Abstract
Large acetabular defects can be reconstructed with various methods depending on size and location of the defect. We prospectively followed our first 37 patients in whom we reconstructed the acetabulum with a trabecular metal augment combined with a trabecular metal shell. Three patients died before completing the minimum 24 months followup while the remaining 34 were followed a minimum of 24 months (mean, 34 months; range, 24–55 months). All defects were classified according to Paprosky. Radiographic signs of osseointegration were classified according to Moore. Quality of life was measured with the SF-12, WOMAC, and Oxford Hip Score. There were 15 men and 19 women with an average age of 64 years. At a minimum of two years followup 32 of the 34 patients required no further surgery for aseptic loosening, while two had rerevision. Of the 32 patients who had not been revised, all had stable cups radiographically. All quality-of-life parameters improved. The early results with tantalum augments are promising but longer followup is required.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Reconstructing acetabular defects in revision hip arthroplasty can be challenging. Small, contained defects can be successfully reconstructed with porous-coated hemispheric cups with or without supplementary allografts [21]. With larger uncontained defects, a cementless cup will not engage with sufficient host bone to provide primary stability even with additional screws [12]. The surgical options include extra-large hemispheric cups [8, 24], high hip center placement [7], impaction grafting with cement [18], structural allografts [19], bilobed oblong cups [4], and reconstruction cages [13, 14].
A new approach to manage uncontained structural acetabular defects is with tantalum acetabular augments. A porous material made of tantalum was developed approximately 10 years ago [2]. Compared with conventional porous materials such as titanium, this material possesses a higher coefficient of friction against bone, low bulk stiffness, high-volume porosity, and more freely communicating pores [3, 5]. Histologic analyses have demonstrated rapid attachment and ingrowth of bone and tissues in canine and small mammal models [2]. The potential advantages of using tantalum acetabular augments for the reconstruction of acetabular defects are the ability to provide biologic fixation of the augment to the host bone, relative ease of reconstruction, and the structural reliability of the metal augment imparted by its inherent resistance to fracture and failure, which may occur over time with structural allograft as a result of revascularization and remodeling. The augment fills the defect and allows insertion of a porous tantalum hemispheric shell [22]. These augments are available in different sizes and shapes and can fill most defects encountered in complex acetabular revision surgery [16, 20].
The purpose of this study was to evaluate (1) the radiographic results specifically looking at osseointegration of the cup/augment construct and position of the the hip center; (2) the clinical results assessed with comprehensive quality of life data; and (3) report complications related to the revision procedure in a prospective cohort of patients who underwent complex acetabular reconstruction with trabecular metal augments and revision shells.
Materials and Methods
We prospectively followed 37 patients with a minimum of 2-year clinical and radiographic followup who underwent acetabular reconstruction with trabecular metal augments (Trabecular Metal Acetabular Augment and Restrictor®; Zimmer, Warsaw, IN) from October 2002 to March 2005 (Table 1). Three patients died of causes unrelated to their hips before completing the minimum 24 months followup; none had undergone a rerevision. That left 34 patients for this report. There were 15 men and 19 women. The average age at operation was 64 years (range, 37–97 years). No patients were lost to followup. The minimum followup period was 24 months (mean, 34 months; range, 24–55 months). The Institutional Ethics Committee approved this study.
Table 1.
Clinical and radiologic results
| Patient number | Paprosky classification | Defect location (acetabular clock) | Followup (months) | Hip center preoperatively | Hip center postoperatively | Radiolucent line augment-bone | Number of screws cup-augment | Comments |
|---|---|---|---|---|---|---|---|---|
| 1 | 2A | 10 to 7 | 53 | Girdlestone | 21 | No | 2/2 | |
| 2 | 3A | 8 to 2 | 51 | 40 | 36 | No | 3/2 | |
| 3 | 2A | 10 to 3 | 48 | 34 | 17 | No | 3/2 | |
| 4 | 3A | 6 to 1 | 55 | 39 | 43 | Yes | 3/3 | Failure, awaiting revision |
| 5 | 3A | 9 to 3 | 50 | 55 | 32 | No | 3/2 | |
| 6 | 3A | 8 to 1 | 47 | 50 | 30 | No | 3/3 | |
| 7 | 3A | 8 to 2 | 0 | 33 | n/a | No | 3/3 | Dead |
| 8 | 3B | 10 to 2 | 48 | 55 | 28 | No | 3/4 | |
| 9 | 2A | 10 to 2 | 26 | 35 | 33 | No | 3/3 | |
| 10 | 3A | 11 to2 | 31 | 40 | 22 | No | 4/2 | |
| 11 | 2C | 10 to 2 | 12 | 29 | 27 | NA | 3/3 | Dead |
| 12 | 3A | 9 to 3 | 48 | 53 | 22 | No | 3/2 | |
| 13 | 3A | 9 to 3 | 42 | 57 | 28 | No | 3/2 | |
| 14 | 3A | 10 to 2 | 42 | 45 | 34 | No | 2/3 | |
| 15 | 3A | 10 to 3 | 42 | 40 | 28 | No | 3/2 | |
| 16 | 3A | 10 to 2 | 39 | 61 | 14 | No | 3/3 | |
| 17 | 3B | 10 to 2 | 29 | 59 | 34 | No | 3/0 | |
| 18 | 2B | 10 to 3 | 37 | 73 | 23 | No | 2/3 | Recurrent dislocation, revision to constrained liner |
| 19 | 3A | 9 to 3 | 36 | 55 | 33 | No | 2/2 | |
| 20 | 3A | 9 to 4 | 37 | 62 | 24 | No | 2/2 | |
| 21 | 2C | 7 to 12 | 36 | 39 | 26 | No | 3/2 | |
| 22 | 3A | 11 to 6 | 35 | 61 | 48 | No | 3/2 | |
| 23 | 3B | 9 to 4 | 24 | 77 | 32 | No | 3/2 | |
| 24 | 3A | 12 to 6 | 35 | 33 | 33 | No | 4/34/2 (rerevision) | Failure, rerevision |
| 25 | 3B | 11 to 3 | 31 | 54 | 23 | No | 3/1 | |
| 26 | 2A | 9 to 12 | 33 | Girdlestone | 19 | No | 3/3 | |
| 27 | 3A | 9 to 4 | 29 | 73 | 44 | No | 3/3 | |
| 28 | 3A | 11 to 3 | 31 | 43 | 30 | No | 2/2 | |
| 29 | 2B | 12 to 6 | 29 | 39 | 18 | No | 3/3 | |
| 30 | 3B | 10 to 2 | 28 | 53 | 33 | No | 3/2 | |
| 31 | 3B | 9 to 2 | 31 | 36 | 24 | No | 3/2 | Pelvic discontinuity |
| 32 | 3A | 11 to 4 | 0 | 41 | n/a | N/A | 3/2 | Dead |
| 33 | 3A | 10 to 4 | 24 | 52 | 24 | No | 3/3 | |
| 34 | 3A | 10 to 5 | 26 | 53 | 28 | No | 4/1 | |
| 35 | 3A | 10 to 2 | 25 | 45 | 29 | No | 2/2 | |
| 36 | 3B | 10 to 3 | 24 | 65 | 33 | No | 3/2 | Pelvic discontinuity |
| 37 | 3B | 10 to 2 | 26 | 45 | 25 | No | 3/2 | Pelvic discontinuity |
NA = Not available.
The patients had a mean of 1.4 (range, 0–6) previous hip arthroplasty procedures performed. The initial diagnosis at the time of the primary THA for the 34 patients was osteoarthritis in 26 patients, developmental dysplasia of the hip in four patients, posttraumatic arthritis (acetabular fracture) in two patients, avascular necrosis of the femoral head in one patient, and ankylosing spondylitis in one patient. The indication for the revision was aseptic loosening in 28 patients, two-stage reconstruction for infection in two patients, mechanical loosening of a cage in one patient, failed structural allograft in one patient, and recurrent dislocation in one patient. In one patient (avascular necrosis of the femoral head), the augment was used as part of the primary hip arthroplasty procedure. In 18 patients, only the acetabular component was revised.
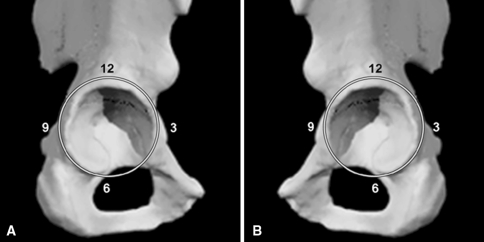
Two surgeons (AS, WK) not involved in the procedures classified all acetabular defects based on preoperative anteroposterior and iliac-ischial oblique (Judet) radiographs using the Paprosky classification system [17]. Intraoperatively, all defects were classified according to the acetabular clock method (Fig. 1). According to the Paprosky classification system [17], 19 defects were classified as Type 3A, eight as Type 3B (two of these had a pelvic discontinuity and needed a posterior column plate), four as Type 2A, two as Type 2B, and one as Type 2C.
Fig. 1A–B.
The figure shows the acetabular clock for the (A) right hip and (B) left hip.
The preoperative assessment of the defect was confirmed at operation. Initially, we used the primary acetabular reamers in increasing diameters to obtain the best possible press-fit of the reamers and trial shells between the anterior and posterior walls of the acetabulum without sacrificing bone stock. Every effort was made to position these primary reamers at the true hip center. The most common locations of the defects were superolateral and posterosuperolateral. The need for an augment was anticipated based on preoperative templating of anteroposterior pelvic radiographs; however, we made the definitive decision to use an augment intraoperatively if an oblong bone defect was recognized that could not support the hemispheric component without augmentation of acetabular bone stock. Trials were used intraoperatively as a guide to decide whether structural support was needed for the revision shell. We used augments when inherent stability of the trials could not be achieved. A trial hemispheric acetabular cup was inserted in the appropriate degree of anteversion and lateral opening so as to measure the remaining defect. We then prepared the superior defect to accept an augment of suitable size such that it had good contact with the remaining host bone and provided the required support for the hemispheric trial cup. Commonly this required secondary reaming with a hemispheric reamer to maximize the augment-bone interface contact and stability. This reaming was done with a reamer that matched the diameter of the augment chosen. The metal augment was secured with a minimum of two titanium cortical screws (AO large fragment set; Synthes (Canada) Ltd., 2566 Meadowpine Boulevard, Mississauga Ontario). If the location of the screw hole within the augment was inconvenient, we created a new screw hole in the augment using a 4-mm high-speed burr. Particulate allograft was packed within the augment. We filled any additional, small contained bone defects by impaction grafting using cancellous allograft while maintaining adequate contact with host bone. We did not adhere to the commonly used 50% rule for cup-host-bone surface contact. In many cases, the trabecular metal revision shell was in contact with an estimated 25% to 30% autogenous host bone (range, 25%–60% on intraoperative estimation). The remainder was in contact with augment, morselized allograft in contained defects and at times fibrous membrane. One patient with a Type 3B defect had two acetabular augments. All other patients needed one augment. Trabecular metal acetabular shells (sizes 48–70; Zimmer) were used in all patients. The most commonly used augment sizes were 58/10 and 58/20 (21 patients) (Table 1).
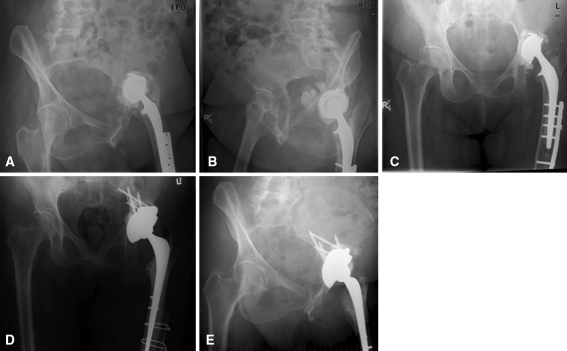
We then inserted the final trabecular metal revision shell and it was secured with multiple screws (Zimmer titanium acetabular screws; Zimmer). Additional holes in the cup for screw placement were created, if required, by using a 4-mm high-speed burr. We did not use bone cement between the augment and the cup. The unused screw holes were filled with bone wax to avoid intrusion of bone cement into the bone-implant interface [10]. We then cemented a polyethylene cup into the shell using Simplex cement with Tobramycin® (Stryker Canada, 45 Innovation Drive, Hamilton, Ontario) (Fig. 2).
Fig. 2A–E.
The figure shows radiographs of a case example of acetabular reconstruction with a trabecular metal augment and shell: (A) preoperative iliac-ischial oblique (Judet) view 1; (B) preoperative iliac-ischial oblique (Judet) view 2; (C) preoperative anteroposterior (AP) pelvis; (D) postoperative AP pelvis; and (E) 50 months followup.
We obtained followup radiographs postoperatively, at 3 months, and then annually. The same two surgeons who evaluated the preoperative radiographs assessed the most recent followup anteroposterior and iliac-ischial oblique radiographs of each patient. We used radiographic signs for osseointegration as described by Moore et al. [15], which involves recording the presence or absence of the following five signs: (1) radiolucent lines; (2) a superolateral buttress; (3) medial stress shielding; (4) radial trabeculae; and (5) inferomedial buttress. When three or more signs are present, the positive predictive value for bone ingrowth is 96.9%, the sensitivity is 89.9%, and the specificity 76.9%. We conducted a careful search for the presence or absence of radiolucent lines at the hemispheric cup-bone interface and the augment-bone interface. A high hip center was defined as being greater than 35 mm above the interteardrop line. Radiographs were available for all 34 patients.
We assessed clinical outcome for all patients with health-related quality-of-life questionnaires. All patients received annual quality-of-life followup questionnaires by mail and responses to Oxford Hip Score [6], the WOMAC Score [1], and the SF-12 [23].
Results
At the time of the latest followup 20 of the 34 acetabular reconstructions showed four of five radiographic signs of osseointegration, 13 showed three signs, and one showed two signs. The patient with two radiographic signs underwent a liner exchange for recurrent dislocation. Intraoperatively, the cup-augment construct was fully ingrown. Two of the 34 patients had clinical and radiological loose acetabular shells and underwent further revision (Table 1). Preoperatively, the hip center was located a mean of 50 mm above the interteardrop line (range, 29–73 mm). Thirty of 33 patients (91%) with implants in situ at the latest followup had a high hip center preoperatively. Postoperatively, the hip center for these patients was located on average 28 mm above the interteardrop line (range, 14–48 mm). Three patients (9%) had a high hip center of more than 35 mm postoperatively. The patient who already underwent a rerevision for a failed cup-augment construct is included here (Table 1).
For 26 of 32 patients who had implants in situ at followup, baseline quality-of-life data (WOMAC, Oxford Hip Score, SF-12) were available. Followup quality-of-life data were available for all 32 patients (Table 2) who still had the original cup-augment construct in situ at a minimum of 24 months. This excludes three patients who died before completing the 2-year followup, one patient who already underwent a rerevision with a new cup-augment construct, and one patient who is awaiting rerevision for a failed augment. The vast majority of patients demonstrated considerable improvement in quality-of-life scores from baseline preoperatively and reported outcomes considered very good or excellent with regard to hip function and generic quality of life.
Table 2.
Quality-of-life outcomes
| Minimum 2-year followup | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|
| WOMAC function | 78.3 | 18.8 | 30.9 | 100.0 |
| WOMAC stiffness | 82.6 | 17.4 | 37.5 | 100.0 |
| WOMAC pain | 89.9 | 15.2 | 40.0 | 100.0 |
| WOMAC global | 81.3 | 15.8 | 33.3 | 100.0 |
| Oxford score | 80.3 | 16.6 | 33.3 | 100.0 |
| SF-12 physical component | 44.1 | 8.5 | 26.3 | 58.9 |
| SF-12 mental component | 53.9 | 9.8 | 28.4 | 67.1 |
| UCLA | 4.9 | 2.1 | 2.0 | 10.0 |
| Satisfaction scores | ||||
| Pain | 95.4 | 19.4 | 0.0 | 100.0 |
| Function | 88.5 | 18.4 | 33.3 | 100.0 |
| Recreational | 85.6 | 20.9 | 33.3 | 100.0 |
| Overall | 95.6 | 11.5 | 66.7 | 100.0 |
| Total score | 92.0 | 13.3 | 58.3 | 100.0 |
| Baseline quality of life | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|
| WOMAC function | 39.8 | 20.8 | 8.8 | 80.9 |
| WOMAC stiffness | 45.0 | 17.7 | 12.5 | 75.0 |
| WOMAC pain | 42.8 | 15.9 | 15.0 | 80.0 |
| WOMAC global | 41.0 | 17.8 | 12.5 | 79.2 |
| Oxford score | 34.6 | 18.0 | 0.0 | 70.5 |
| SF-12 physical component | 30.4 | 9.5 | 18.8 | 51.3 |
| SF-12 mental component | 45.0 | 12.1 | 20.4 | 64.2 |
| Standard response mean | < 0.8 | > 0.8 (large effect) | Percentage of large effect |
|---|---|---|---|
| WOMAC function | 4 | 18.0 | 75 |
| WOMAC stiffness | 5 | 18.0 | 75 |
| WOMAC pain | 1 | 22.0 | 92 |
| WOMAC global | 4 | 18.0 | 75 |
| Oxford score | 4 | 20.0 | 83 |
| SF-12 physical component | 7 | 14.0 | 56 |
| SF-12 mental component | 12 | 9.0 | 36 |
| Effect size | < 0.8 | > 0.8 (large effect) | Percentage of large effect |
|---|---|---|---|
| WOMAC function | 4 | 18.0 | 75 |
| WOMAC stiffness | 5 | 18.0 | 75 |
| WOMAC pain | 1 | 22.0 | 92 |
| WOMAC global | 2 | 20.0 | 83 |
| Oxford score | 2 | 22.0 | 92 |
| SF-12 physical component | 5 | 16.0 | 64 |
| SF-12 mental component | 12 | 9.0 | 36 |
Two patients had complications related to the revision surgery. One patient sustained a dislocation postoperatively that was treated with closed reduction. No further dislocation occurred. One patient developed recurrent dislocations 2 years postoperatively and underwent revision surgery to a constrained liner without further dislocation to date. The first failure in the present study was a 47-year-old male patient who underwent an acetabular revision procedure to reconstruct a Type 3A defect with an augment. He showed radiographic signs of loosening 12 months postoperatively and underwent a revision procedure with a new cup-augment construct. At the index procedure, immediate postoperative films showed minimal host-bone contact. He was reconstructed with another augment-revision shell combination. Intraoperatively, it was verified that host-bone contact was made with remaining available host bone. At the time of the latest followup, radiographs showed four signs of osseointegration and the patient was asymptomatic (16 months after the rerevision procedure). The second failure in this series was a 51-year-old male patient. He was initially treated with a cage and a structural allograft that subsequently failed 1 year later. A further acetabular reconstruction was performed with a trabecular metal augment and shell (Type 3A defect). Postoperatively, he functioned well until a radiograph for this study 55 months later revealed complete failure of the augment and cup. He is now awaiting revision surgery.
Discussion
Reconstruction of the acetabulum in the face of a large defect continues to pose a challenge at revision surgery. This study was conducted to evaluate the early clinical and radiological results of acetabular defect reconstruction with trabecular metal augments and shells. Although the followup was on average only 34 months (range, 24–55 months), the results so far are promising. Another limitation is the relatively small sample size but this report is still the largest one reported in the literature. Ideal would be comparison with results of other types of reconstruction, such as allografts from the same institution. However, even in a tertiary referral center such as the authors’ institution, it would be difficult to obtain a large enough sample size with similar defects to make a comparison.
Numerous other reconstructive options have been reported with mixed results. These include placement of a smaller hemispheric cup at a high hip center [7], a reconstruction cage [11], a bilobed oblong cup [4], extra large cementless cup (jumbo) [24], impaction grafting with a cemented cup [18], and use of a structural allograft [9].
As a result of the limitations and inconsistent results reported with traditional reconstruction, the combination of a hemispheric trabecular metal cup and augments appears an attractive technique for reconstruction of large acetabular defects. Biologic fixation of the new acetabular cup and near anatomic restoration of the hip center are important goals in acetabular revision surgery. Trabecular metal cups and augments are an attractive option to achieve these goals and two articles report favorable results [16, 20] at 3 years. We have been using trabecular metal augments in combination with trabecular metal cups since 2002. This technique has a number of advantages. The augment fills the defect, obviating the need for a structural allograft. The augment combined with a hemispheric cup increases the surface area for host-bone contact and facilitates ingrowth.
This method also restores the hip center to near normal and improves hip biomechanics as demonstrated. We were able to restore the average center of rotation from 50 mm preoperatively to an average of 28 mm postoperatively with only three patients out of 33 displaying a high hip center postoperatively versus 30 preoperatively.
This study supports some of the potential advantages of trabecular metal used as a revision shell in combination with a segmental trabecular metal augment. In addition to the excellent stability achieved in the 32 cases, radiographic evidence of absence of medial stress shielding indicated the low modulus of these shells might be advantageous to the remaining host bone.
The patients who received augments in this study had a marked improvement in quality of life and expressed a high degree of satisfaction (Table 2). There are some limitations of the technique we describe. The acetabular shells are not fixed to the augments with screws or cement. Potential micromotion at this interface could lead to debris generation. Further followup will be necessary to ascertain if this has any clinical significance. Furthermore, this technique is more expensive than alternative techniques and cages. Until mid- to long-term data demonstrate its efficacy, the authors cannot state this treatment method will be cost-effective.
Although at times it is still necessary to use structural allograft and/or a reconstructive cage, our use of those reconstructive options has diminished in recent years. We found encouraging short-term clinical and radiographic outcomes by incorporating these trabecular metal augments and trabecular metal revision shells. By using the acetabular clock, we found almost all patients having augments had defects involving the dome and posterior wall. In almost all cases, this corresponded to at least four hands of the clock. Careful intraoperative evaluation with trial components was essential and suggested all of these cases would be manageable with the trabecular metal augment and hemispheric revision acetabular shell. Given these encouraging results, the combination of trabecular metal augments and revision shells is our treatment of choice for large uncontained defects, mainly Paprosky Type 3A and Type 3B. This technique, in our hands, is relatively straightforward, reliable, and associated with very good clinical and radiographic results at 2 to 5 years followup. We await longer-term followup as well as additional reports from other centers to validate our early outcomes with this new reconstructive regimen.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed]
- 2.Black J. Biological performance of tantalum. Clin Mater. 1994;16:167–173. [DOI] [PubMed]
- 3.Bobyn JD, Poggie RA, Krygier JJ, Lewallen DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe, TJ, Christie MJ, Nasser S, Wood JE, Stulberg SD, Tanzer M. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86(Suppl 2):123–129. [DOI] [PubMed]
- 4.Chen WM, Engh CA Jr, Hopper RH Jr, McAuley JP, Engh CA. Acetabular revision with use of a bilobed component inserted without cement in patients who have acetabular bone-stock deficiency. J Bone Joint Surg Am. 2000;82:197–206. [DOI] [PubMed]
- 5.Christie MJ. Clinical applications of trabecular metal. Am J Orthop. 2002;31:219–220. [PubMed]
- 6.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185–190. [PubMed]
- 7.Dearborn JT, Harris WH. High placement of an acetabular component inserted without cement in a revision total hip arthroplasty. Results after a mean of ten years. J Bone Joint Surg Am. 1999;81:469–480. [DOI] [PubMed]
- 8.Dearborn JT, Harris WH. Acetabular revision arthroplasty using so-called jumbo cementless components: an average 7-year follow-up study. J Arthroplasty. 2000;15:8–15. [DOI] [PubMed]
- 9.Dewal H, Chen F, Su E, Di Cesare PE. Use of structural bone graft with cementless acetabular cups in total hip arthroplasty. J Arthroplasty. 2003;18:23–28. [DOI] [PubMed]
- 10.Garbuz D. Revision total hip: a novel modular cementless acetabular system for reconstruction of severe acetabular bone loss. Operative Techniques in Orthopaedics. 2004;14:117–120. [DOI]
- 11.Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19:436–446. [DOI] [PubMed]
- 12.Gross AE. Revision arthroplasty of the acetabulum with restoration of bone stock. Clin Orthop Relat Res. 1999;369:198–207. [DOI] [PubMed]
- 13.Gross AE, Goodman S. The role of cages and rings: when all else fails. Orthopedics. 2004;27:969–970. [DOI] [PubMed]
- 14.Gross AE, Goodman S. The current role of structural grafts and cages in revision arthroplasty of the hip. Clin Orthop Relat Res. 2004;429:193–200. [DOI] [PubMed]
- 15.Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;444:176–183. [DOI] [PubMed]
- 16.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–208. [DOI] [PubMed]
- 17.Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9:33–44. [DOI] [PubMed]
- 18.Schreurs BW, Bolder SB, Gardeniers JW, Verdonschot N, Slooff TJ, Veth RP. Acetabular revision with impacted morsellised cancellous bone grafting and a cemented cup. A 15- to 20-year follow-up. J Bone Joint Surg Br. 2004;86:492–497. [PubMed]
- 19.Shinar AA, Harris WH. Bulk structural autogenous grafts and allografts for reconstruction of the acetabulum in total hip arthroplasty. Sixteen-year-average follow-up. J Bone Joint Surg Am. 1997;79:159–168. [DOI] [PubMed]
- 20.Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty. 2006;21:83–86. [DOI] [PubMed]
- 21.Templeton JE, Callaghan, JJ, Goetz DD, Sullivan PM, Johnston RC. Revision of a cemented acetabular component to a cementless acetabular component. A ten to fourteen-year follow-up study. J Bone Joint Surg Am. 2001;83:1706–1711. [DOI] [PubMed]
- 22.Unger AS, Lewis RJ, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthroplasty. 2005;20:1002–1009. [DOI] [PubMed]
- 23.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [DOI] [PubMed]
- 24.Whaley AL, Berry DJ, Harmsen WS. Extra-large uncemented hemispherical acetabular components for revision total hip arthroplasty. J Bone Joint Surg Am. 2001;83:1352–1357. [DOI] [PubMed]