Abstract
Metal-on-metal bearing total hip arthroplasty is performed more commonly than in the past. There may be manufacturing differences such as clearance, roughness, metallurgy, and head size that affect performance. In a prospective, randomized trial, we compared 2-year postoperative ion levels for a 28-mm metal-on-polyethylene bearing with 28-mm and 36-mm metal-on-metal bearings. We measured serum, erythrocyte, and urine ion levels. We observed no difference in the ion levels for the 28-mm and 36-mm metal-on-metal bearings. The ion levels in these patients were lower than reported for most other metal-on-metal bearings. Although both erythrocyte and serum cobalt increased, erythrocyte chromium and erythrocyte titanium did not increase despite a four- to sixfold serum chromium and a three- to fourfold serum titanium increase. This may represent a threshold level for serum chromium and serum titanium below which erythrocytes are not affected.
Level of Evidence: Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metal-on-metal bearing total hips have been approved for use in the United States since 1999 and use is increasing [26, 27]. Some long-term followup studies of the McKee-Farrar metal-on-metal hip have demonstrated results equivalent to metal-on-polyethylene hip replacements [3, 20]. More recently, midterm results on second-generation metal-on-metal hips are available and appear encouraging [6, 7, 19, 21, 24, 29–31].
However, concerns about metal ion release, cancer, osteolysis, and hypersensitivity remain [5, 9, 11, 15, 16, 25, 31, 33, 35]. Additionally, there may be performance issues related to manufacturing choices such as clearance, femoral head size, carbon content, and chrome cobalt processing that are reflected in the metal ion levels of patients with these implants [8, 15, 17, 32]. Because clinical, surgical, biologic, and manufacturing factors can affect the bearing performance, there is a need for well-designed clinical studies that control as many variables as possible.
Well-designed clinical outcome studies will likely take 5 to 10 years to demonstrate a difference in either the occurrence of osteolysis or revision rates for metal-on-metal hips. However, measurement of ion levels from patients may allow an early glimpse of metal-on-metal bearing performance [17]. To date, variations in metal ion levels have been associated with head size, cup abduction angle, T cell counts, and lymphocyte changes [2, 4, 12, 13, 22]. In addition, a variation in ion levels reported in different studies has been attributed to design differences such as head-to-cup clearance and variations in metallurgy [23, 28, 34].
We designed a prospective, randomized blinded study with three arms to first evaluate serum, erythrocyte, and urine ion levels using a metal-on-polyethylene bearing as a control along with 28-mm and 36-mm metal-on-metal as the study bearings; we expected the ion levels for the two metal-on-metal bearings would be higher than the ion levels for the metal-on-polyethylene and hoped the magnitude of this elevation would be less than reported for other metal-on-metal designs. Second, we asked whether there was a difference between the ion levels when comparing the 28-mm metal-on-metal with the 36-mm metal-on-metal bearing surface. Third, we wanted to identify any relationships between serum, erythrocyte, and urine metal ion levels.
Materials and Methods
From October 2003 until October 2005, we offered enrollment to patients 40 to 80 years of age with unilateral noninflammatory degenerative joint disease and without a preexisting arthroplasty that might affect ion levels. One hundred six enrolled patients were randomized and had surgery. Two patients withdrew, five were lost to followup, one had a femoral revision for failure of femoral ingrowth, and seven had a contralateral hip arthroplasty before the 2-year evaluation. This left 91 patients with unilateral hip arthroplasty in the cohort. Thirty-four patients were included in the metal-on-polyethylene control group, 25 had a 28-mm metal-on-metal hip, and 32 had a 36-mm metal-on-metal bearing hip. Patients, laboratory personnel, and clinical assistants were blinded to the implant. This prospective, randomized study was Institutional Review Board-approved and carried out at two institutions.
All patients had either an AML or a Prodigy (DePuy Orthopaedics, Warsaw, IN) extensively porous-coated chrome-cobalt femoral implant. The acetabular shell was a titanium porous-coated Pinnacle design (DePuy Orthopaedics). This Pinnacle acetabular component can accept a polyethylene liner or a metal-on-metal insert. The polyethylene liner is secured with a self-locking peripheral taper and dome contact. The metal inserts are held with the same self-locking taper. Only patients with a pelvis that could accommodate a 52-mm acetabular shell were enrolled because that is the smallest shell that can accept a 36-mm metal-on-metal liner (Fig. 1).
Fig. 1.
This figure shows extensively porous-coated femoral stems, 28-mm and 36-mm femoral heads, and the Pinnacle shell with the three different bearing surfaces.
The polyethylene used in the control group was a Marathon (DePuy Orthopaedics) polyethylene, which is crosslinked with 5 Mrads of external beam radiation, heated to melt temperature to extinguish free radicals, and then gas plasma-sterilized. The metal-on-metal acetabular liners and femoral balls are wrought high-carbon alloy. The carbon content is considered high at 0.15% to 0.35% meeting the ASTM F1537 Alloy 2 specifications. Both the femoral heads and the acetabular liners are highly polished. The only difference between the 28- and the 36-mm metal-on-metal bearing surfaces is the 28-mm bearing surface has a 60 μm ± 20-μm clearance, and the 36-mm bearing surface has a 100 μm ± 20-μm clearance. The femoral heads, acetabular liners, and femoral components are composed of 59% to 68% cobalt and 27% to 30% chromium.
We determined patient functional status preoperatively, at 6 months, 1 year, and 2 years with a Harris hip score (HHS), WOMAC score, and a SF-12 score. Femoral stability was graded with the technique described by Engh and the acetabular components were considered loose if they had circumferential radiolucencies, migration more than 3 mm, or a change in inclination of more than 5° [10]. We used these validated clinical measures to confirm the quality of the randomization.
Blood and 24-hour urine samples were collected at preoperative, 6-month, 1- and 2-year intervals. The protocol has been previously described [28]. Care was taken to prevent metal contamination from the needle or collection tubes. All specimens were analyzed on a high-resolution inductively coupled mass spectrometer at one institution.
The metal ion level distributions were asymmetric; therefore, nonparametric tests were used and we therefore used the Mann-Whitney test to compare samples from each of the three treatment groups. When ion levels at two time intervals for the same group were considered, the Wilcoxon test was used. We used the Statistical Package for the Social Sciences (version 8.0 for Windows; SPSS Inc, Chicago, IL) for the analysis.
Results
We observed no difference in HHS at the preoperative or 2-year postoperative visit among any of the three groups (p = 0.057, 0.246, and 0.274) (Table 1). However, at 2 years, the 28-mm metal-on-metal group had lower WOMAC and SF-12 physical (p = 0.052 and 0.033) and higher SF-12 mental scores (p = 0.015) than the metal-on-polyethylene group. One asymptomatic patient has a fibrous stable femoral component. All other acetabular and femoral components exhibited no radiographic signs of loosening.
Table 1.
Two-year mean clinical evaluation scores
| Treatment group | Metal-on-polyethylene | Metal-on-metal, 28 mm | Metal-on-metal, 36 mm | Intergroup p value* (MOP and MOM28) | Intergroup p value* (MOP and MOM36) | Intergroup p value* (MOM28 and MOM 36) |
|---|---|---|---|---|---|---|
| Harris hip | 96 ± 6 | 92 ± 10 | 95 ± 9 | 0.057 | 0.246 | 0.274 |
| WOMAC | 89 ± 19 | 88 ± 11 | 88 ± 18 | 0.052 | 0.674 | 0.129 |
| SF-12 PCS | 51 ± 9 | 44 ± 12 | 48 ± 11 | 0.033 | 0.281 | 0.224 |
| SF-12 MCS | 55 ± 6 | 58 ± 7 | 56 ± 7 | 0.015 | 0.279 | 0.262 |
*Intergroup p value = difference in mean evaluation scores at the 2-year postoperative interval between the metal-on-polyethylene group and each of the metal-on-metal groups and between the two metal-on-metal groups using the Mann-Whitney test; means and standard deviations are shown; no differences between groups were seen for any of the clinical evaluations at 2-year followup with the exception of the WOMAC (0.052), SF-12 physical (0.033) and mental scores (0.015) between the metal-on-polyethylene (MOP) group and the 28-mm metal-on-metal (MOM) group.
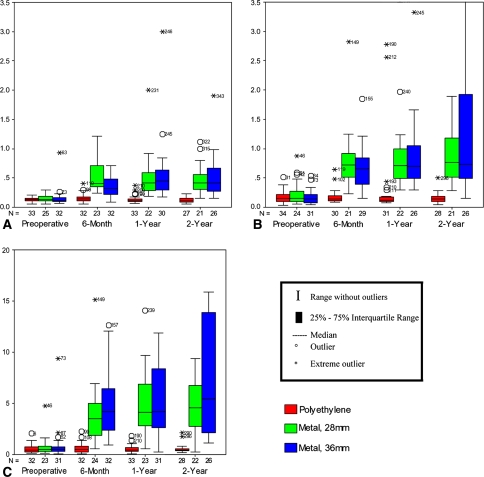
Erythrocyte, serum, and urine cobalt intragroup and intergroup comparisons along with probability values are reported (Table 2) (Fig. 2A–C). In summary, 28-mm and 36-mm metal-on-metal 2-year cobalt serum, erythrocyte, and urine ion levels were higher than the 2-year metal-on-polyethylene levels (all intergroup p values < 0.001). There was no difference between the 28-mm and the 36-mm metal-on-metal serum, erythrocyte, or urine cobalt levels at 2 years (intergroup p = 0.831, 0.915, and 0.612). Erythrocyte cobalt increased 3.2-fold and 3.8-fold from preoperatively to 2 years postoperatively for the 28-mm metal-on-metal and 36-mm metal-on-metal groups. This represented an erythrocyte cobalt increase for individual patients on average of only 0.33 μg/L (range, 0.08–0.49 μg/L) and 0.29 μg/L (range, 0.14–0.64 μg/L) for the 28-mm metal-on-metal and 36-mm metal-on-metal groups, respectively. Serum cobalt increased 4.8-fold and 5.2-fold from preoperatively to 2 years postoperatively for the 28-mm metal-on-metal and 36-mm metal-on-metal groups, respectively. This represented a serum cobalt increase for individual patients on average of only 0.61 μg/L (range, 0.33–1.05 μg/L) and 0.48 μg/L (range, 0.20–1.78 μg/L) for the 28-mm metal-on-metal and 36-mm metal-on-metal groups, respectively.
Table 2.
Cobalt ion concentration in erythrocytes, serum, and urine
| Treatment group | Median preoperative concentration* (interquartile range) | Median 6-month concentration (interquartile range) | Median year 1 concentration (interquartile range) | Median year 2 concentration (interquartile range) | Intragroup p value|| | Intergroup p value¶ (MOP and MOM) | Intergroup p value (MOM28 and MOM 36) |
|---|---|---|---|---|---|---|---|
| Erythrocyte cobalt | |||||||
| MOP† | 0.13 (0.11–0.16) | 0.14 (0.11–0.18) | 0.11 (0.10–0.14) | 0.11 (0.09–0.15) | 0.249 | ||
| MOM28‡ | 0.13 (0.11–0.19) | 0.41 (0.35–0.73) | 0.42 (0.27–0.59) | 0.42 (0.30–0.60) | < 0.001 | < 0.001 | |
| MOM36§ | 0.11 (0.09–0.16) | 0.32 (0.21–0.51) | 0.45 (0.30–0.65) | 0.42 (0.27–0.71) | < 0.001 | < 0.001 | 0.915 |
| Serum cobalt | |||||||
| MOP | 0.15 (0.10–0.22) | 0.15 (0.11–0.20) | 0.12 (0.10–0.17) | 0.14 (0.09–0.19) | 0.252 | ||
| MOM28 | 0.16 (0.09–0.29) | 0.72 (0.49–0.98) | 0.72 (0.48–1.02) | 0.77 (0.48–1.19) | < 0.001 | < 0.001 | |
| MOM36 | 0.14 (0.08–0.22) | 0.66 (0.37–0.85) | 0.70 (0.49–1.10) | 0.73 (0.47–1.99) | < 0.001 | < 0.001 | 0.831 |
| Urine cobalt | |||||||
| MOP | 0.41 (0.26–0.75) | 0.50 (0.27–0.80) | 0.42 (0.29–0.78) | 0.44 (0.35–0.60) | 0.809 | ||
| MOM28 | 0.48 (0.29–1.03) | 3.52 (1.83–5.01) | 4.13 (2.79–7.51) | 4.55 (2.74–6.86) | < 0.001 | < 0.001 | |
| MOM36 | 0.48 (0.32–0.78) | 4.16 (2.29–6.50) | 4.20 (2.56–8.48) | 5.42 (2.07–14.22) | 0.001 | < 0.001 | 0.612 |
*All concentrations are given in μg/L; †MOP = patients randomized to metal-on-polyethylene THA; ‡MOM28 = patients randomized to metal-on-metal, 28-mm THA; §MOM36 = patients randomized to metal-on-metal, 36-mm THA; ||intragroup p value = difference in preoperative and 2-year postoperative cobalt ion concentrations within each group using the Wilcoxon test; ¶intergroup p value = difference in cobalt ion concentrations at the 2-year postoperative interval between the metal-on-polyethylene group and each of the metal-on-metal groups and between the two metal-on-metal groups using the Mann-Whitney test.
Fig. 2A–C.
Cobalt ion levels in (A) erythrocytes, (B) serum, and (C) urine among the three treatment groups at the preoperative, 6-month, 1-year, and 2-year intervals. All metal ion level units are in micrograms/liter. The number of cases in each group is denoted by the N. The upper limits of normal for cobalt in erythrocytes, serum, and urine are 0.23 μg/L, 0.40 μg/L, and 1.25 μg/L, respectively. At 2 years, cobalt serum, erythrocyte, and urine ion levels in the two metal-on-metal groups were higher compared with the metal-on-polyethylene group (all p < 0.001). There was no difference at 2 years in these same ion levels comparing the 28-mm and 36-mm metal-on-metal groups (p = 0.915, 0.831, 0.612).
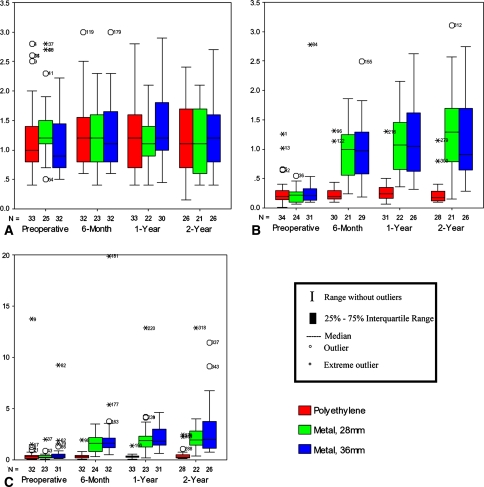
Erythrocyte, serum, and urine chromium intragroup and intergroup comparisons along with probability values are reported (Table 3) (Fig. 3A–C). Although serum chromium ion levels increased at 2 years for the two metal-on-metal groups (intragroup p < 0.001 and p = 0.001), there was surprisingly no elevation in erythrocyte chromium ions (intragroup p = 0.609, 0.198, and 0.525) nor any difference in the erythrocyte chromium ions comparing the metal-on-polyethylene with the metal-on-metal groups (intergroup p = 0.864 and 0.527). There were no differences between the 2-year chromium serum, erythrocyte, or urine levels when comparing the 28-mm with the 36-mm metal-on-metal groups (intergroup p = 0.600, 0.427, and 0.788). Although the serum chromium ion levels increased 5.9-fold and 4.3-fold from preoperatively to 2 years, the actual magnitude of this change for individual patients was on average only 1.15 μg/L (range, 0.62–1.43 μg/L) and 0.56 μg/L (range, 0.36–1.58 μg/L) for the 28-mm and 36-mm metal-on-metal groups, respectively. The 2-year urine chromium level was 9.6- and 5.6-fold greater than the preoperative level for the 28-mm and 36-mm metal-on-metal groups, respectively.
Table 3.
Chromium ion concentration in erythrocytes, serum, and urine
| Treatment group | Median preoperative concentration* (interquartile range) | Median 6-month concentration (interquartile range) | Median year 1 concentration (interquartile range) | Median year 2 concentration (interquartile range) | Intragroup p value|| | Intergroup p value¶ (MOP and MOM) | Intergroup p value (MOM28 and MOM 36) |
|---|---|---|---|---|---|---|---|
| Erythrocyte chromium | |||||||
| MOP† | 1.00 (0.75–1.45) | 1.20 (0.80–1.58) | 1.20 (0.70–1.60) | 1.10 (0.70–1.73) | 0.609 | ||
| MOM28‡ | 1.20 (1.10–1.55) | 1.20 (0.80–1.60) | 1.10 (0.87–1.42) | 1.10 (0.60–1.70) | 0.198 | 0.864 | |
| MOM36§ | 0.90 (0.70–1.47) | 1.10 (0.80–1.68) | 1.20 (1.00–1.85) | 1.20 (0.78–1.63) | 0.525 | 0.527 | 0.427 |
| Serum chromium | |||||||
| MOP | 0.20 (0.13–0.29) | 0.20 (0.14–0.32) | 0.24 (0.13–0.37) | 0.17 (0.12–0.29) | 0.904 | ||
| MOM28 | 0.22 (0.10–0.28) | 0.99 (0.49–1.30) | 1.07 (0.65–1.46) | 1.29 (0.77–1.72) | < 0.001 | < 0.001 | |
| MOM36 | 0.21 (0.12–0.34) | 0.97 (0.55–1.39) | 1.06 (0.61–1.63) | 0.91 (0.64–1.78) | 0.001 | < 0.001 | 0.600 |
| Urine chromium | |||||||
| MOP | 0.30 (0.15–0.49) | 0.30 (0.18–0.50) | 0.31 (0.22–0.37) | 0.27 (0.19–0.53) | 0.819 | ||
| MOM28 | 0.24 (0.18–0.46) | 1.64 (0.74–2.19) | 1.88 (1.20–2.40) | 1.92 (1.44–2.90) | < 0.001 | < 0.001 | |
| MOM36 | 0.36 (0.18–0.58) | 1.65 (1.19–2.17) | 1.80 (1.33–3.04) | 2.02 (1.08–3.74) | < 0.001 | < 0.001 | 0.788 |
*All concentrations are given in μg/L; †MOP = patients randomized to metal-on-polyethylene THA; ‡MOM28 = patients randomized to metal-on-metal, 28-mm THA; §MOM36 = patients randomized to metal-on-metal, 36-mm THA; ||intragroup p value = difference in preoperative and 2-year postoperative chromium ion concentrations within each group using the Wilcoxon test; ¶intergroup p value = difference in chromium ion concentrations at the 2-year postoperative interval between the metal-on-polyethylene group and each of the metal-on-metal groups and between the two metal-on-metal groups, using the Mann-Whitney test.
Fig. 3A–C.
Chromium ion levels in (A) erythrocytes, (B) serum, and (C) urine among the three treatment groups at the preoperative, 6-month, 1-year, and 2-year intervals. All metal ion level units are in micrograms/liter. The number of cases in each group is denoted by the N. The upper limits of normal for chromium in erythrocytes, serum, and urine are 3.0 μg/L, 0.3 μg/L, and 0.8 μg/L, respectively. Although serum chromium ion levels increased at 2 years for the 28-mm and 36-mm metal-on-metal groups (all p ≤ 0.001), there was no corresponding elevation either within groups (p = 0.198 and 0.525) or between groups for erythrocyte chromium (p = 0.864 and 0.527). There was no difference comparing the two metal-on-metal groups (p = 0.427, 0.600, 0.788).
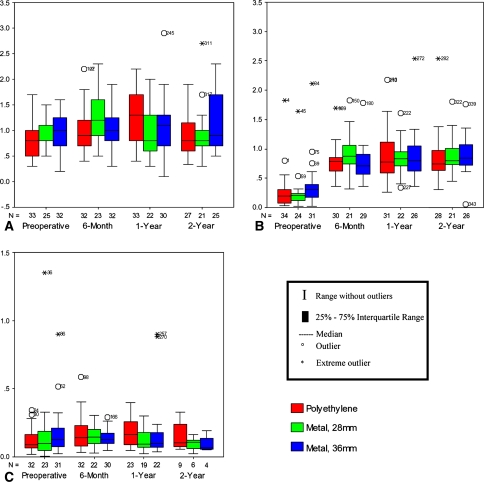
Erythrocyte, serum, and urine titanium intragroup and intergroup comparisons along with probability values are reported (Table 4; Fig. 4A–C). Although erythrocyte (intragroup p = 0.391, 0.647, and 0.388) and urine titanium values (intragroup p = 0.192, 0.686, and 0.465) did not change over time, all three study groups had an elevation in serum titanium from preoperatively to 2 years postoperatively (all intragroup p values < 0.001). There were no differences for any ion levels comparing the two metal-on-metal groups (intergroup p = 0.549, 0.974, and 0.669).
Table 4.
Titanium ion concentration in erythrocytes, serum, and urine
| Treatment group | Median preoperative concentration* (interquartile range) | Median 6-month concentration (interquartile range) | Median year 1 concentration (interquartile range) | Median year 2 concentration (interquartile range) | Intragroup p value|| | Intergroup p value¶ (MOP and MOM) | Intergroup p value (MOM28 and MOM 36) |
|---|---|---|---|---|---|---|---|
| Erythrocyte titanium | |||||||
| MOP† | 0.80 (0.50–1.00) | 0.90 (0.70–1.20) | 1.30 (0.80–1.70) | 0.80 (0.60–1.20) | 0.391 | ||
| MOM28‡ | 1.10 (0.80–1.10) | 1.20 (0.90–1.60) | 0.80 (0.60–1.33) | 0.80 (0.70–1.05) | 0.647 | 0.668 | |
| MOM36§ | 1.00 (0.70–1.28) | 1.00 (0.80–1.30) | 1.10 (0.70–1.30) | 0.90 (0.70–1.70) | 0.388 | 0.204 | 0.549 |
| Serum titanium | |||||||
| MOP | 0.20 (0.08–0.32) | 0.79 (0.61–0.87) | 0.78 (0.59–1.13) | 0.74 (0.63–0.98) | < 0.001 | ||
| MOM28 | 0.21 (0.11–0.25) | 0.87 (0.74–1.10) | 0.84 (0.70–1.00) | 0.80 (0.70–1.05) | < 0.001 | 0.271 | |
| MOM36 | 0.31 (0.16–0.40) | 0.71 (0.56–0.92) | 0.80 (0.62–1.06) | 0.85 (0.72–1.09) | < 0.001 | 0.257 | 0.974 |
| Urine titanium | |||||||
| MOP | 0.09 (0.06–0.17) | 0.14 (0.08–0.24) | 0.16 (0.09–0.26) | 0.11 (0.07–0.27) | 0.192 | ||
| MOM28 | 0.10 (0.04–0.23) | 0.14 (0.09–0.20) | 0.10 (0.07–0.20) | 0.11 (0.05–0.13) | 0.686 | 0.479 | |
| MOM36 | 0.12 (0.07–0.23) | 0.12 (0.10–0.18) | 0.10 (0.07–0.18) | 0.07 (0.05–0.16) | 0.465 | 0.164 | 0.669 |
*All concentrations are given in μg/L; †MOP = patients randomized to metal-on-polyethylene THA; ‡MOM28 = patients randomized to metal-on-metal, 28-mm THA; §MOM36 = patients randomized to metal-on-metal, 36-mm THA; ||intragroup p value = difference in preoperative and 2-year postoperative titanium ion concentrations within each group using the Wilcoxon test; ¶intergroup p value = difference in titanium ion concentrations at the 2-year postoperative interval between the metal-on-polyethylene group and each of the metal-on-metal groups and between the two metal-on-metal groups using the Mann-Whitney test.
Fig. 4A–C.
Titanium ion levels in (A) erythrocytes, (B) serum, and (C) urine among the three treatment groups at the preoperative, 6-month, 1-year, and 2-year intervals. All metal ion level units are in micrograms/liter. The number of cases in each group is denoted by the N. The upper limits of normal for titanium in erythrocytes, serum, and urine are 1.96 μg/L, 0.28 μg/L, and 0.4 μg/L, respectively. At 2 years, although serum titanium levels increased in all three treatment groups (all p < 0.001), no differences were found for erythrocyte titanium either within groups (p = 0.391, 0.647, 0.388) or between the metal-on-polyethylene and metal-on-metal groups (p = 0.668 and 0.204). There was no difference comparing the two metal-on-metal groups (p = 0.549, 0.974, 0.669).
Discussion
This is a prospective, randomized blinded study with a metal-on-polyethylene control group and two metal-on-metal bearing groups that were compared. The femoral and the acetabular implants, along with the acetabular liner locking mechanism and the femoral head taper, were identical, theoretically allowing a true comparison of a single variable, that being the articular bearing surface. In addition, patients with preexisting total joint replacements, which could affect baseline ion levels, were excluded. Lastly, we used a previously validated technique to evaluate metal ion levels that eliminated the chance of metal contamination and analyzed the samples on a high-resolution inductively coupled plasma mass spectrometer (ICPMS). The ICPMS has very low detection limits and allowed testing of both serum and erythrocyte levels in all samples of the study patients.
Our purpose was to report and compare the ion levels. We expected to see elevations for the patients with a metal-on-metal bearing and wanted to compare the ion levels with these implants with the levels reported for other metal-on-metal implants. Second, we wanted to compare the ion levels for the two metal-on-metal head sizes. Lastly, we wanted to explore the relationship among serum, erythrocyte, and urine ion levels. Like other studies, we found cobalt and chromium ion levels increased for patients with a metal-on-metal bearing [1, 2, 4, 12, 14, 18, 22]. However, the 2-year ion levels were as low or lower than most reports, likely indicating a bearing surface that is performing as well as others. Second, although large-head metal-on-metal bearings should have lower wear rates, there was no difference in ion levels for the 28-mm metal-on-metal and the 36-mm metal-on-metal implants 2 years postoperatively. Lastly, this randomized study is unique because both serum and erythrocyte ion levels were measured. Although serum cobalt, titanium, and chromium were elevated for the metal-on-metal groups, erythrocyte chromium and titanium did not increase.
With regard to the ion levels with this particular metal-on-metal design, we found lower erythrocyte chromium, cobalt, and titanium levels for the two metal-on-metal bearing groups than those reported by the other prospective, randomized study that measured erythrocyte ion levels [28]. In that study, erythrocyte cobalt increased 7.9-fold and urine cobalt increased 35-fold. The patients in this study with 28-mm metal-on-metal and 36-mm metal-on-metal bearings had a 3.2- to 3.8-fold increase in erythrocyte cobalt. The urine cobalt in this study increased 9.5- to 11.3-fold. These cobalt increases are roughly half that of the other study. The differences in chromium ion levels between the two studies are even more dramatic. In MacDonald’s study, there was a 2.3-fold increase in erythrocyte chromium and a 17.4-fold increase in urine chromium [28]. With the metal-on-metal bearings analyzed in this study, there was no increase in the erythrocyte chromium and a 5.6- to 9.6-fold increase in the urine chromium. Serum ion levels have been more commonly reported than erythrocyte ion levels [1, 2, 4, 12, 14, 18, 22]. Median serum cobalt levels have ranged from 0.7 μg/L to 3 μg/L in various studies [1, 2, 4, 12, 14, 18, 22]. The 28-mm metal-on-metal serum cobalt level of 0.77 μg/L and the 36-mm metal-on-metal level of 0.73 μg/L compare favorably with those in the literature. Likewise, serum chromium levels reported in the literature have ranged from 1.1 μg/L to 4.2 μg/L; the values from the current study of 1.29 μg/L for the 28-mm metal-on-metal and 0.91 μg/L for the 36-mm metal-on-metal compare favorably with those in the literature. There is only one prospective, randomized study, that we are aware of, looking at serum cobalt ion levels in patients with a metal-on-metal implant [1]. In that study, the 2-year serum cobalt level was 0.75 μg/L, which is the same as the values reported in this study.
Our second purpose was to compare the ion levels for the metal-on-metal 28- and 36-mm heads. We found no difference in the ion levels measured for the metal-on-metal 28-mm and the metal-on-metal 36-mm groups. In general, larger femoral heads in THA provide improved joint stability with greater range of motion before impingement, improved head-to-neck ratios, and greater jump distances. Hip simulator analysis with all manufacturing parameters being equal would predict lower wear with larger diameter metal-on-metal hip bearings and researchers would expect this to be reflected by lower ion levels. However, one clinical study actually demonstrated higher ion levels with larger head diameters [4]. Although the ion levels in this study were not lower for the 36-mm metal-on-metal group, they were not higher than the 28-mm metal-on-metal group. It is reassuring to know that with the bearing studied, surgeons can get the benefits of larger heads without increased ion levels. Although it is reassuring that the levels were not different, the question remains why the 36-mm metal-on-metal ion levels were not lower. In this study, the carbon content, metal processing, and roughness of the surfaces were kept constant. Only the head size and clearance were different. It is possible the benefits of the larger 36-mm bearing surface were negated by its 40-μm larger clearance. This would be one explanation why there was no difference in the in vivo ion levels. It is also possible that ion levels measured 2 years postoperatively are determined mainly by the run-in period of the metal-on-metal bearing, which generates more debris than the steady-state wear phase of a metal-on-metal bearing [14]. Assuming this is true, it may take 5 years of followup, at which point the bearings would be in a steady state of wear to see a difference in ion levels. Lastly, the study was designed with the power to see a 50% reduction in ion levels from the metal-on-metal 28-mm to the metal-on-metal 36-mm groups. A smaller difference in ion levels would likely require many more patients.
The final purpose of this study was to better understand the relationship between serum and erythrocyte metal ion levels in patients with metal-on-metal bearing surfaces. The majority of studies on metal ions have either analyzed serum or whole blood. In this study, analysis of both erythrocyte ion levels and serum ion levels in the same patients revealed an interesting finding. Serum and erythrocyte cobalt ion levels increased from preoperatively to 2 years for both metal-on-metal groups. However, although the serum chromium and titanium increased for both metal-on-metal groups, the erythrocyte chromium and titanium did not increase. This raises the possibility of a threshold level for serum chromium and titanium, below which erythrocyte chromium and titanium are not affected. This possibility is compatible with relatively low ion levels in our patients with metal-on-metal bearing surfaces. One study has looked at whole blood, serum, and erythrocyte ion levels in patients with total hip resurfacing [34]. In that study, the serum and erythrocyte values for patients with metal-on-metal hips were similar to the values in our patients with metal-on-metal bearing surfaces. However, because it was not a prospective, randomized study with preoperative and postoperative testing, the authors did not discover the absence of an erythrocyte chromium increase.
In conclusion, we did not see a difference in 2-year ion levels when comparing the patients with 28-mm metal-on-metal with the patients with 36-mm metal-on-metal with this hip system. However, the patients’ ion levels were as low as reported for any other metal-on-metal bearing hip, providing an early indication this bearing is performing well. Although the metal-on-metal groups had an increase in erythrocyte cobalt, serum cobalt, serum chromium, and serum titanium, there was no increase in the erythrocyte chromium or erythrocyte titanium, indicating a possible threshold level for bearing wear or for serum levels of chromium and titanium. Longer followup of this prospective, randomized cohort will help our understanding of the time-related changes in ion levels for patients with a metal-on-metal hip bearing.
Footnotes
The institution of one or more of the authors (CAE Jr, SS, CAE) has received funding from Inova Health Services (Fairfax, VA). One or more of the authors (CAE Jr, SJM, CAE) has received funding from DePuy Orthopaedics (Warsaw, IN) for this study. The authors have an agreement with the sponsor (DePuy Orthopaedics) that allows them to review all data before submission.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2168–2173. [DOI] [PubMed]
- 2.Brodner W, Grubl A, Jankovsky R, Mesinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19:66–70. [DOI] [PubMed]
- 3.Brown SR, Davies WA, DeHeer DH, Swanson AB. Long-term survival of McKee-Farrar total hip prostheses. Clin Orthop Relat Res. 2002;402:157–163. [DOI] [PubMed]
- 4.Clarke MT, Lee PTH, Arora A, Villar RN. Levels of metal ions after small and large diameter metal-on-metal hip arthroplasty. J Bone Joint Surg Br. 2003;85:913–917. [PubMed]
- 5.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. [DOI] [PubMed]
- 6.Delaunay CP. Metal-on-metal bearings in cementless primary total hip arthroplasty. J Arthroplasty. 2004;19:35–40. [DOI] [PubMed]
- 7.Dorr LD, Wan, Z, Longjohn DB, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. J Bone Joint Surg Am. 2000;82:789–798. [DOI] [PubMed]
- 8.Dowson D, Hardaker C, Flett M, Isaac G. A hip joint stimulator study of the performance of metal-on-metal joints. J Arthroplasty. 2004;19:124–130. [DOI] [PubMed]
- 9.Dunstan E, Sanghrajka AP, Tilley S, Unwin P, Blunn G, Cannon SR, Briggs TWR. Metal ion levels after metal-on-metal proximal femoral replacements—a 30 year follow-up. J Bone Joint Surg Br. 2005;87:628–631. [DOI] [PubMed]
- 10.Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–128. [PubMed]
- 11.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. [DOI] [PubMed]
- 12.Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:88–93. [DOI] [PubMed]
- 13.Hart AJ, Hester T, Sinclair K, Powell JJ, Goodship AE, Pele L, Fersht NL, Skinner J. The association between metal ions from resurfacing and reduced T-cell counts. J Bone Joint Surg Br. 2006;88:449–454. [DOI] [PubMed]
- 14.Heisel C, Silva M, Skipor AK, Jacobs JJ, Schmalzried TP. The relationship between activity and ions in patients with metal-on-metal bearing hip prostheses. J Bone Joint Surg Am. 2005;87:781–787. [DOI] [PubMed]
- 15.Huo MH, Gilbert NF. What’s new in hip arthroplasty. J Bone Joint Surg Am. 2005;87:2133–2146. [DOI] [PubMed]
- 16.Jacobs JJ, Hallab NJ, Skipor AK, Urban RM. Metal degradation products—a cause for concern in metal-metal bearings? Clin Orthop Relat Res. 2003;417:139–147. [DOI] [PubMed]
- 17.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19:59–65. [DOI] [PubMed]
- 18.Jacobs JJ, Skipor AK, Doorn PF, Campbell P, Schmalzried TP, Black J, Amstutz HC. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res. 1996;329:256S–263S. [DOI] [PubMed]
- 19.Jacobs M, Gorab R, Mattingly D, Trick L, Southworth C. Three-to six-year results with the Ultima metal-on-metal hip articulation for primary total hip arthroplasty. J Arthroplasty. 2004;19:48–53. [DOI] [PubMed]
- 20.Jacobsson SA, Djerf K, Wahlstrom O. Twenty-year results of McKee-Farrar versus Charnley prosthesis. Clin Orthop Relat Res. 1996;329(Suppl):S60–S68. [DOI] [PubMed]
- 21.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. [DOI] [PubMed]
- 22.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:78–83. [DOI] [PubMed]
- 23.Lhotka C, Szekeres T, Steffan I, Zhuber K, Zweymuller K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. J Orthop Res. 2003;21:189–195. [DOI] [PubMed]
- 24.Long WT, Dorr LD, Gendelman V. An American experience with metal-on-metal total hip arthroplasties. J Arthroplasty. 2004;19:29–34. [DOI] [PubMed]
- 25.MacDonald SJ. Can a safe level for metal ions in patients with metal-on-metal total hip arthroplasties be determined? J Arthroplasty. 2004;19:71–77. [DOI] [PubMed]
- 26.MacDonald SJ. Metal-on-metal total hip arthroplasty—the concerns. Clin Orthop Relat Res. 2004;429:86–93. [DOI] [PubMed]
- 27.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;19:12–16. [DOI] [PubMed]
- 28.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282–296. [DOI] [PubMed]
- 29.Migaud H, Jobin A, Chantelot C, Giraud F, Laffargue P, Duquennoy A. Cementless metal-on-metal hip arthroplasty in patients less than 50 years of age. J Arthroplasty. 2004;19:23–28. [DOI] [PubMed]
- 30.Milosev I, Trebse R, Kovac S, Cor A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006;88:1173–1182. [DOI] [PubMed]
- 31.Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–1521. [DOI] [PubMed]
- 32.Rieker CB, Schon R, Kottig P. Development and validation of a second-generation metal-on-metal bearing—laboratory studies and analysis of retrievals. J Arthroplasty. 2004;19:5–11. [DOI] [PubMed]
- 33.Tharani R, Dorey F, Schmalzried TP. The risk of cancer following total hip or knee arthroplasty. J Bone Joint Surg Am. 2001;83:774–780. [DOI] [PubMed]
- 34.Vendittoli PA, Mottard S, Roy AG, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br. 2007;89:441–448. [DOI] [PubMed]
- 35.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. [DOI] [PubMed]