Abstract
Substantial bone loss is frequently encountered with revision hip arthroplasty. A proximal femoral allograft may be used to reconstitute bone stock in the multiply revised femur with segmental bone loss of greater than 5 cm. We retrospectively reviewed 92 patients (93 hips) who underwent such proximal femoral allografts. The average age at the surgery was 61 years. The average number of previous revision procedures was 2.5. Six patients were lost to followup. Thirty-four of 36 deceased patients had the original proximal femoral allograft at the time of death. The minimum followup for the 50 remaining patients was 15 years (average, 16.2 years; range, 15–22 years). Analysis included survivorship and radiographic assessment. Of the 50 patients reviewed, two had a failed reconstruction due to infection, six for aseptic loosening, three for nonunion, and four for dislocation. Revision of the proximal femoral allograft for all reasons excluding the acetabulum was performed in seven patients. At last followup, 42 patients (84%) had a well-functioning construct. Proximal femoral allograft for revision hip arthroplasty in femoral segmental bone loss is a durable alternative in most patients for a complex problem.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Revision hip arthroplasty in the setting of proximal femoral deficiency is one of the major challenges in hip replacement surgery [3]. Methods used in this situation, with some success, include impaction allografting techniques [4, 17, 21, 24, 26], distal press-fit fixation [27, 28], massive endoprosthetic reconstruction [23, 29, 33], and replacement with a proximal femoral allograft (PFA) [2, 9–12, 14, 16, 18, 34]. Mega prostheses survivorship range in survival from 58% to 84% with average followup from 5 to 10 years.
The senior author (AEG) has successfully used allograft bone for the reconstruction of deficient bone stock in revision hip surgery since 1984 [2, 10–12]. With the advent of modular femoral components, our use of PFA has decreased to about six per year, but for the young patient or when the bone loss extends well into the diaphysis, this is our treatment of choice.
We report the survival rate, functional outcomes (WOMAC), and complications (infection, loosening, nonunion, resorption, dislocation) for proximal femoral bone loss.
Materials and Methods
From our prospectively collected database, we identified 92 patients (93 hips) with a PFA of greater than 5 cm in length implanted to replace deficient bone stock during revision hip surgery between 1986 and 1992. Our indication for a PFA in revision hip surgery is a femur with full circumferential bone loss of at least 5 cm in length, extending into the diaphysis. This included 39 men and 54 women (one woman had the procedure bilaterally) with an average age of 63 years (range, 49–81 years) at the time of revision. The average number of previous hip revision surgeries was 2.5 (range, 1–5) in this group. The average length of the allograft was 14.7 cm (range, 8.5–32 cm). Six patients were lost to followup. The minimum followup for the 86 remaining patients was 15 years (average, 17.8 years; range, 15–22 years). Thirty-six patients (40%) had died after the minimum followup but were included in the analysis; 34 of these 36 had the original PFA at the time of death and two had been revised. This left 50 patients still alive at the time of this review. No patient had died as a direct complication of the procedure.
We classified femoral defects at time of surgery according to the Gross classification: [10, 30] Type 1 defect, no substantial bone loss; Type 2 defect, contained (cavitary) bone loss; Type 3 defect, segmental (full circumferential) bone loss from the proximal femur that is less than 5 cm in length and involves the calcar and the lesser trochanter but does not extend into the diaphysis; Type 4 defect, segmental (full circumferential) bone loss of greater than 5 cm in length extending into the diaphysis; and Type 5 defect, same as in Type 4 with the addition of a periprosthetic fracture. In this study, we used PFAs to treat Type 4 and 5 defects (Table 1) [19].
Table 1.
Treatment algorithm for femoral defects
| Gross’ classification | Type of defect | Treatment alternatives |
|---|---|---|
| Type 1 | No significant bone loss | Conventional cemented or uncemented femoral component |
| Type 2 | Contained (cavitary) bone loss | Proximally porous-coated implantExtensively porous-coated implants for ingrowthExtensively grit-blasted titanium implants for ongrowthImpaction grafting with a cemented componentModular implants for proximal or extensive ingrowth or ongrowthLong-stemmed cemented implants |
| Type 3 | Segmental (full circumferential) bone loss from the proximal femur that is less than 5 cm in length and involves the calcar and the lesser trochanter but does not extend into the diaphysis | As in Type 2 with a calcar replacement option |
| Type 4 | Segmental (full circumferential) bone loss of greater than 5 cm in length extending into the diaphysis | Allograft-prosthesis composite or tumor prosthesis |
| Type 5 | As in Type 4 with the addition of a periprosthetic fracture | Allograft-prosthesis composite or tumor prosthesis |
All procedures were conducted by the senior surgeon (AEG), using the same technique. All allografts were stored at −70°C and irradiated with 2.5 mrad; cultures of the graft were taken before immersion in warm 50% Betadine® solution. Graft preparation was performed on a separate sterile surgical table by a second surgical team. We recommend using fresh-frozen allograft from an American Association of Tissue Banks accredited tissue bank [31]. We use irradiation to be certain of graft sterility since grafts are not processed by other methods i.e., sterilizing solutions etc. The dose of irradiation does not substantially affect graft strength [31]. Graft immunogenicity is not a factor in the clinical situation. The femoral head was excised at the base of the lesser trochanter, facilitating insertion of the implant and allowing room for adjusting the version. Lengthening of the leg was not carried out via the neck cut, but rather by the length of the allograft below the lesser trochanter. The allograft should be cut long at first. A stable graft-host junction is necessary. Either a step cut or an oblique graft host osteotomy can be used to obtain stability. An oblique osteotomy is easier and allows adjustment of the version without having to make major changes to the osteotomy. An oblique osteotomy should be as long as possible, at least 2 cm in length. Occasionally there is enough of a canal diameter discrepancy between the graft and the host that the graft can be telescoped into the host canal for a couple of centimeters, making the step cut or oblique osteotomy unnecessary.
We excised the graft greater trochanter, allowing for reattachment of the host trochanter. Reaming was then carried out. We milled the calcar region until the implant could be seated. We reamed the cortex only enough to allow insertion of the implant. The host canal is almost always larger than the allograft canal, and if the surgeon attempts to use an implant large enough to obtain a press fit distally, the allograft will have to be excessively reamed and hence weakened. We most commonly used a 13.5- or 14-mm-diameter stem, which did not usually provide a press fit distally. We judged this unnecessary since the implant was cemented into the allograft, and once the allograft-host junction united, the entire construct was stable. The implant was 300 mm in length and smooth for cementation into the allograft (Long Stem GROSS Hip, Johnson & Johnson Inc., Warsaw, IN).
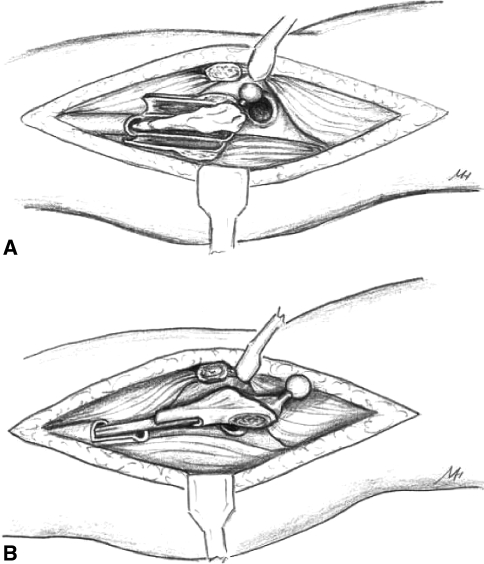
Since the implant is cemented into the allograft but not the host bone, we are dependent on the stability of the graft-host junction to stabilize the construct. Cementing proximally into the allograft and distally into the host would interfere with graft-host union (by stress shielding the graft-host junction leading to graft resorption) [15]. We cemented the femoral component into the allograft on a separate table using a cement gun inserted into the allograft. The cement was pressurized by plugging the canal distally with a finger. The implant was then inserted, in the correct version, which was predetermined (and marked) along with the required length at the trial reduction. After the implant was seated, the cement was cleaned off the distal stem and also off the surface of the osteotomy, using damp sponges. The graft-implant composite was then ready for insertion into the host. Additional fine-tuning of length of the graft and version of the osteotomy may be necessary depending on the final trial reductions (Fig. 1).
Fig. 1A–B.
Diagrams illustrate surgical approach. (A) Trochanteric slide osteotomy with longitudinal split of the proximal femur is shown. (B) Insertion of the PFA composite with a step cut at the junction is shown.
For the revision, we used a straight lateral incision, incorporating old scars if possible. We prefer to use a trochanteric slide for exposure [7, 8]. Often the proximal femur is so deficient that the trochanteric fragment is very thin, but it is important to keep it in continuity with the abductors and vastus lateralis. We have modified the trochanteric slide to reduce the incidence of posterior dislocation [8]. We left the posterior capsule and external rotators intact by leaving about 1 cm of posterior greater trochanter attached to the femur. After the trochanteric osteotomy was completed, the vastus lateralis was reflected off the septum down to the level at which the coronal femoral split was to be performed. This was determined by preoperative planning and intraoperative visualization of the junction of the deficient and healthy host femur. The trochanter was retracted anteriorly and the femur was then split in the coronal plane down to the level considered healthy enough not to require replacing by allograft. At the level of healthy femur, transverse cuts were made anteriorly and posteriorly, each extending about a quarter of the way around the femur, leaving the medial half of the femur intact. The deficient femur was then pried open using multiple osteotomes. At the level of the horizontal cut, the medial half of the femur stays intact and can be used as the step cut or oblique osteotomy. The hip was then dislocated and the old femoral component removed.
Any residual bone in the deficient proximal femur was left with the soft tissue attachments intact, so it could be used as a vascularized bone graft to wrap around the allograft, especially at the graft-host junction where it enhanced union.
Before preparation of the allograft, the femoral implant was inserted into the host canal, without the allograft, and reduced into the trial cup, the leg length was measured, and the length of allograft was then determined. The allograft-prosthetic composite was inserted into the host and fixed at the junction with cerclage wires. Any available residual host femur with its soft tissue attachments was also cerclaged around the junction to act as vascularized autograft for enhanced union. Any autograft bone obtained during the removal of the loose implant or from host bone reaming can be added to the host-allograft bone junction.
If the junction was not perfectly stable, a cortical strut was cerclaged to the junction as a biologic plate. The greater trochanter was attached to the allograft with two 1.6-mm stainless steel cerclage wires. The vastus lateralis was reattached to the septum.
The patient was nonweightbearing using crutches or a walker until there was radiographic union (trabecular bridging) between host and allograft (usually 8 to 12 weeks). Patients were seen at 6 weeks, 12 weeks, 6 months, and then annually. At 8 to 12 weeks, physiotherapy was started, including abductor strengthening and gait training. Intravenous cefazolin (1 g every 6 hours) was administered intraoperatively and for 5 days postoperatively, and then cephalexin (500 mg every 6 hours) orally for 5 days. Antibiotics were not given in the cement.
Functional outcome evaluation was conducted using the short-form WOMAC and SF–12 questionnaire in 31 of the 50 surviving patients (62%) (34% of the entire group); functional evaluation was performed only for patients personally interviewed by the authors at an average followup of 16.2 years (range, 5–20 years). Clinical and radiographic data on the remaining 19 patients surviving after the minimum of 15 years followup were obtained from our records.
We (OS, AEG) assessed the most recent radiographs of the 50 patients, looking specifically for allograft-host union, periosteal and endosteal graft resorption, implant loosening, trochanteric union, and allograft fracture (Figs. 2, 3). The allograft was divided into zones as described by Gruen et al. [13], excluding Zones 1 and 4 (the site of the absent allograft trochanter and the site of allograft-host union). Resorption of the graft was measured in these zones and classified as mild resorption (partial thickness loss less than 1 cm in length), moderate resorption (partial thickness loss greater than 1 cm in length), and severe resorption (full thickness loss of any length). Implant stability was assessed based on implant migration of more than 2 mm and cement mantle fracture [2].
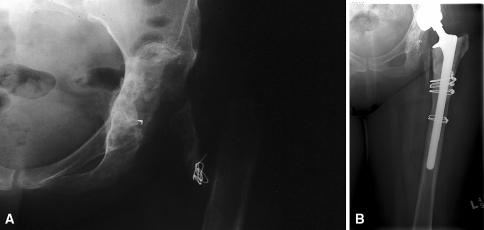
Fig. 2A–B.
(A) A radiograph shows the hip of a patient who had an excisional arthroplasty for the treatment of an infected THA. (B) A 19-year followup radiograph shows the hip after treatment with a THA using a PFA.
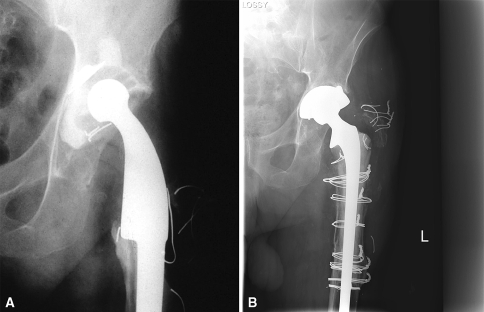
Fig. 3A–B.
(A) A radiograph demonstrates aseptic loosening of a tumor prosthesis. (B) A 17-year followup radiograph shows the hip after revision of the tumor prosthesis with a PFA.
A successful PFA was defined as a stable implant that had not undergone revision at last followup. Failure was defined as revision femoral surgery necessitating removal and/or revision of the PFA for any reason.
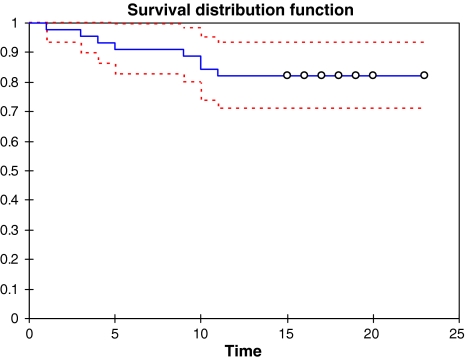
SPSS was used to conduct a Kaplan-Meier survival analysis on the data. Survival time was entered as the time variable, while failure/no failure was entered as the status variable. Cases were categorized as failed or not failed. For PFAs that failed, survival time was noted as the number of years postsurgery that failure occurred, while PFAs that had not failed were noted as the number of years postsurgery at which followup had indicated a positive outcome without any need for further surgery (Fig. 4). Details regarding the specific causes of failure (eg, infection, etc) were not included in this analysis. A successful construct, defined as a stable implant with no need for rerevision surgery at the time of latest followup.
Fig. 4.
A Kaplan-Meier survivorship analysis is shown. The dashed lines represent the 95% confidence interval for the survival rate. Survival rate at 15 years was 82.2%(CI 71.1–93.4).
Results
The survival was 82.2 % (CI 71.1–93.4) at 15 years. Mean survival time was 17.6 years (CI 16.0–19.3). Revision of the PFA for all reasons excluding the acetabulum was performed in seven patients (14%). We observed a successful construct in 42 of the 50 patients (84%).
In the 31 patients who underwent functional evaluation, the average short-form WOMAC score was 64.6 (range, 94.7–33.72) and the average SF–12 score was 34.7 (Physical Component Summary) and 51.4 (Mental Component Summary).
Failures due to infection occurred in two of the 50 patients (4%), and of these, one was successfully revised with another PFA as a two-stage revision. The second patient was treated with antibiotic suppression. Aseptic loosening occurred in six patients (12%). Most of these cases were revised with a second PFA, but in one case, the PFA was solidly fixed and the femoral prosthesis was revised, leaving the original PFA in place. Nonunion between allograft and host bone occurred in five patients (6%), requiring grafting and plate fixation. A stable trochanteric union was achieved in 75% of cases with trochanteric migration in 26%. Minor resorption of the graft was seen in 29 patients (58%) in our series, but only one of our patients had severe resorption leading to failure of the allograft. Dislocation occurred in four patients (8%). One patient (2%) had a pulmonary embolus. One patient suffered a nerve injury and another patient a vascular injury, which was repaired at the time of surgery.
Discussion
In some difficult femoral revisions, massive bone loss precluding impaction grafting or distal fixation can be managed by a PFA or, in some situations, a tumor prosthesis. We presented our long-term results of PFAs, including survival, functional results, and complications rate.
Our study had certain limitations. We prospectively collected all data, but the study was not randomized and we had no control group with alternate approaches. Despite the prospective data collection, functional and radiographic outcomes were not available for all patients.
Major deficient femoral bone at the site of a failed hip arthroplasty is not an uncommon finding. In most cases, the loss is minimal and can be treated successfully with a long-stemmed femoral component to bypass the deficit [28]. In the setting of massive femoral bone loss, the options are limited. Massive endoprostheses have been utilized in this setting, but problems with this technique include instability, stress shielding, and inadequate fixation if the remnant distal femur is excessively short, leading to early femoral component loosening or fracture [6, 29, 33]. Such components are bone substituting and a need to revise them can lead to even greater host bone loss. In addition, for stabilization by porous coat or cement, at least 6 cm of diaphyseal host bone is necessary.
Impaction grafting is another well-described technique that has some success in patients with femoral bone loss [17, 21, 32]. While radiographic and histologic studies have suggested neovascularization of impacted allograft bone in the proximal femur [22], when there is substantial segmental bone loss, concerns regarding prosthesis migration and periprosthetic fracture remain [24, 25]. We believe impaction grafting is best used for those patients in whom there is contained or limited circumferential bone loss, whereas a PFA or endoprosthesis is a more appropriate option for substantial uncontained bone loss down to and including the diaphysis.
We previously reported using a structural allograft with uncontained defects in 63 THAs revised with a PFA without rerevision of 78% at an average of 11 years’ followup (stable implant with no need for rerevision surgery at the time of latest followup) [2]. Our current longer-term outcome data in a larger group of patients parallel those of earlier results. The longer-term results of other papers, although in smaller series, also show PFA in the setting of deficient proximal femoral bone has a favorable long-term outcome (Table 2) [3, 9, 14, 16]. The operative technique we have previously described seems to have some bearing on the longevity of the graft. Although we observed resorption of the graft to some extent in 58% of hips, in only one was this sufficiently severe to result in failure of the allograft. Our results contrast to other series where the femoral component was cemented into the allograft and host bone or where the femoral component did not bypass the allograft-host junction [14, 33]. We believe loading of the allograft-host site is favorable for union and also protects against graft resorption (Table 2).
Table 2.
Comparison of outcomes of PFA from the literature
| Study | Technique | Number of hips | Followup (years) | Infection (%) | Fracture (%) | Nonunion (%) | Severe resorption (%) | Loosening (%) | Femoral revision (%) | Dislocations (%) | Failure (%) | Success (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chandler et al. [3] | PFADistal press fit | 30 | 2 | 3.3 | 13.6 | 3.3 | 10 | 16.6 | 10 | 90 | ||
| Fox et al. [5] | PFA for tumorPlate or distal fixation | 56 | 7.9 | 11 | 81 | |||||||
| Langlais et al. [20] | PFA for tumorCemented distally | 21 | 6 | 0 | 28.6 | 4.8 | 14.3 | 14.3 | 0 | 81 | ||
| Haddad et al. [14] | PFACemented distally | 40 | 8.8 | 5 | 2.5 | 8 | 17.5 | 0 | 10 | 10 | 10 | 90 |
| Zehr et al. [33] | PFA for tumor | 14 | 10 | 16.7 | 5.6 | 0 | 0 | 14.3 | 76 | |||
| Megaprosthesis for tumor | 10 | 10 | 5.9 | 11.8 | 5.9 | 17.7 | 47 | 58 | ||||
| Zmolek and Dorr [34] | Press fit ± plate | 15 | 2 | 6.7 | 18 | 26.7 | 5.5 | 26.7 | 73.3 | |||
| Safir et al. | PFANo distal fixation | 50 | 16 | 4 | 0 | 6 | 2 | 12 | 14 | 8 | 16 | 84 |
PFA = proximal femoral allograft.
Our functional outcome analysis demonstrated relatively low scores for both WOMAC and SF-12. We believe the increased age and disability after multiple hip surgeries of this subgroup of patients account for this outcome.
The overall infection rate of this series was 4.8%. Although this infection rate seems high, it was not unexpected given the complexity of the surgery. Staged revision to another structural prosthetic-allograft composite for infected hip arthroplasty is a recognized technique that we employed successfully for the treatment for our infected allografts [1].
Nonunion is one of the more common complications when using a PFA (Table 2) [2, 5, 9, 11, 12, 14, 16, 18, 20]. The cause of the nonunions was presumably motion of the allograft-host junction. All but one of our patients went on to healing of the junction with additional surgery. We believe it is very important to achieve rigid stabilization of the junction at the time of the initial surgery. We now routinely utilize allograft struts and morselized autograft around the junction if there is concern with stability at the time of operation. Autograft bone from reamings of the femur and acetabulum is also laid around the junction and it is important to maintain the remnants of the native femur as a vascularized sleeve around the graft.
Dislocation of the construct was our most common major complication. This has been noted also in other series (Table 2) [3, 14, 18]. It is crucial to prevent malrotation of the prosthesis-allograft construct by taking care to orient the step cut appropriately. We now routinely perform a modification of the trochanteric osteotomy to avoid release of the posterior capsular structures in an effort to reduce the dislocation rate. Maintaining the soft tissue attachment to the osteotomized femur and using the remnant as a vascularized graft also aid in the prevention of dislocation. If the patient has no abductor mechanism, a constrained socket is used for the acetabulum. Bracing has not been used.
Our data demonstrate PFA for revision hip arthroplasty in femoral segmental bone is a durable solution in most patients at a minimum followup of 15 and mean of 16.2 years.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alexeeff M, Mahomed N, Morsi E, Garbuz D, Gross A. Structural allograft in two-stage revisions for failed septic hip arthroplasty. J Bone Joint Surg Br. 1996;78:213–216. [PubMed]
- 2.Blackley HR, Davis AM, Hutchison CR, Gross AE. Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip: a nine to fifteen-year follow-up. J Bone Joint Surg Am. 2001;83:346–354. [DOI] [PubMed]
- 3.Chandler H, Clark J, Murphy S, McCarthy J, Penenberg B, Danylchuk K, Roehr B. Reconstruction of major segmental loss of the proximal femur in revision total hip arthroplasty. Clin Orthop Relat Res. 1994;298:67–74. [PubMed]
- 4.Duncan CP, Masterson EL, Masri BA. Impaction allografting with cement for the management of femoral bone loss. Orthop Clin North Am. 1998;29:297–305. [DOI] [PubMed]
- 5.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term follow up of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. [DOI] [PubMed]
- 6.Gerrand CH, Bell RS, Griffin AM, Wunder JS. Instability after major tumor resection: prevention and treatment. Orthop Clin North Am. 2001;32:697–710, ix-x. [DOI] [PubMed]
- 7.Glassman AH, Engh CA, Bobyn JD. A technique of extensile exposure for total hip arthroplasty. J Arthroplasty. 1987;2:11–21. [DOI] [PubMed]
- 8.Goodman S, Pressman A, Saastamoinen H, Gross A. Modified sliding trochanteric osteotomy in revision total hip arthroplasty. J Arthroplasty. 2004;19:1039–1041. [DOI] [PubMed]
- 9.Graham NM, Stockley I. The use of structural proximal femoral allografts in complex revision hip arthroplasty. J Bone Joint Surg Br. 2004;86:337–343. [DOI] [PubMed]
- 10.Gross AE, Blackley H, Wong P, Saleh K, Woodgate I. The role of allografts in revision arthroplasty of the hip. Instr Course Lect. 2002;51:103–113. [PubMed]
- 11.Gross AE, Hutchison CR. Proximal femoral allografts for reconstruction of bone stock in revision arthroplasty of the hip. Orthop Clin North Am. 1998;29:313–317. [DOI] [PubMed]
- 12.Gross AE, Hutchison CR. Proximal femoral allografts for reconstruction of bone stock in revision hip arthroplasty. Orthopedics. 1998;21:999–1001. [DOI] [PubMed]
- 13.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 14.Haddad FS, Garbuz DS, Masri BA, Duncan CP. Structural proximal femoral allografts for failed total hip replacements: a minimum review of five years. J Bone Joint Surg Br. 2000;82:830–836. [DOI] [PubMed]
- 15.Haddad FS, Garbuz DS, Masri BA, Duncan CP, Hutchison CR, Gross AE. Instructional course lectures, the American Academy of Orthopaedic Surgeons. Femoral bone loss in patients managed with revision hip replacement: results of circumferential allograft replacement. J Bone Joint Surg Am. 1999;81:420–436. [DOI] [PubMed]
- 16.Haddad FS, Spangehl MJ, Masri BA, Garbuz DS, Duncan CP. Circumferential allograft replacement of the proximal femur: a critical analysis. Clin Orthop Relat Res. 2000;371:98–107. [DOI] [PubMed]
- 17.Halliday BR, English HW, Timperley AJ, Gie GA, Ling RS. Femoral impaction grafting with cement in revision total hip replacement: evolution of the technique and results. J Bone Joint Surg Br. 2003;85:809–817. [PubMed]
- 18.Head WC, Hillyard JM, Emerson RH Jr, Peters PC Jr. Proximal femoral allografts in revision total hip arthroplasty. Semin Arthroplasty. 1993;4:92–98. [PubMed]
- 19.Kellett CF, Boscainos PJ, Maury A, Pressman A, Cayen B, Zalzal P, Backstein D, Gross A. Proximal femoral allograft treatment of Vancouver type-B3 periprosthetic femoral fractures after total hip arthroplasty: surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2, Pt 1):68–79. [DOI] [PubMed]
- 20.Langlais F, Lambotte JC, Collin P, Thomazeau H. Long-term results of allograft composite total hip prostheses for tumors. Clin Orthop Relat Res. 2003;414:197–211. [DOI] [PubMed]
- 21.Lind M, Krarup N, Mikkelsen S, Horlyck E. Exchange impaction allografting for femoral revision hip arthroplasty: results in 87 cases after 3.6 years’ follow-up. J Arthroplasty. 2002;17:158–164. [DOI] [PubMed]
- 22.Linder L. Cancellous impaction grafting in the human femur: histological and radiographic observations in 6 autopsy femurs and 8 biopsies. Acta Orthop Scand. 2000;71:543–552. [DOI] [PubMed]
- 23.Malkani AL, Sim FH, Chao EY. Custom-made segmental femoral replacement prosthesis in revision total hip arthroplasty. Orthop Clin North Am. 1993;24:727–733. [PubMed]
- 24.Masterson EL, Duncan CP. Subsidence and the cement mantle in femoral impaction allografting. Orthopedics. 1997;20:821–822. [DOI] [PubMed]
- 25.Meding JB, Ritter MA, Keating EM, Faris PM. Impaction bone grafting before insertion of a femoral stem with cement in revision total hip arthroplasty: a minimum two-year follow-up study. J Bone Joint Surg Am. 1997;79:1834–1841. [DOI] [PubMed]
- 26.Morgan HD, McCallister W, Cho MS, Casnellie MT, Leopold SS. Impaction allografting for femoral component revision: clinical update. Clin Orthop Relat Res. 2004;420:160–168. [DOI] [PubMed]
- 27.Paprosky WG, Aribindi R. Hip replacement: treatment of femoral bone loss using distal bypass fixation. Instr Course Lect. 2000;49:119–130. [PubMed]
- 28.Paprosky WG, Greidanus NV, Antoniou J. Minimum 10-year-results of extensively porous-coated stems in revision hip arthroplasty. Clin Orthop Relat Res. 1999;369:230–242. [DOI] [PubMed]
- 29.Parvizi J, Sim FH. Proximal femoral replacements with megaprostheses. Clin Orthop Relat Res. 2004;420:169–175. [DOI] [PubMed]
- 30.Saleh KJ, Holtzman J, Gafni A, Saleh L, Davis A, Resig S, Gross AE. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am. 2001;83:1040–1046. [DOI] [PubMed]
- 31.Tomford WM. Disease transmission, sterilization and the clinical use of musculoskeletal tissue allografts. In: Tomford WM, ed. Musculoskeletal Tissue Banking. New York, NY: Raven Press; 1993:209–230.
- 32.van Biezen FC, ten Have BL, Verhaar JA. Impaction bone-grafting of severely defective femora in revision total hip surgery: 21 hips followed for 41–85 months. Acta Orthop Scand. 2000;71:135–142. [DOI] [PubMed]
- 33.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. [DOI] [PubMed]
- 34.Zmolek JC, Dorr LD. Revision total hip arthroplasty: the use of solid allograft. J Arthroplasty. 1993;8:361–370. [DOI] [PubMed]