | 35 | 102 | .1752 |
| 79% | 62% | 74% | 71% | ||
| Body mass index | 25.9 | 27.3 | 27.3 | 26.9 | .1996 |
| HHS – preoperative | 43.5 | 39.6 | 41.6 | 41.5 | .3402 |
| HHS – 15 year | 91.4 | 86.7 | 93.3 | 90.3 | .0332 |
| Cup revision | 9 | 16 | 9 | 34 | .3959 |
| 21% | 30% | 19% | 24% | ||
| Bone type* | |||||
| A | 24 (56%) | 15 (33%) | 8 (19%) | 47 (36%) | .0066 |
| B | 15 (35%) | 24 (52%) | 30 (70%) | 69 (52%) | |
| C | 4 (9%) | 7 (15%) | 5 (12%) | 16 (12%) | |
| Not available | 0 | 7 | 4 | 11 | |
| Stem size | |||||
| 5–6 | 2 (5%) | 2 (4%) | 3 (6%) | 7 (5%) | .2988 |
| 7–8 | 15 (35%) | 19 (36%) | 18 (38%) | 52 (36%) | |
| 9–10 | 24 (56%) | 28 (53%) | 20 (43%) | 72 (50%) | |
| 11 | 2 (5%) | 4 (8%) | 6 (13%) | 12 (8%) | |
Group 1 = minimal remodeling changes; Group 2 = cortical hypertrophy and cancellous condensation; Group 3 = cortical porosis with or without cortical hypertrophy; HHS = Harris hip score; * bone type percentages are valid percentages (missing data not included).
Each study center was provided with a detailed study protocol prior to initiation of the study to ensure that all clinical and radiographic data were obtained in a standardized manner pre- and postoperatively and annually thereafter. Protocol details included instructions on film and x-ray machine preparation and patient positioning for both anteroposterior and lateral radiographs. Comprehensive radiographic assessments were completed at 1, 5, 10, and 15 years postoperatively. Dorr bone type was estimated from the preoperative anteroposterior radiographs only [11]. Briefly, Type A bone has thick cortices with a distinct medial fin on the AP radiograph. Type B has less bone volume from the medial and posterior fins, and Type C bone has virtually lost the medial and posterior fin. The radiographs were blinded and then reviewed by two experienced hip arthroplasty surgeons (WNC, JAD) individually. If the two assessments were not in agreement, the radiographs were then rereviewed by both surgeons and consensus obtained. The radiographic review by Gruen zone [20] included analysis of bone condensation, cortical hypertrophy, pedestal formation, and cortical porosis. Cancellous condensation, cortical hypertrophy, and pedestal formation have been defined and described previously [9, 10]. Briefly, cancellous condensation is defined as an increase in density in the endosteum. Cortical hypertrophy is defined as an increase in the diameter of the cortex, and pedestal formation is defined as a shelf of new endosteal bone either partially or completely bridging the intramedullary canal. Cortical porosis is defined as a loss of endosteal definition and a decrease in bone mineralization resulting in a homogeneous but somewhat sparse (washed-out) appearance of the remaining cortex. Sixty four percent of hips had Type B or C bone. The percentage breakdown by group was 44% of Group 1, 58% of Group 2, and 74% of Group 3 with Type B or C bone (Table 1).
Quantitative measurements included measurement of the outer diameter (OD) and inner diameter (ID) of the femur (canal width), cortical thickness, and stem width (SW) in millimeters. Cortical index and canal fill are calculated as percentages on the operative side at three distinct levels: just below the lesser trochanter, at midstem, and 1 cm above the tip of the component at each postoperative interval (Fig. 1). Cortical index is calculated as OD − ID/OD x 100, and canal fill is calculated as SW/ID x 100. All quantitative measurements of the radiographs were made by a bioengineer (NB) at the sponsoring institution (Stryker Orthopaedics).
Fig. 1.
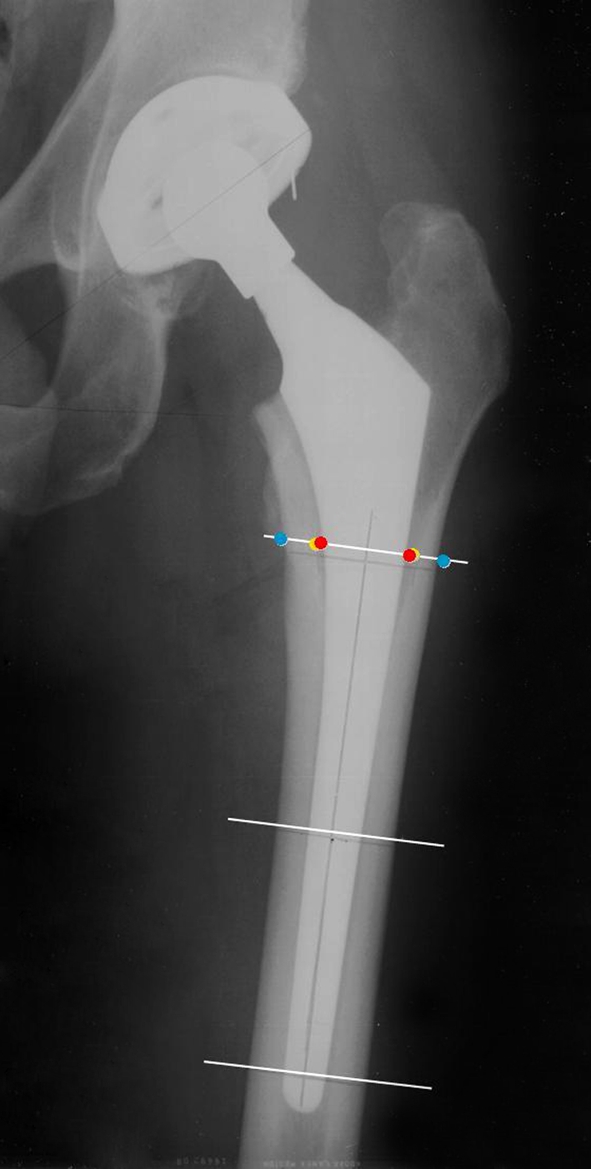
This anteroposterior radiograph shows the three levels of measurement: just below the lesser trochanter, at midstem, and 1 cm above the tip of the implant. Measurements include the outer diameter of the femur, the inner diameter of the femur (canal width), stem width, and cortical thickness at each of the three levels.
Fifty-seven of the 129 patients had a unilateral THA and had not subsequently undergone THA on the contralateral side. Of that subgroup, 38 patients (67%) had all requisite films on the contralateral side allowing for quantitative measurements of the cortical index, cortical thickness, and outer diameter of the femur at the same defined areas of the femur and at the same time periods as the operated side. Relative differences in bone measurement values from baseline to 15 years across the three groups were calculated for the operative side and for the contralateral, nonoperative side. A positive value indicates a relative increase at 15 years, and a negative value indicates a relative decrease at 15 years. Similarly we compared the relative differences in bone measurement values by group between the implant and nonimplant sides from baseline to 15 years, ie, the changes on the implant side minus the changes on the contralateral side. A positive value indicates a greater relative change on the operative side, whereas a negative value indicates a greater relative change on the contralateral side.
We compared demographics, bone type, and radiographic measures across groups. Analysis of variance was used to compare continuous variables (age, body mass index, Harris Hip Scores, cortical index, canal fill, femoral outer diameter, and cortical thickness) between the three groups, and the Fisher’s exact test was used to compare categorical data (sex, diagnosis, cup revisions, bone type, stem size, and cortical hypertrophy by number of zones). We performed secondary analyses including only those hips with osteoarthrosis (n = 102) since this group is the largest single diagnostic group in this study as well as in the general population undergoing THA. All statistical analyses were performed using SAS® software (Version 9.1; SAS Institute Inc, Cary, NC).
Results
Of the 143 hips followed 15 years post-THA, 43 (30%) demonstrated minimal remodeling changes with cancellous condensation but no cortical hypertrophy or porosis (Group 1) (Fig. 2); 53 (37%) showed remodeling changes of cancellous condensation and progressive cortical hypertrophy (Group 2) (Fig. 3); and 47 (33%) showed evidence of cortical porosis at 15 years (Group 3) (Fig. 4), 44 of which also demonstrated progressive cortical hypertrophy (Fig. 5). More hips in Group 3 demonstrated cortical hypertrophy in greater than two zones than those in Group 2 at both 10 (p = 0.006) and 15 years (p = 0.003). Three and five of the 53 hips in Group 2 had greater than two zone cortical hypertrophy at 10 and 15 years respectively compared to 12 and 16 of the 47 hips in Group 3 at the same time periods. Cortical porosis was first evident in one hip at 5 years postimplantation and seven more hips at 10 years, with development in the remaining 39 hips between the 10 and 15 year assessments (Fig. 6).
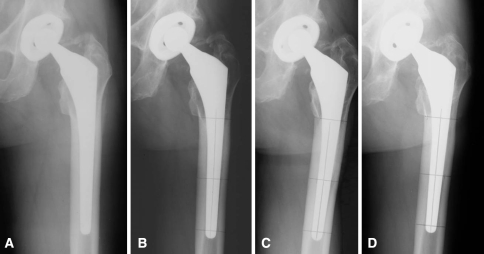
Fig. 2A–D.
This AP radiographic series shows a 49-year-old man in Group 1 with osteoarthrosis of the left hip with minimal remodeling changes from (A) early postoperative through (B) 5 and (C) 10 years to (D) his most recent followup at 15 years postoperatively.
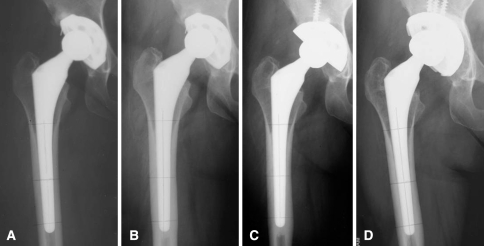
Fig. 3A–D.
This AP radiographic series shows a 48-year-old woman in Group 2 with developmental dysplasia of the right hip at (A) early postoperative and the development of cortical hypertrophy at (B) 5 years postoperatively, with those radiographic changes still evident at (C) 10 and (D) 15 years postoperatively.
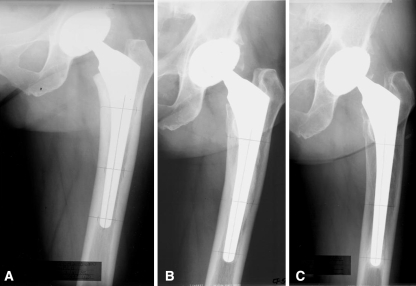
Fig. 4A–C.
This AP radiographic series shows a 51-year-old woman in Group 3 with osteoarthrosis of the left hip at (A) early postoperative, the development of cortical hypertrophy at (B) 5 years postoperatively, and (C) the later development of periprosthetic cortical porosis at 15 years postoperatively.
Fig. 5.
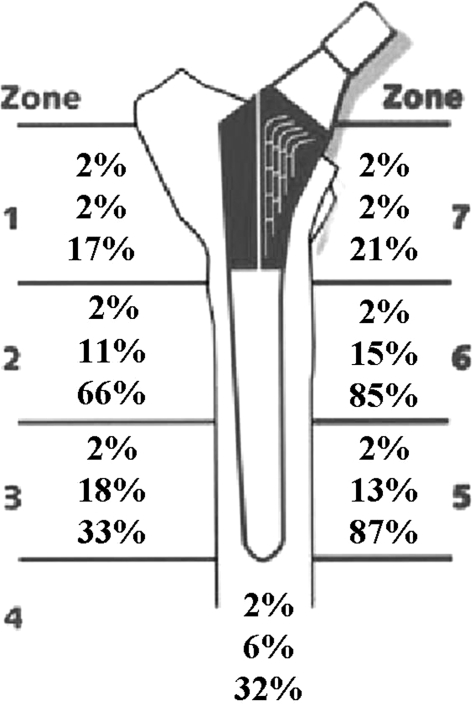
A diagram illustrates the percentage of hips with radiographic evidence of cortical hypertrophy (n = 97) by Gruen zone at 5, 10, and 15 years post-THA with this proximally HA-coated tapered titanium stem. The increasing cortical hypertrophy over time indicates remodeling, for those hips in Groups 2 and 3 that demonstrated remodeling changes, is a dynamic process with this stem.
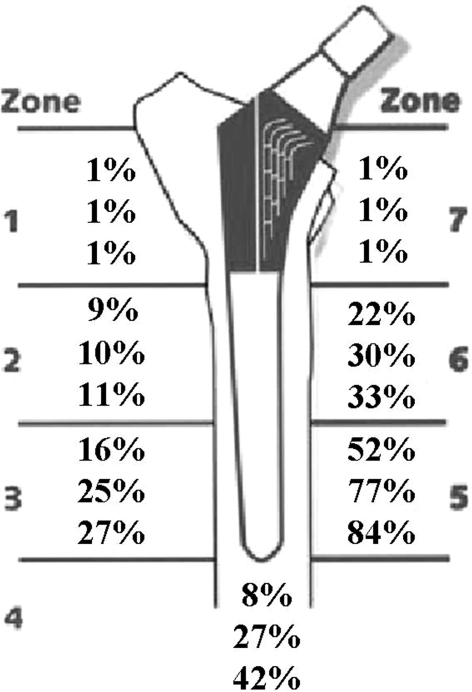
Fig. 6.
A diagram illustrates the percentage of hips with radiographic evidence of cortical porosis (n = 47) by Gruen zone at 5, 10, and 15 years post-THA with this proximally HA-coated tapered titanium stem. Cortical porosis, which was evident in only a small percentage of hips at 5- and 10- years, increased greatly, particularly in Gruen zones 2, 5, and 6, at 15 years in those hips in Group 3.
At 15-year followup, Group 2 had a lower average Harris hip score, primarily due to non-hip-related, comorbid conditions in five patients (Table 1). The number of cup revisions for any reason did not differ between groups (Table 1). All femoral components were well-fixed with no radiographic evidence of loosening at 15 year followup. More hips with minimal radiographic remodeling changes (Group 1) at 15 years had Type A bone, whereas those with remodeling changes and/or cortical porosis had more Type B or C bone (p = 0.0066) (Table 1). There were no differences in stem sizes by group.
Hips with minimal radiographic remodeling changes (Group 1) over 15 years had a higher cortical index at midstem both at baseline (early postoperative) (p = 0.0003) and at 15 years (p = 0.0113) than those with radiographic remodeling changes with or without cortical porosis (Groups 2 and 3) (Table 2). Cortical thickness was also greater in Group 1 than in Groups 2 and 3 beneath the lesser trochanter (p = 0.0374), at midstem (p = 0.0005), and above the tip of the prosthesis (p = 0.0333) at baseline only. There was no difference in canal fill between groups at baseline, but the percentage of canal fill was less in Group 3 than in Groups 1 and 2 at midstem (p = 0.0001) and above the tip of the prosthesis (p = 0.0084) at 15-year followup. Relative differences in bone measurement values from baseline to 15 years across the three groups are shown on the operative side (Table 3) and for the contralateral side (Table 4). On the implant side, there was a greater increase in the outer diameter of the femur in Group 3 than in Groups 1 and 2 at all three levels of measurement, with no differences in cortical index or cortical thickness between groups. Conversely, on the contralateral, nonoperated side, there was no difference between groups in the outer diameter of the femur over time, but there was a decrease in the cortical index and cortical thickness below the lesser trochanter in Group 3. Relative differences in bone measurement values were compared by group on the implant and contralateral sides from baseline to 15 years (Table 5). There was a greater increase on the operative side in Group 3 with regard to the outer diameter of the femur at all three levels of measurement and an increase in the cortical thickness below the lesser trochanter in Group 3 over 15 years.
Table 2.
Radiographic measurements at baseline (early postoperative) and 15 years by group
| Parameter | Baseline | 15-year followup | ||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | p value | Group 1 | Group 2 | Group 3 | p value | |
| Cortical index | ||||||||
| Below lesser trochanter | 31.2 | 30.1 | 28.1 | 0.1153 | 26.2 | 24.8 | 24.9 | 0.7342 |
| Midstem | 50.1 | 47.5 | 45.7 | 0.0003 | 49.7 | 49.0 | 44.0 | 0.0113 |
| Above tip | 53.7 | 51.7 | 50.5 | 0.0729 | 53.8 | 55.3 | 50.3 | 0.0290 |
| Canal fill | ||||||||
| Below lesser trochanter | 73% | 72% | 74% | 0.5179 | 67% | 65% | 68% | 0.3321 |
| Midstem | 87% | 87% | 86% | 0.7979 | 86% | 83% | 74% | 0.0001 |
| Above tip | 82% | 82% | 80% | 0.6191 | 80% | 80% | 72% | 0.0084 |
| Femur outer diameter (mm) | ||||||||
| Below lesser trochanter | 33.5 | 33.0 | 32.1 | 0.2734 | 34.5 | 33.6 | 33.7 | 0.4774 |
| Midstem | 28.8 | 27.2 | 27.2 | 0.0829 | 29.7 | 29.5 | 31.3 | 0.0598 |
| Above tip | 27.8 | 26.4 | 26.6 | 0.1314 | 29.1 | 28.2 | 29.7 | 0.1677 |
| Cortical thickness (mm) | ||||||||
| Below lesser trochanter | 10.5 | 10.0 | 9.0 | 0.0374 | 9.1 | 8.4 | 8.3 | 0.5361 |
| Midstem | 14.5 | 13.0 | 12.5 | 0.0005 | 15.0 | 14.5 | 13.7 | 0.2951 |
| Above tip | 15.0 | 13.7 | 13.5 | 0.0333 | 15.8 | 15.7 | 15.0 | 0.5203 |
Table 3.
Changes in radiographic measurements from baseline (early postoperative) to 15 years on the implant side
| Parameter | Group 1 | Group 2 | Group 3 | p Value |
|---|---|---|---|---|
| Number of hips | 42 | 53 | 43 | |
| Cortical index | ||||
| Below lesser trochanter | −5.05 | −5.38 | −3.60 | 0.4317 |
| Midstem | −0.27 | 1.58 | −1.20 | 0.3184 |
| Above tip | 0.12 | 3.42 | 0.26 | 0.0997 |
| Femur outer diameter | ||||
| Below lesser trochanter | 0.95 | 0.60 | 1.84 | 0.0166* |
| Midstem | 0.80 | 2.25 | 4.33 | 0.0001‡ |
| Above tip | 1.10 | 1.99 | 2.89 | 0.0055† |
| Cortical thickness | ||||
| Below lesser trochanter | −1.43 | −1.56 | −0.53 | 0.2522 |
| Midstem | 0.37 | 1.50 | 1.35 | 0.2435 |
| Above tip | 0.63 | 2.08 | 1.53 | 0.0999 |
A positive number indicates an increase at 15 years, and a negative number indicates a decrease at 15 years; *p < 0.05; †p < 0.01; ‡p < 0.001.
Table 4.
Changes in radiographic measurements from baseline (early postoperative) to 15 years in the contralateral side
| Parameter | Group 1 | Group 2 | Group 3 | p Value |
|---|---|---|---|---|
| Number of hips | 11 | 13 | 14 | |
| Cortical index | ||||
| Below lesser trochanter | −0.05 | 0.33 | −4.66 | 0.0069* |
| Midstem | −1.45 | 0.09 | −2.15 | 0.0812 |
| Above tip | −0.98 | −0.55 | −3.38 | 0.1459 |
| Femur outer diameter | ||||
| Below lesser trochanter | 0.06 | −0.41 | −0.03 | 0.7497 |
| Midstem | −0.30 | −0.50 | −0.09 | 0.8387 |
| Above tip | −0.04 | −0.77 | 0.12 | 0.4832 |
| Cortical thickness | ||||
| Below lesser trochanter | 0.06 | 0.05 | −1.69 | 0.0058* |
| Midstem | −0.49 | −0.18 | −0.57 | 0.9055 |
| Above tip | −0.26 | −0.48 | −0.70 | 0.8046 |
A positive number indicates an increase at 15 years, and a negative number indicates a decrease at 15 years; *p < 0.01.
Table 5.
Changes in radiographic measurements from baseline (early postoperative) to 15 years on the implant side minus changes on contralateral side for paired hips
| Parameter | Group 1 | Group 2 | Group 3 | p Value |
|---|---|---|---|---|
| Number of hips | 11 | 13 | 13 | |
| Cortical index | ||||
| Below lesser trochanter | −5.86 | −4.64 | 2.20 | 0.1529 |
| Midstem | 1.99 | 1.48 | 3.91 | 0.7957 |
| Above tip | 1.62 | 2.05 | 4.00 | 0.7296 |
| Femur outer diameter | ||||
| Below lesser trochanter | −0.01 | 0.34 | 2.05 | 0.0137* |
| Midstem | 0.59 | 1.60 | 5.41 | 0.0001† |
| Above tip | 0.63 | 1.68 | 3.90 | 0.0129* |
| Cortical thickness | ||||
| Below lesser trochanter | −2.11 | −1.39 | 1.78 | 0.0358* |
| Midstem | 0.85 | 1.17 | 3.76 | 0.0860 |
| Above tip | 0.75 | 1.52 | 3.20 | 0.1167 |
These numbers represent differences in absolute values between the operative and nonoperative sides. A positive number indicates a greater relative change on the operative side, and a negative number indicates a greater relative change on the nonoperative side; *p < 0.05; †p < 0.001.
Secondary analyses including only the subgroup of hips with a diagnosis of osteoarthrosis demonstrated a greater percentage (p = 0.0216) of women were in the cortical porosis group at 15 years. Of the 90 patients (102 hips) with osteoarthrosis, 50 (56%) were female, with 36%, 54%, and 79% of the hips in Groups 1, 2, and 3, respectively. All other analyses using this subgroup yielded results similar to those reported with the total study group.
Discussion
The rationale for this study arose from the observation of some unexpected radiographic findings in a few hips, first noted to appear at about 10 years postimplantation with this proximally HA-coated tapered titanium stem. Those changes were a progressive and distal migration of cortical hypertrophy in hips that showed early evidence of hypertrophy and a generalized periprosthetic bone loss in a few patients. We then designed this study to retrospectively examine the plain radiographs of this entire patient cohort followed a minimum of 15 years post-THA to evaluate the incidence and nature of late remodeling changes. The primary research questions in this retrospective examination of serial plain radiographs were to determine how many hips showed radiographic signs of late remodeling around this proximally HA-coated femoral component and what patient or implant factors are associated with these late remodeling changes.
The major limitation of this study is the ability to quantify the late remodeling changes seen in the femur with this proximally HA-coated tapered titanium stem from plain radiographs. Unfortunately, since the radiographic changes prompting this study were not anticipated, only plain radiographs were available for this retrospective analysis. Yet the data in this study confirm those of other studies using dual-energy xray absorptiometry [2, 27, 43] and quantitative CT [5, 36, 37]. The associated finding of progressive cortical hypertrophy in this study was quantifiable and lends credence to these study results. One other limitation of this study is that bone type was estimated from the anteroposterior radiograph only, because many of the lateral radiographs were inadequate to clearly visualize the necessary bony landmarks.
The results of our retrospective analysis of late bone remodeling revealed patients fell into one of three subgroups. Thirty percent of hips showed minimal remodeling changes (cancellous condensation but no cortical hypertrophy or porosis) from the time of implantation to 15-year followup. This group tended to have more men and more Type A bone than the other two remodeling groups. The remaining 70% of patients showed early evidence of adaptive remodeling typical of that previously described, that is, cortical hypertrophy in Gruen Zones 2 through 6 progressively and evidence of cancellous condensation in at least one Gruen zone (2, 3, 5, or 6) [10]. Of the 70% of hips with remodeling changes, 47 hips (33% of total) also had developed generalized cortical porosis at 15 years post-THA. Regardless of these radiographic changes, no hips in this study showed evidence of implant loosening and all patients are functioning well clinically at 15 years.
In contrast to previous suggestions [1, 25, 35, 38, 44, 46] we found adaptive remodeling did not stop after the first few years. In a number of hips with cortical hypertrophy, the area of cortical hypertrophy was noted to have moved distally over the course of time and the general appearance of these radiographs at 15 years appears more similar to that of an extensively fixed rather than a proximally fixed implant. One could speculate these stems became extensively osseointegrated due to stress transmission moving from proximal to distal over time, resulting in proximal stress shielding and manifesting as cortical porosis at 10 to 15 years postimplantation. This same phenomenon was noted in a study by Geesink [18] using this same stem in which bone apposition was noted to progress distally over time, even at 10 years. In yet another study of a proximally HA-coated femoral component with a curved stem (ABG®; Stryker UK Ltd, Newbury, UK) the authors reported that the location of cortical thickening and densification over the course of time suggested implant/bone stress transfer moved distally in 51 of 67 stems after 10 years [36].
Another notable and unexpected finding of this study was that cortical porosis, which was evident in only one hip before 10 years and seven more hips at 10 years postimplantation, was seen in 1/3 (47) of hips at 15-year followup. No attempt was made to differentiate stress shielding secondary to adaptive bone remodeling around the implant from metabolic bone changes occurring with the normal aging process in this study, but it is presumed both factors contributed to the bone loss. In cases where there was a nonoperated, contralateral side with sufficient radiographs, cortical index, cortical thickness, and the outer diameter of the femur were measured and compared to the operated side. Side-to-side comparisons over time indicated a relative increase in the outer diameter of the femur on the operated side in Group 3, likely reflecting the increase in cortical hypertrophy in that group. An interesting finding is related to the apparent age-related decrease in cortical thickness seen over 15 years in the area below the lesser trochanter on both the implant and contralateral sides. The decrease was greater on the contralateral side, possibly indicating preservation of cortical thickness through stress transfer on the implant side. With regard to the adaptation of more flexible bone around a relatively stiffer implant, in a canine model, Geesink [18] reported a thinning of the outer cortex through stress shielding over time, but also the formation of a new cortex directly in contact with the implant with trabecular bridges between the two dense bone areas. Radiographically these adaptive changes could be described or appear as cortical porosis.
Hips demonstrating generalized cortical porosis were more likely to have had Type B or C bone (less distinct and thinner cortices) at the time of implantation, and when including only those patients with a diagnosis of osteoarthrosis, there were more women in the cortical porosis subgroup. Poorer bone quality on either the operated or contralateral side at the time of implantation reportedly correlated with a greater loss of bone density later in a number of other studies of cementless stems [15, 29, 37]. In one retrieval study of 11 pairs of femurs, one of which had been implanted with an extensively porous-coated stem at an average 5.9 years, women had greater bone loss than men (31.2% versus 12.3%, respectively) [41]. One study of nonoperated femurs using CT reported a correlation between increased porosity and advancing age in women and a similar, but weaker correlation in men. That same study also reported an age-related decrease in cortical thickness in women but no such relationship in men [5].
A question that arises from the findings of this study is whether the combination of continued adaptive bone remodeling around a hip stem in conjunction with the generalized porosis of aging will eventually threaten the implant stability. The aim of THA has always been to provide a long-lasting, ideally single, implant for the patient requiring THA. As the technology has improved, so the indications for the timing of THA have increased to include much younger patients. On the other end of the spectrum, patients are also living longer, more active lives. Thus, it is imperative we begin to examine how the bone loss associated with normal aging may affect the longevity of a hip implant. This study cohort was relatively young at the time of their THA (average age, 51 years); therefore, these patients may require 30 years or more from their THA. At 15 years postimplantation, we have seen the development of cortical porosis in about 1/3 of the patients in which this proximally HA-coated femoral component was implanted. All implants in this study remain well-fixed and the patients are functioning well at 15 years. In addition, this same implant has performed equally well in other mid- to long-term studies [7, 21, 27]. Longer-term followup is needed to determine if this generalized bone loss will eventually threaten the stability of the implant. Studies of other implants long-term have looked at radiographic remodeling changes [32–34, 40] but not specifically in relationship to changes in bone quality associated with aging. In fact, often such long-term followup studies rely on telephone interview with the patient or family member and do not include radiographic followup.
Our data suggest certain patients, in particular women with poorer bone quality at the time of arthroplasty, may be more likely to develop periprosthetic cortical porosity after 10 years or more. Given these results and knowing implants of various designs remodel differently, the question arises whether one implant design is preferable over another in patients with a particular profile undergoing THA. Design considerations include metal (titanium versus cobalt-chrome) and fixation methods (porous versus HA coating and proximal versus extensive coating). For example, in this study the distal portion of the stem was grit-blasted, and it is possible in some cases of progressive remodeling this portion of the stem became osseointegrated, whereas this may not have occurred had the distal portion of the stem been polished. This study examined only a single implant design and a single patient cohort. Radiographs of other implant designs and fixation methods need to be examined in a similar manner to determine the incidence and effect of cortical porosity in a variety of ages, races, both sexes, and all bone types. In addition, other patient factors such as activity levels and medication use such as hormone replacement therapy or other drugs that might affect bone quality need to be examined. Age-related bone changes alone are complex, but when combined with implant-related bone changes, future studies will need to include large numbers of patients and implants to determine which factors are significant and in what combinations they might have either a beneficial or deleterious effect on the surrounding bone.
The aim of this proximally HA-coated tapered titanium stem was to preserve bone, yet the radiographs of 1/3 of these hips indicate a generalized loss of bone, partially due to continued bone remodeling secondary to the implant and partially due to the bone loss associated with aging. Poorer bone quality at the time of arthroplasty and female gender appear to be risk factors. Thus, the question arises whether a particular patient profile can be identified in future studies as one with a higher risk of developing cortical porosis in the long term. Additional studies should examine not only patient factors but implant design factors, which could then lead to the use of an optimal implant design for the subset of patients at risk for late remodeling changes. The current iteration of this stem has a plasma-spray coating proximal in addition to the HA-coating. It remains to be seen what effect, if any, this modification will have on long-term remodeling. Nonetheless, at 15 years postimplantation, this proximally HA-coated implant remains well-fixed and without clinical symptoms in this entire cohort of 143 hips. We will continue to follow these patients and evaluate their clinical scores and radiographs to determine if and when bone loss associated with normal aging might threaten the stability of their hip implant.
Acknowledgments
We thank Rama Ramakrishnan, Jianhua Shen, Nilam Babaria, and William L. Jaffe, MD, for their expert contributions to this study.
Footnotes
One or more of the authors (WNC, JAD, RGG, JRF, MN) have received funding from Stryker Orthopaedics, Mahwah, NJ.
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ang C, Das De S, Goh JC, Low SL, Bose K. Periprosthetic bone remodeling after cementless total hip replacement: a prospective comparison of two different implant designs. J Bone Joint Surg Br. 1997;79:675–679. [DOI] [PubMed]
- 2.Bloebaum RD, Liau DW, Lester DK, Rosenbaum TG. Dual-energy x-ray absorptiometry measurement and accuracy of bone mineral after unilateral total hip arthroplasty. J Arthroplasty. 2006;21:612–622. [DOI] [PubMed]
- 3.Bobyn JD, Glassman AH, Goto H, Krygier JJ, Brooks CE, Miller JE. The effect of stem stiffness on femoral bone resorption after canine porous-coated total hip arthroplasty. Clin Orthop Relat Res. 1990;261:196–213. [PubMed]
- 4.Bobyn JD, Mortimer ES, Glassman AH, Engh CA, Miller JE, Brooks CE. Producing and avoiding stress shielding: laboratory and clinical observations of non-cemented total hip arthroplasty. Clin Orthop Relat Res. 1992;274:79–96. [PubMed]
- 5.Bousson V, Bergot C, Meunier A, Barbot F, Parlier-Cuau C, Laval-Jeantet AM, Laredo JD. CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity. Radiology. 2000;217:179–187. [DOI] [PubMed]
- 6.Cameron HU. The 3–6 year results of a modular noncemented low-bending stiffness hip implant: a preliminary study. J Arthroplasty. 1993;8:239–243. [DOI] [PubMed]
- 7.Chang J-K, Chen C-H, Huang K-Y, Wang G-J. Eight-year results of hydroxyapatite-coated hip arthroplasty. J Arthroplasty. 2006;21:541–546. [DOI] [PubMed]
- 8.Cutter J. Living well to 100. MedicineNet Web site. Available at: http:www.medicinenet.com/script/main/art.asp?articlekey=51451. Accessed October 29, 2007.
- 9.D’Antonio JA, Capello WN, Crothers OD, Jaffe WL, Manley MT. Early clinical experience with hydroxyapatite-coated femoral implants. J Bone Joint Surg Am. 1992;74:995–1008. [PubMed]
- 10.D’Antonio JA, Capello WH, Manley MT. Remodeling of bone around hydroxyapatite-coated stems. J Bone Joint Surg Am. 1996;78:1226–1234. [DOI] [PubMed]
- 11.Dorr LD, Absatz M, Gruen TA, Saberi MT, Doerzbacher JF. Anatomic porous replacement hip arthroplasty: first 100 consecutive cases. Semin Arthroplasty. 1990;1:77–86. [PubMed]
- 12.Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop Relat Res. 1988; 231:7–28. [PubMed]
- 13.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement: the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. [DOI] [PubMed]
- 14.Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–128. [PubMed]
- 15.Engh CA, McGovern TG, Bobyn JD, Harris WH. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg Am. 1992;74:1009–1020. [PubMed]
- 16.Engh CA, O’Connor D, Jasty M, McGovern TF, Bobyn JD, Harris WH. Quantification of implant micromotion, strain shielding, and bone resorption with porous-coated anatomic medullary locking femoral prostheses. Clin Orthop Relat Res. 1992; 285:13–29. [PubMed]
- 17.Finlay JB, Rorabeck CH, Bourne RB, Tew WM. In vitro analysis of proximal femoral strains using PCA femoral implants and a hip-abductor muscle stimulator. J Arthroplasty. 1989;4:335–345. [DOI] [PubMed]
- 18.Geesink RGT. Osteoconductive coatings for total joint arthroplasty. Clin OrthopRelat Res. 2002;395:53–65. [DOI] [PubMed]
- 19.Glassman AH, Crowninshield RD, Schneck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;393:128–136. [DOI] [PubMed]
- 20.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 21.Huiskes R. The various stress patterns of press-fit, ingrown, and cemented femoral stems. Clin Orthop Relat Res. 1990;261:27–38. [PubMed]
- 22.Huiskes R, van Rietbergen B. Preclinical testing of total hip stems: the effects of coating placement. Clin Orthop Relat Res. 1995;319:64–76. [PubMed]
- 23.Huiskes R, Weinans H, Dalstra M. Adaptive bone remodeling and biomechanical design considerations for noncemented total hip arthroplasty. Orthopedics. 1989;12:1255–1267. [DOI] [PubMed]
- 24.Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;274:124–134. [PubMed]
- 25.Kiratli BJ, Checovich MM, McBeath AA, Wilson MA, Heiner JP. Measurement of bone mineral density by dual energy x-ray absorptiometry in patients with the Wisconsin hip, an uncemented femoral stem. J Arthroplasty. 1996; 11:184–193. [DOI] [PubMed]
- 26.Korovessis P, Droutsas P, Piperos G, Michael A, Baikousis A, Stamatakis M. Course of bone mineral content changes around cementless Zweymueller total hip arthroplasty: A 4 year follow-up study. Arch Orthop Trauma Surg. 1997;116:60–65. [DOI] [PubMed]
- 27.Kroger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O. Evaluation of periprosthetic bone using dual-energy x-ray absorptiometry: precision of the method and effect of operation on bone mineral density. J Bone Miner Res. 1996; 11:1526–1530. [DOI] [PubMed]
- 28.Life expectancy by age, 1850–2004. Infoplease Web site. Available at: http://www.infoplease.com/ipa/A0005140.html. Accessed April 9, 2008.
- 29.Maloney WJ, Sychterz C, Bragdon C, McGovern T, Jasty M, Engh CA, Harris WH. Skeletal response to well fixed femoral components inserted with and without cement. Clin Orthop Relat Res. 1996;333:15–26. [DOI] [PubMed]
- 30.McAuley JP, Sychterz CJ, Engh CA. Influence of porous coating level on proximal femoral remodeling. Clin Orthop Relat Res. 2000;371:146–153. [DOI] [PubMed]
- 31.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in tensile properties of cortical bone: the relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–1205. [DOI] [PubMed]
- 32.Mullis MM, Norbury W, Dowell JK, Heywood-Waddington M. Thirty-year results of a prospective study of Charnley total hip arthroplasty by the posterior approach. J Arthroplasty. 2007;22:833–839. [DOI] [PubMed]
- 33.Nagi ON, Kumar S, Aggarwal S. The uncemented isoelastic/isotitan total hip arthroplasty: a 10–15 years follow-up with bone mineral density evaluation. Acta Orthop Belg. 2006;72:55–64. [PubMed]
- 34.Nercessian OA, Martin G, Joshi RP, Su BW, Eftekhar NS. A 15- to 25-year follow-up study of primary Charnley low-friction arthroplasty: a single surgeon series. J Arthroplasty. 2005;20:162–167. [DOI] [PubMed]
- 35.Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res. 1997;339:121–131. [DOI] [PubMed]
- 36.Oosterbos CJ, Rahmy AI, Tonino AJ, Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand. 2004;75:127–133. [DOI] [PubMed]
- 37.Pandit S, Graydon A, Bradley L, Walker C, Pitto R. Computed tomography assisted osteodensitometry in total hip arthroplasty. ANZJ Surg. 2006;76:778–781. [DOI] [PubMed]
- 38.Rosenthall L, Bobyn JD, Brooks CE. Temporal changes of periprosthetic bone density in patients with a modular noncemented femoral prosthesis. J Arthroplasty. 1999;14:71–76. [DOI] [PubMed]
- 39.Sabo D, Reiter A, Simank HG, Thomsen M, Lukoschak M, Ewerbeck V. Periprosthetic mineralization around cementless total hip endoprosthesis: longitudinal study and cross-sectional study on titanium threaded acetabular cup and cementless Spotorno stem with DEXA. Calcif Tissue Int. 1998;62:177–182. [DOI] [PubMed]
- 40.Schulte KR, Callaghan JJ, Kelley SS, Johnston RC. The outcome of Charnley total hip arthroplasty with cement after a minimum twenty-year follow-up. The results of one surgeon. J Bone Joint Surg Am. 1993;75:961–975. [DOI] [PubMed]
- 41.Sychterz CJ, Engh CA. The influence of clinical factors on periprosthetic bone remodeling. Clin Orthop Relat Res. 1996;322:285–292. [DOI] [PubMed]
- 42.Tanzer M, Chan S, Brooks CE, Bobyn JD. Primary cementless total hip arthroplasty using a modular femoral component: a minimum 6-year follow-up. J Arthroplasty. 2001;16(Suppl):64–70. [DOI] [PubMed]
- 43.Trevisan C, Bigoni M, Randelli G, Marinoni EC, Peretti G, Ortolani S. Periprosthetic bone density around fully hydroxyapatite coated femoral stem. Clin Orthop Relat Res. 1997;340:109–117. [DOI] [PubMed]
- 44.Venesmaa PK, Kroger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhav EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy x-ray absorptiometry: a 3 year follow-up study. J Bone Miner Res. 2001;16:1056–1061. [DOI] [PubMed]
- 45.Wixson RL, Stulberg SD, Van Flandern GJ, Puri L. Maintenance of proximal bone mass with an uncemented femoral stem: analysis with dual-energy x-ray absorptiometry. J Arthroplasty. 1997;12:365–372. [DOI] [PubMed]
- 46.Yamaguchi K, Masuhara K, Ohzono K, Sugano N, Nishii T, Ochi T. Evaluation of periprosthetic bone-remodelling after cementless total hip arthroplasty: the influence of the extent of porous coating. J Bone Joint Surg Am. 2000;82:1426–1431. [DOI] [PubMed]