Abstract
Today most emergency room radiographs are computerized, making digital image enhancement a natural advancement to improve fracture diagnosis. We compared the diagnosis of nondisplaced proximal femur fractures using four different image enhancement methods using standard DICOM (Digital Imaging and Communications in Medicine) after window-leveling optimization. Twenty-nine orthopaedic residents and specialists reviewed 28 pelvic images consisting of 25 occult proximal femur fractures and three images with no fracture, using four different image filters and the original DICOM image. For intertrochanteric fractures, the Retinex filter outperforms the other filters and the original image with a correct fracture type diagnosis rate of 50.6%. The Retinex filter also performs well for diagnosis of other fracture types. The Retinex filter had an interobserver agreement index of 53.5%, higher than the other filters. Sensitivity of fracture diagnosis increased to 85.2% when the Retinex filter was combined with the standard DICOM image. Correct fracture type diagnosis per minute for the Retinex filter was 1.43, outperforming the other filters. The Retinex filter may become a valuable tool in clinical settings for diagnosing fractures.
Level of Evidence: Level I, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
In recent years, traditional radiographic film gradually has been replaced by digital images. Digital technology allows many useful image manipulations. Some of these manipulations are basic and mandatory, eg, change of brightness, contrast, and zoom. More advanced manipulations such as image enhancement for soft or bony tissues are available but lack evidence to support their superiority in diagnosis of fractures.
Numerous studies describe the use of digital image enhancement, most for chest or dental radiography [3, 6, 8, 12, 17, 18, 24, 25, 32, 34, 35, 37, 39, 40]. Image enhancement outperformed standard DICOM images, eg, it enabled better lung and skeletal tumor detection [3, 6, 18], better craniofacial soft tissue measures [8], improved detection of lung and mediastinal nodules [11], and superior dental caries identification [39]. Other studies have only presented various filters without studying their performance in a clinical setting [17, 23, 24, 32, 34, 35].
Nondisplaced proximal femur fractures can be difficult to diagnose and in some cases the fracture is overlooked until displacement occurs [1, 21, 27–31, 38]. The actual number of missed hip fractures is unknown but has been reported from 2% to 10% [28, 36]. In patients with suspected hip fractures and normal-appearing radiographs, bone scans, CT, and MRI currently are used for additional diagnosis [2, 4, 5, 7, 9–11, 13, 14, 16, 20, 22, 26, 41]. MRI is considered the best diagnostic modality with reported sensitivity of 97% to 100% and specificity 96% to 100% [4, 9, 11, 41]. However, these modalities are either high-cost (MRI) or expose the patients to high levels of radiation (CT, bone scan).
Digital image enhancement offers a low-cost option to increase correct fracture diagnosis rates. This modality uses the standard radiograph performed in the emergency room and does not require exposure of patients to high levels of radiation. Evidence for better fracture diagnosis achieved by digital image enhancement is important as it may easily be incorporated into emergency room practice.
We compared the efficiency of four different mathematical algorithms, and the usual DICOM radiograph, for diagnosis of occult proximal femur fractures (subcapital, intertrochanteric, and base of neck). Based on specific fracture type, we compared: (1) the probability for correct fracture-type identification, by filter, (2) interobserver agreement of fracture type, and (3) correct fracture-type identification per minute. Regardless of fracture type (comparing only femurs without a fracture versus any fracture) we: (4) compared the sensitivity, specificity, and positive and negative predictive values, between the filters, and (5) identified factors contributing to correct diagnosis (fracture or no fracture).
Materials and Methods
In this study, we used the radiographs from a cohort of 28 patients with 11 subcapital fractures, five base of neck fractures, nine intertrochanteric fractures, and three normal (without fracture) radiographs as a control group. The final diagnosis for 17 of the patients was based on fracture displacement several days after presentation (Fig. 1) and for eight patients by other imaging modalities (MRI, CT, or bone scan). These images were digitally enhanced by four filters and contrast and brightness optimal adjustments performed to the standard DICOM format. We then randomly presented the 140 images (28 × 5) to 29 blinded physicians who determined the diagnoses. Overall 4060 images were analyzed. Approval for the use of the radiographic images was provided by the Chaim Sheba Medical Center Helsinki committee, which concluded the nature of the study was such that written patient consent was not required.
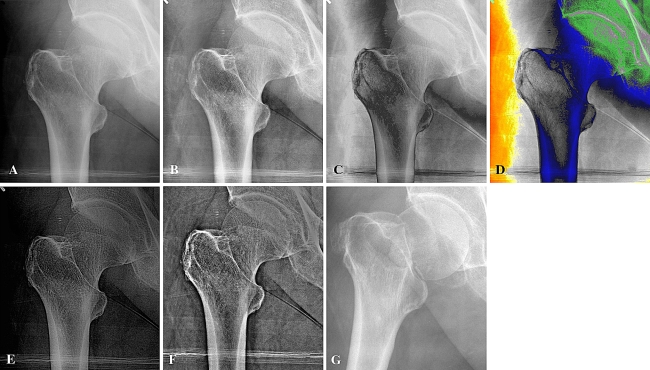
Fig. 1A–G.
(A) A DICOM image after optimal brightness and contrast adjustment, (B) adaptive histogram equalization filter, (C) triple gray filter, (D) color filter, (E) bone filter, and (F) Retinex filter images of a nondisplaced subcapital fracture are shown. This fracture was not diagnosed at presentation to the emergency room. (G) One day after the first radiograph was taken, the patient presented to the emergency room with a displaced subcapital fracture. In the DICOM image, the image brightness is not equal, whereas with the histogram equalization filter the brightness of the image is equally distributed. With the triple gray filter the image was parted into three zones and rebuilt, whereas with the color filter the image was parted into seven zones and rebuilt. The color filter was dropped after the pilot study. With the bone filter the bony trabeculae are enhanced, whereas with the Retinex filter bone elements are enhanced according to a multiscale level.
The study was conducted in two hospitals in Israel, The Chaim Sheba Medical Center and the Tel-Aviv Medical Center. Twenty-nine physicians participated in the study, included in two groups based on professional experience. The junior group (n = 12 [41.4%]) consisted of eight orthopaedic surgery residents with as much as 24 months residency training, three physicians in internship, and one family medicine resident. The senior group (n = 17 [59.6%]) consisted of 12 orthopaedic surgery residents with more than 2 years residency and five specialists in orthopaedic surgery. The mean age of the participants was 36 years (range, 26–59 years; standard deviation, 6.9 years), and the mean time of experience (measured from the beginning of residency) was 5.5 years (66.5 months; range, 0 for internships to 33 years for the most veteran specialist). We assured each physician was familiar with the nomenclature of proximal femur fracture by reviewing a diagram of the different proximal femur fractures.
Reviewing the Picture Archiving System from 2003 to 2006, there were 41 patients with nondisplaced proximal femur fractures. By using the rule of two as described by Laurin et al. [19], patients with two radiographic examinations of the hip or pelvis in a 3-week period before proximal femur surgery were reviewed. Twenty patients with proximal femur fractures who were misdiagnosed as not having fractures on their first visit to the emergency department were identified. Seventeen of these patients were later admitted with a displaced proximal femur fracture. An additional 21 patients with a nondisplaced hip fracture were diagnosed clinically at the emergency room and were admitted for additional examination. The control group consisted of three pelvic images of patients, who were referred to the emergency room with low back pain, excluding a fall.
We conducted a pilot study in which three orthopaedic residents (ND, ST, IBB) read 41 radiographic images. As a result of this pilot survey, the color filter (Fig. 1D) was dropped, and the pelvic images were zoomed and focused on the fracture side. The 41 radiographic images were too burdensome for the physicians to view, therefore, images in which the fracture was too evident were excluded; this left 25 radiographs of nondisplaced proximal femur fractures and three radiographs without fractures for review. We arbitrarily chose the number of radiographs without fractures.
All 25 fracture images were reviewed by three expert reviewers (IBB [senior resident], SD, AC [heads of orthopaedic surgery departments). A visibility score was given to the remaining images on a scale of 0 to 3: 0 = no sign of fracture, 1 = minimal sign of fracture (eg, cortex discontinuity), 2 = nondisplaced fracture (eg, hairline fracture), 3 = obvious fracture. This score was not validated previously (Pearson’s correlation = 0.47–0.78).
Using MATLAB® software (Version 7; The MathWorks, Inc, Natick, MA), we developed four different image enhancement filters that were applied to the pelvic radiographs. The original DICOM images were used as the gold standard for comparison with the filtered images (DICOM group, Fig. 1A). For the DICOM group, the window level (contrast and brightness) of the images was adjusted manually by one of the authors (IBB) to make the fracture site as visible as possible.
The image enhancement filters used were adaptive histogram equalization (Heq group) (Fig. 1B) [35], for which an internal MATLAB® algorithm was used; a triple gray filter (GgG group) (Fig. 1C), which parted the 4096 shades of gray in the regular image to three equal parts and the images obtained were combined smoothly; a color filter, in which gray-scale partition (as in the GgG filter) was used with colors for each partition (Fig. 1D); a bone filter (BF group) (Fig. 1E), in which we enhanced the bone trabeculae in the radiograph by detecting their spatial frequency; and a multiscale Retinex filter (Retinex group) (Fig. 1F), which separates the image into few high-pass sub-bands and recombines the sub-band images with a weighting parameter that reflects the importance of the features highlighted in that sub-band image to a clinician (Appendix 1) [23].
The study was conducted using software specifically tailored to the survey needs. All 28 images were displayed using four filters and the original DICOM image. Every participant had to diagnose the 140 images with a limit of 1 minute for each image. We recorded the diagnosis time of each image. To determine the image display order, for each participant, a constrained randomization algorithm was used, ie, no consecutive images were presented with the same fracture using different filters.
All of the images were anteroposterior radiographs of the proximal femur without a lateral view of the hip. The participants had to choose a diagnosis from a list or write a freehand diagnosis if it did not match the list. A level of confidence was noted for each image diagnosis on a scale of 1 to 5 in increasing confidence order (1 = suspected, 5 = absolutely sure). This score was not validated previously (Pearson’s correlation, –0.11–0.56; median, 0.16). We used the same portable computer (AMILO; Fujitsu Siemens, Maarssen, The Netherlands) to test all subjects.
Correct fracture-type classification probabilities are presented as frequency counts (percent). Comparisons of correct fracture-type classification probabilities were performed by the chi square test. All p values presented are two-sided. We used R software Version 2.5.1 (Vienna, Austria) for all analyses [33].
Interobserver agreement for each filter was calculated as the mean (±standard deviation) percent of similar fracture-type classification among all pairs of reviewers. It was compared using analysis of variance. Post hoc multiple corrections were performed by comparisons against the best [15].
The efficiency index was calculated as the number of correct answers divided by the total reviewer time (in minutes). This index is the number of correct answers per minute, and is an important criterion for filter performance; it represents the time efficiency of the filters. A linear regression model was fitted using filter type, reviewer experience group (juniors or seniors), and their interactions to detect which of these factors influenced the index.
We calculated diagnosis test indices (sensitivity, specificity, and positive and negative predictive values) by grouping the answers to fractures versus no fractures. The indices among all groups were compared with the combined performance of the original DICOM group and Retinex filter group. For this combined use of filters, we defined a fracture diagnosis if so diagnosed using at least one of the filters (Retinex or DICOM groups). The comparisons were made using proportion approximation to the normal distribution calculating covariance for comparisons with the DICOM or the Retinex filter. For the other filters, independence was assumed. We performed correction for multiple comparisons using the Dunnett method with the combined filter used as the common compared treatment.
Multivariate analysis was performed using logistic regression for correct diagnosis, ie, fracture or no fracture. A correct diagnosis was considered identifying any type of fracture or no fracture. The covariates inserted in the model were experience group, visibility score (0 for no fracture, 1 to 3 by increasing visibility), time of diagnosis greater than 30 seconds, filter type, confidence index, type of fracture, and interaction terms for time with experience group and visibility index. Results of the logistic regression are presented as odds ratio (OR), with the appropriate 95% confidence interval and p value.
We also performed a logistic regression using time longer than 30 seconds as the dependent variable. Explanatory covariates were correct fracture diagnosis, senior versus junior groups, visibility score, and interaction between the last two.
Results
The mean time to answer all the questions concerning the 140 images was 53 minutes (range, 34–82 minutes; standard deviation, 13 minutes), with an average time of 23 seconds per question. The senior group answered the survey on average 10 minutes faster (p = 0.04) than the junior group, with means of 49.1 minutes and 59.6 minutes, respectively. The average time for answering a question decreased as the visibility index improved. There was a tendency to answer faster when the confidence level increased.
Overall correct fracture-type identification probability was 47.3%. The correct identification probabilities of intertrochanteric fractures for the DICOM, Retinex, GgG, Heq, and BF groups were 113 (43.3%), 132 (50.6%), 77 (29.5%), 121 (46.4%), and 119 (45.6%), respectively (Table 1) (p = 0.001). We found the Retinex filter had interobserver fracture-type agreement of 53.5%, which was greater than any other filter (Table 1) (p = 0.001).
Table 1.
Correct fracture classification by filter and fracture type
| Fracture type | DICOM | Retinex | GgG | Heq | BF | p value |
|---|---|---|---|---|---|---|
| Subcapital (n = 319) | 182 (57%) | 185 (58%) | 163 (51%) | 188 (59%) | 178 (56%) | 0.3 |
| Intertrochanteric (n = 261) | 113 (43%)* | 132 (50%)* | 77 (29%) | 121 (46%) | 119 (45%) | 0.001 |
| Base of neck (n = 145) | 40 (27%) | 37 (25%) | 39 (27%) | 38 (26%) | 43 (30%) | 0.94 |
| Interobserver agreement† | 48% (± 11%)‡ | 54% (± 10%)‡ | 51.6% (± 10.5%)‡ | 47 % (± 12%)‡ | 48% (± 10%)‡ | 0.001§ |
* p = 0.114; †interobserver agreement was calculated only for frames in which at least one of the testers was correct in diagnosis of fracture type; values are expressed as mean (± standard deviation); ‡adjusted p < 0.05 (pair-wise comparisons with the best, ie, Retinex); §using analysis of variance; GgG = triple gray filter; Heq = adaptive histogram equalization filter; BF = bone filter.
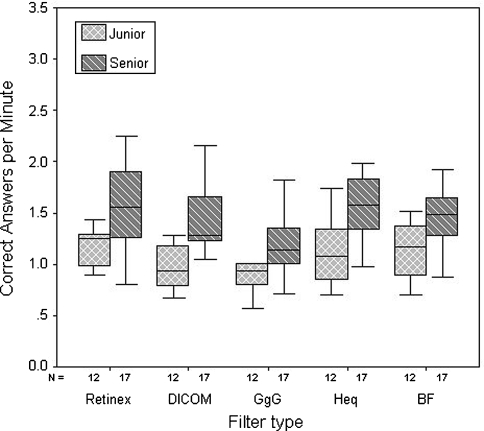
The seniors had higher correct fracture identification per minute (1.45) as compared with the juniors’ experience group (1.09; p = 0.001) (Fig. 2). Mean efficiency indices for DICOM, Retinex, GgG, Heq, and BF groups were 1.28, 1.43, 1.08, 1.37, and 1.36, respectively. The Retinex filter had the highest efficiency index, meaning highest correct fracture diagnosis per minute (p = 0.0016).
Fig. 2.
A graph compares box plots of correct fracture-type identification per minute for senior and junior physicians with each filter used in the study. The middle lines in the box plots represent the median, the box limits are the first and third quartiles, and the bars are extreme values. The senior physicians have higher correct fracture-type identification per minute than the junior physicians (p = 0.001). As compared with the DICOM filter, the triple gray and Retinex filters have lower and higher correct fracture-type identifications per minute, respectively (p = 0.025 and p = 0.09, respectively). GgG = triple gray filter; Heq = adaptive histogram equalization filter; BF = bone filter.
The Retinex filter generally outperformed the other filters with sensitivity, specificity, and positive and negative predictive values of 75.4%, 66.7%, 95%, and 24.6%, respectively (Table 2). The sensitivity of the filter obtained from combining the DICOM filter with the Retinex filter was 85.2%, outperforming all other filters (adjusted p = 0.01).
Table 2.
Diagnosis test indices for the filters
| Index | DICOM (n = 812) | Retinex (n = 812) | Heq (n = 812) | GgG (n = 812) | BF (n = 812) | DICOM + Retinex (n = 812) |
|---|---|---|---|---|---|---|
| Sensitivity | 73%* | 75%* | 72%* | 62%* | 72%* | 85%* |
| Specificity | 64% | 66% | 55% | 60% | 48% | 53% |
| PPV | 94% | 95% | 93% | 93% | 92% | 94% |
| NPV | 22% | 24% | 19% | 16% | 17% | 30% |
All fracture types were combined and diagnosis was restricted to fracture versus no fracture. As the Retinex filter outperformed the other filters, we checked the performance of combining the DICOM with Retinex filter, so that fracture diagnosis with any of them would be considered a fracture; *adjusted p < 0.05 (Dunnett’s method), compared with DICOM + Retinex; GgG = triple gray filter; Heq = adaptive histogram equalization filter; BF = bone filter; PPV = positive predictive value; NPV = negative predictive value.
The following factors increased the odds for correct diagnosis (fracture or without fracture) (Table 3): senior physicians (OR = 1.25; p = 0.015), diagnosis time longer than 30 seconds for each frame (OR = 1.6; p = 0.013), each visibility score (OR = 2.81; p = 0.001), and each confidence index (OR = 1.13; p = 0.001). The GgG filter (OR = 0.59; p = 0.001) decreased the probability for correct diagnosis. The OR of the other filters did not differ from that of the DICOM image. A senior physician observing an image for more than 30 seconds reduced his odds to diagnose correctly (OR = 0.62; p = 0.004).
Table 3.
Multivariate logistic regression analyzing factors*
| Variable | OR | OR 95% CI | p value |
|---|---|---|---|
| Physician experience | |||
| Junior | 1 | ||
| Senior | 1.25 | 1.04–1.5 | 0.015 |
| Visibility score | 2.81 | 2.48–3.19 | 0.001 |
| Time to review an image | |||
| < 30 seconds | 1 | ||
| > 30 seconds | 1.60 | 1.1–2.31 | 0.013 |
| Filter type | |||
| DICOM | 1 | ||
| Retinex | 1.11 | 0.88–1.41 | 0.38 |
| GgG | 0.59 | 0.47–0.75 | 0.001 |
| Heq | 0.90 | 0.71–1.13 | 0.363 |
| BF | 0.87 | 0.69–1.09 | 0.232 |
| Confidence index | 1.13 | 1.06–1.21 | 0.001 |
| Type of fracture | |||
| None | 1 | ||
| Base of neck | 0.15 | 0.11–0.22 | 0.001 |
| Intertrochanter | 0.49 | 0.36–0.67 | 0.001 |
| Subcapital | 0.20 | 0.15–0.27 | 0.001 |
| Seniors and time > 30 seconds | 0.62 | 0.45–0.86 | 0.004 |
| Visibility score and time > 30 seconds | 0.82 | 0.68–0.98 | 0.028 |
* Odds ratio > 1 indicates increasing chance for correct diagnosis (fracture or no fracture); OR = odds ratio; CI = confidence interval; GgG = triple gray filter; Heq = adaptive histogram equalization filter; BF = bone filter.
To explain the last result, we examined factors that influenced the odds to review an image more than 30 seconds. Junior physicians increased the odds by a factor of 1.37 (p = 0.0013). Each visibility score and correct diagnosis reduced the odds by factors of 0.89 (p = 0.0008) and 0.75 (p = 0.0003), respectively. However, senior physicians did not view images with low visibility score longer (p = 0.23).
Discussion
Digital image enhancement is a relatively simple but important advance in diagnosis of fractures. We believe it offers better diagnosis of fractures without higher radiation exposure. We examined several filters for diagnosis of nondisplaced proximal femur fracture. The filters were compared by probability for correct fracture-type identification, interobserver agreement, correct fracture-type identification per minute, diagnosis test indices, and multivariate model for correct diagnosis.
Our study had several limitations. The images used did not necessarily represent the cases viewed in the emergency room by the orthopaedic physician. Thus, the data do not represent the fracture types or the normal to abnormal image ratios. This presents difficulty in assessing the positive and negative predictive values of the tests, because these indices depend on the incidence of fractures in the studied population. However, the focus of the analysis is on indices that are less influenced from fracture prevalence such as the OR, sensitivity, and specificity. The limited number of normal images was attributable to trying not to overburden the reviewers, ie, most of them reviewed the images for almost an hour.
Our data suggest digital image enhancement may be a useful tool for improving fracture diagnosis. Most of the literature regarding digital image enhancement are descriptive and do not offer comprehensive comparisons of filters (Table 4) [6, 8, 12, 18, 35, 39, 40]. For chest radiography, digital image enhancement leads to better tumor detection and better nodule detection rate [6, 18]. In dental surgery, digital image enhancement improves the metric measures for facial soft tissue structures [8]. It also provides better detection rates of caries [39]. Our data suggest the Retinex filter has the highest probability for correct diagnosis of intertrochanteric fractures. Combining this filter with the original DICOM image yields a high sensitivity (85%), outperforming the other filters. This finding is further emphasized as the DICOM images presented were optimally adjusted manually (contrast and brightness). This manual adjustment partially explains the relatively good diagnosis based on the DICOM images.
Table 4.
Studies of digital image enhancement
| Study | Radiograph type | Aim | Results |
|---|---|---|---|
| Braunstein et al. [3] | Skeletal xray | Evaluate the adaptive histogram equalization (AHE) filter | AHE was better in diagnosing periosteal reaction and cortical breakthrough. |
| Cohn et al. [6] | Chest xray | Evaluate the filter, unsharp masking. | Better chest tumor detection and 2:1 compression rate. |
| Eppley and Sadove [8] | Craniofacial | Compare craniofacial metrics using image enhancement. | Better soft tissue relationships with image enhancement. Similar bony relationship. |
| Gotfredsen et al. [12] | Dental caries | Evaluate the use of image enhancement. | Visualix images were enhanced more frequently then those obtained from other systems. |
| Kim et al. [18] | Chest xray | Nodule identification. | Proposed image enhancement improves lung and mediastinal nodule detection. |
| Rehm et al. [35] | Chest xray | Present the ASAHE algorithm | The algorithm did not change rate of chest nodule detection. |
| Shrout et al. [39] | Dental caries | Identifying dental caries by image enhancement. | Digital enhancement showed superior identification of dental caries. |
| Stahl et al. [40] | Orthopaedics | Presenting nonlinear multiscale enhancement algorithm. | Anecdotal examples, not clinically assessed. |
| Current study | Orthopaedics | Comparing several filters: (1) fracture diagnosis; (2) interobserver agreement; (3) correct diagnosis per minute; (4) sensitivity and specificity; (5) multivariate analysis. | The Retinex filter outperformed the other filters used. By combining the Retinex filter with the standard DICOM image, sensitivity for correct diagnosis increased to 85%. |
The study design enabled exploration of some important issues, previously unnoticed, regarding occult proximal femur fractures. As was expected, the senior physicians tended to view images for shorter times, with higher correct diagnosis per minute than junior physicians. Although the junior group increased its OR for correct diagnosis by extending viewing time of each image, a reverse effect was observed in the senior physicians group. One might argue senior examiners are able to recognize and focus on occult fractures better; however, as we assessed the visibility of each fracture, we were able to reject this hypothesis.
We believe the Retinex filter can be used as a complement to diagnosis based on a standard DICOM image, enhancing its sensitivity to occult fractures. We suggest that if no fracture is seen on the DICOM image but there is clinical evidence to suggest an occult fracture (inability of an elderly patient to walk or pain at axial loading with limited range of motion, etc), then the Retinex filter should be used. When a fracture is seen, it should be treated. If no fracture is seen with the Retinex filter, MRI or other imaging modalities should be used.
Acknowledgments
We thank Professor Shmuel Dekel, Dr. Niv Dreiangel, and Dr. Shay Tenenbaum for assistance with this study. IBB and AH contributed equally to this work.
Appendix 1. Description of the filters used in this study
The original DICOM picture (Fig. 1A) was used as the gold standard for comparison with the filtered images. The window level (contrast and brightness) for all of the images was adjusted manually to make the fracture site as visible as possible. The adjusted pictures were saved as bit map files.
The adaptive histogram equalization (Fig. 1B) filter was not developed by us. We used the integral algorithm of the MATLAB® software. It was tested previously on chest radiographs. Because the dynamic range of radiographs is approximately 1024 to 4096 gray levels and standard screens normally show 256 gray levels, these image gray levels to the screen gray levels must be mapped. To map the image, it was divided into square blocks of 32 × 32 pixels. On each block, mapping of the window level for each pixel was performed; the center of each block was used as the transformation calculated for the block. For each pixel in the image, we set the gray level transformation as the bilinear interpolation from the block centers. The result is an image that locally shows the pattern in all frequencies at maximum contrast; however, on a global scale, it has a nonlinear transformation compressing the dynamic range to 256 gray levels.
The idea behind the triple gray filter (Fig. 1C) was to triple the number of the gray shades seen when the radiograph is displayed on a standard screen. As mentioned before, a normal computer screen displays eight-bit depth, equal to 256 steps of gray; however, in a DICOM image, each pixel contains 12-bit depth, which means approximately 4096 shades of gray. The image was parted into three images containing 1/3 of the gray scale of the original image. On each part, 1365 shades of gray instead of 4096 in the original image were displayed with 256 gray levels. To combine the three images smoothly, the middle part was left normal and the center and the peripheral parts were inverted. This filter can be dynamic by changing the thresholds between the parts, but for the test the parameters were constant for all of the images.
Because most of the physicians use a color computer screen, we tried to present the picture in colors to show more information (Fig. 1D). The same manipulation on the image was performed as for the triple gray filter, only the image was parted into seven zones according to the gray level. We left the middle part gray and used colors to display the other parts. Eventually the image was rebuilt. This filter was excluded from the final survey after a pilot survey showed this filter was significantly inferior to the other filters.
With the bone filter (Fig. 1E), we attempted to enhance the bone trabeculae in the radiograph by detecting the spatial frequency band of the trabeculae and enhancing their average frequency in the image using a spatial filter. To reduce the dynamic range, we weakened the low frequencies in the image (using a single spatial filter). After enhancing the trabecular grid in the image and suppressing the low frequencies, we wanted to avoid the tedious action of setting the window leveling; we applied an adaptive histogram equalization filter on the resulting image. The result is an easy to calculate image in which the trabecular grid is visible and enhanced.
The multiscale Retinex filter (Fig. 1F) is designed for dynamic range compression. The filter is applicable in cases in which the image is unable to encompass the wide dynamic range present in most natural scenes. The human visual system is tolerant to spectral changes in lighting and conditions. A human observer is able to separate the lighting conditions of the scene from the texture details related to the features in the scene. Therefore, the lighting variations across the image are largely compressed, whereas the texture variations are compressed with much lower values. The multiscale Retinex method is based on such a model of the human visual system. It separates the image into a few high-pass sub-bands, thus examining the image at different resolutions and decreasing the effects of illumination, which is considered smooth over the image. Global window leveling optimization then is applied to each image sub-band to fit the relevant gray scale values over the available display gray scale values. Thus, the irrelevant gray scale values related to external objects (metal implants) are fitted with constant saturation value. The resulting filtered image finally is attained by recombining the sub-band images with a weighting parameter that reflects the importance of the features highlighted in that sub-band image.
Footnotes
One or more of the authors (RN, DR) has or may receive payments or benefits from a commercial entity (Surgix Orthopedic Solutions Ltd) related to this work.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alba E, Youngberg R. Occult fractures of the femoral neck. Am J Emerg Med. 1992;10:64–68. [DOI] [PubMed]
- 2.Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197:263–267. [DOI] [PubMed]
- 3.Braunstein EM, Capek P, Buckwalter K, Bland P, Meyer CR. Adaptive histogram equalization in digital radiography of destructive skeletal lesions. Radiology. 1988;166:883–885. [DOI] [PubMed]
- 4.Chana R, Noorani A, Ashwood N, Chatterji U, Healy J, Baird P. The role of MRI in the diagnosis of proximal femoral fractures in the elderly. Injury. 2006;37:185–189. [DOI] [PubMed]
- 5.Clinkscales CM. MRI in the diagnosis of occult hip fractures in the elderly. Orthopedics. 1994;17:578. [DOI] [PubMed]
- 6.Cohn M, Trefler M, Young TY. Enhancement and compression of digital chest radiographs. J Thorac Imaging. 1990;5:92–95. [DOI] [PubMed]
- 7.Egund N, Nilsson LT, Wingstrand H, Stromqvist B, Pettersson H. CT scans and lipohaemarthrosis in hip fractures. J Bone Joint Surg Br. 1990;72:379–382. [DOI] [PubMed]
- 8.Eppley BL, Sadove AM. Computerized digital enhancement in craniofacial cephalometric radiography. J Oral Maxillofac Surg. 1991;49:1038–1043. [DOI] [PubMed]
- 9.Evans PD, Wilson C, Lyons K. Comparison of MRI with bone scanning for suspected hip fracture in elderly patients. J Bone Joint Surg Br. 1994;76:158–159. [PubMed]
- 10.Fairclough J, Colhoun E, Johnston D, Williams LA. Bone scanning for suspected hip fractures: a prospective study in elderly patients. J Bone Joint Surg Br. 1987;69:251–253. [DOI] [PubMed]
- 11.Frihagen F, Nordsletten L, Tariq R, Madsen JE. MRI diagnosis of occult hip fractures. Acta Orthop. 2005;76:524–530. [DOI] [PubMed]
- 12.Gotfredsen E, Wenzel A, Grondahl HG. Observers’ use of image enhancement in assessing caries in radiographs taken by four intra-oral digital systems. Dentomaxillofac Radiol. 1996;25:34–38. [DOI] [PubMed]
- 13.Guanche CA, Kozin SH, Levy AS, Brody LA. The use of MRI in the diagnosis of occult hip fractures in the elderly: a preliminary review. Orthopedics. 1994;17:327–330. [DOI] [PubMed]
- 14.Holder LE, Schwarz C, Wernicke PG, Michael RH. Radionuclide bone imaging in the early detection of fractures of the proximal femur (hip): multifactorial analysis. Radiology. 1990;174:509–515. [DOI] [PubMed]
- 15.Hsu J, ed. Multiple Comparison,Theory and Methods. London, England: Chapman & Hall; 1996.
- 16.Hughes SS, Voit G, Kates SL. The role of computerized tomography in the diagnosis of an occult femoral neck fracture associated with an ipsilateral femoral shaft fracture: case report. J Trauma. 1991;31:296–298. [DOI] [PubMed]
- 17.Khocht A. Computerized image capture, storage, retrieval, and enhancement in digital radiography. Pract Proced Aesthet Dent. 2004;16:477–478. [PubMed]
- 18.Kim JH, Im JG, Han MC, Min BG, Lee CW. Improved visualization of stimulated nodules by adaptive enhancement of digital chest radiography. Acad Radiol. 1994;1:93–99. [DOI] [PubMed]
- 19.Laurin S, Jonsson K, Jonsson R. [Low frequency of missed or invisible hip fracture in X-ray examination: the rule-of-2-a simple method for quality assessment][in Swedish]. Lakartidningen. 2004;101:2423–2425. [PubMed]
- 20.Lim KBL, Eng AKH, Chng SM, Tan AGS, Thoo FL, Low CO. Limited magnetic resonance imaging (MRI) and the occult hip fracture. Ann Acad Med Singapore. 2002;31:607–610. [PubMed]
- 21.Lindberg EJ, Macias D, Gipe BT. Clinically occult presentation of comminuted intertrochanteric hip fractures. Ann Emerg Med. 1992;21:1511–1514. [DOI] [PubMed]
- 22.Lubovsky O, Liebergall M, Mattan Y, Weil Y, Mosheiff R. Early diagnosis of occult hip fractures MRI versus CT scan. Injury. 2005;36:788–792. [DOI] [PubMed]
- 23.Meylan L, Susstrunk, S. High dynamic range image rendering using a Retinex-based adaptive filter. IEEE Trans Image Process. 2006;15:2820–2830. [DOI] [PubMed]
- 24.Moore CJ. Measurements from digital scanned projection radiographs: couch detail and detector artefact removal by simple image enhancement. Br J Radiol. 1985;58:756–759. [DOI] [PubMed]
- 25.Oestmann JW, Greene R, Rubens JR, Pile-Spellman E, Hall D, Robertson C, Llewellyn HJ, McCarthy KA, Potsaid M, White G. High frequency edge enhancement in the detection of fine pulmonary lines: parity between storage phosphor digital images and conventional chest radiography. Invest Radiol. 1989;24:643–646. [DOI] [PubMed]
- 26.Pandey R, McNally E, Ali A, Bulstrode C. The role of MRI in the diagnosis of occult hip fractures. Injury. 1998;29:61–63. [DOI] [PubMed]
- 27.Parker M, Johansen A. Hip fracture. BMJ. 2006;333:27–30. [DOI] [PMC free article] [PubMed]
- 28.Parker MJ. Missed hip fractures. Arch Emerg Med. 1992;9:23–27. [DOI] [PMC free article] [PubMed]
- 29.Pathak G, Parker MJ, Pryor GA. Delayed diagnosis of femoral neck fractures. Injury. 1997;28:299–301. [DOI] [PubMed]
- 30.Perron AD, Miller MD, Brady WJ. Orthopedic pitfalls in the ED: radiographically occult hip fracture. Am J Emerg Med. 2002;20:234–237. [DOI] [PubMed]
- 31.Pool FJ, Crabbe JP. Occult femoral neck fractures in the elderly: optimisation of investigation. N Z Med J. 1996;109:235–237. [PubMed]
- 32.Powlis WD, Brikman I, Seshadri SB, Bloch P. Portal radiographs: digital enhancement of contrast. Radiology. 1988;169:839–841. [DOI] [PubMed]
- 33.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2007.
- 34.Reddy MS, Bruch JM, Jeffcoat MK, Williams RC. Contrast enhancement as an aid to interpretation in digital subtraction radiography. Oral Surg Oral Med Oral Pathol. 1991;71:763–769. [DOI] [PubMed]
- 35.Rehm K, Seeley GW, Dallas WJ, Ovitt TW, Seeger JF. Design and testing of artifact-suppressed adaptive histogram equalization: a contrast-enhancement technique for display of digital chest radiographs. J Thorac Imaging. 1990;5:85–91. [DOI] [PubMed]
- 36.Rizzo PF, Gould ES, Lyden JP, Asnis SE. Diagnosis of occult fractures about the hip: magnetic resonance imaging compared with bone-scanning. J Bone Joint Surg Am. 1993;75:395–401. [DOI] [PubMed]
- 37.Scholz A, Langer M, Zwicker C, Felix R. Picture quality of a digital urogram using image enhancement radiography [in German]. Digitale Bilddiagn. 1989;9:31–35. [PubMed]
- 38.Schultze J. Occult fracture of the femoral neck fractures of the proximal femur [in German]. Nuklearmedizin. 1998;37:80–82. [PubMed]
- 39.Shrout MK, Russell CM, Potter BJ, Powell BJ, Hildebolt CF. Digital enhancement of radiographs: can it improve caries diagnosis? J Am Dent Assoc. 1996;127:469–473. [DOI] [PubMed]
- 40.Stahl M, Aach T, Dippel S. Digital radiography enhancement by nonlinear multiscale processing. Med Phys. 2000;27:56–65. [DOI] [PubMed]
- 41.Verbeeten KM, Hermann KL, Hasselqvist M, Lausten GS, Joergensen P, Jensen CM, Thomsen HS. The advantages of MRI in the detection of occult hip fractures. Eur Radiol. 2005;15:165–169. [DOI] [PubMed]