Abstract
Local attempts to repair a cartilage lesion could cause increased levels of anabolic and catabolic factors in the synovial fluid. After repair with regenerated cartilage, the homeostasis of the cartilage ideally would return to normal. In this pilot study, we first hypothesized levels of synovial fluid markers would be higher in patients with cartilage lesions than in patients with no cartilage lesions, and then we hypothesized the levels of synovial fluid markers would decrease after cartilage repair. We collected synovial fluid samples from 10 patients before autologous chondrocyte transplantation of the knee. One year later, a second set of samples was collected and arthroscopic evaluation of the repair site was performed. Fifteen patients undergoing knee arthroscopy for various symptoms but with no apparent cartilage lesions served as control subjects. We measured synovial fluid matrix metalloproteinase-3 (MMP-3) and insulinlike growth factor-I (IGF-I) concentrations with specific activity and enzyme-linked immunosorbent assays, respectively. The levels of MMP-3 and IGF-I were higher in patients having cartilage lesions than in control subjects with no cartilage lesions. One year after cartilage repair, the lesions were filled with repair tissue, but the levels of MMP-3 and IGF-I remained elevated, indicating either graft remodeling or early degeneration.
Level of Evidence: Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Autologous chondrocyte transplantation (ACT) is a cell-based method for repair of cartilage lesions. Little is known about the biochemical mediators in synovial fluid affecting healing of cartilage lesions [21]. Normal cartilage turnover is a result of homeostasis between anabolic and catabolic factors. Both types of factors can be secreted by chondrocytes and by the cells of the synovial lining. With inflammation, osteoarthritis, and trauma, this homeostasis is disturbed and the balance is shifted toward a catabolic state. In cartilage repair, this increased turnover must be shifted toward an anabolic state for the repair to be successful.
MMP-3 (or stromelysin-1) is one of the calcium-dependent zinc-containing proteinases stimulated by interleukin-1 and responsible for degradation of extracellular matrix. It has a wide variety of substrates and it hydrolyzes extracellular matrix components, such as aggrecan, fibronectin, laminin, and collagens III, IV, IX, X, and XI [24, 34]. MMP-3 also activates other pro-MMPs, such as MMP-1, 7, 8, 9, and 13 [18]. MMP-3 reportedly plays a major role in the turnover of young human cartilage and early osteoarthritis [24]. The predominant forms of MMP-3 in the synovial fluid is usually the latent proenzymes [32].
IGF-I is thought to have a major anabolic effect on cartilage metabolism [15]. It induces synthesis of collagen Type II and core protein [33]. IGF-I stabilizes the chondrocytic phenotype, has a mitogenic effect on chondrocytes [16], and enhances cartilage repair [8, 9]. IGF-I levels are elevated in osteoarthritis, especially during the early stages [26]. A local attempt to repair a cartilage lesion could cause increased levels of anabolic and catabolic factors. After successful repair with regenerated cartilage, the homeostasis of the cartilage ideally would return to normal to prevent future degeneration.
We therefore first hypothesized levels of synovial fluid markers would be higher in patients with cartilage lesions than in patients with other knee complaints but no cartilage lesions. We also hypothesized the levels of synovial fluid markers would decrease after resurfacing Grades 3–4 full thickness cartilage lesions.
Materials and Methods
We collected synovial fluid samples from patients before and 1 year after ACT and analyzed the synovial fluid concentration of MMP-3 and IGF-I. Control samples were collected from a set of patients undergoing arthroscopy for various symptoms but without obvious cartilage lesions. The treatment group had symptoms we judged related to the cartilage lesions. During a study period in 1999–2000, 17 patients underwent ACT and arthroscopy 1 year afterward. All patients had full-thickness cartilage lesions (International Cartilage Repair Society [ICRS], classification Grades 3–4 [27]) of the femur or patella and had lesions filled with repair tissue at 1 year. We were able to obtain paired synovial fluid samples from 10 of these patients, who form the experimental group. These 10 patients had lesions with a mean size of 9.4 cm2 (range, 2.5–22.4 cm2). The control group consisted of 15 patients undergoing elective knee arthroscopy (Table 1). Before the procedure, we obtained a synovial fluid sample during spinal anesthesia. During arthroscopy, the cartilage lesions and other joint abnormalities were recorded. Only samples from patients with intact cartilage were included in the study. This was a pilot study to find possible hypotheses for further research. Therefore, no power analysis was performed beforehand. All patients provided informed consent.
Table 1.
Demographics of study groups
| Group | Number | Age (years) | Gender | Lesion size (cm²) | Type of injury/diagnosis |
|---|---|---|---|---|---|
| Lesion | 14 | 39 | Female | 3 | Sports-related trauma |
| Lesion | 16 | 39 | Male | 4 | Work-related trauma |
| Lesion | 19 | 17 | Male | 6 | Osteochondritis dissecans |
| Lesion | 21 | 47 | Male | 10 | Work-related trauma |
| Lesion | 26 | 17 | Male | 12 | Osteochondritis dissecans |
| Lesion | 27 | 40 | Male | 3 | Trauma, partial ACL rupture |
| Lesion | 28 | 34 | Male | 3 | Osteochondritis dissecans |
| Lesion | 31 | 29 | Male | 20 | Osteochondritis dissecans |
| Lesion | 35 | 17 | Female | 12 | Unknown trauma |
| Lesion | 36 | 43 | Female | 22 | Osteoarthritis |
| Control | 1004 | 41 | Male | Ruptured medial meniscus; ACL rupture | |
| Control | 1007 | 27 | Male | Ruptured medial meniscus | |
| Control | 1009 | 36 | Female | ACL rupture; ruptured medial meniscus | |
| Control | 1010 | 20 | Male | Infrapatellar fat pad syndrome and medial plica | |
| Control | 1012 | 33 | Male | Medial plica | |
| Control | 1013 | 22 | Male | Ruptured lateral meniscus | |
| Control | 1018 | 25 | Female | Knee pain, medial plica | |
| Control | 1019 | 32 | Female | Knee pain | |
| Control | 1021 | 21 | Male | Ruptured medial meniscus | |
| Control | 1022 | 36 | Female | Synovial plica | |
| Control | 1026 | 39 | Male | Ruptured lateral meniscus | |
| Control | 1031 | 32 | Male | Ruptured medial meniscus | |
| Control | 1035 | 43 | Male | Ruptured medial meniscus | |
| Control | 79 | 19 | Male | Cartilage lesion of the ankle (knee arthroscopy for cartilage biopsy) | |
| Control | 70 | 21 | Male | Cartilage lesion of the opposite knee (knee arthroscopy for cartilage biopsy) |
There were three female and seven male patients in the ACT group with an average age of 32 years (range, 17–43 years). There were four female and 11 male patients in the control group with an average age of 30 years (range, 19–41 years). There were no differences in age and gender between the groups (Table 1).
The ACT consisted of two operative procedures [3]. A cartilage biopsy specimen was obtained arthroscopically from a nonweightbearing area and sent for chondrocyte isolation and culture [3, 19, 20]. A few weeks after cartilage harvesting, we performed arthrotomy. The lesion was débrided to the subchondral bone, avoiding bleeding from the bony surface. We harvested a periosteal flap from the medial part of the tibia or from one of the femoral epicondyles. This flap was sutured with the cambium layer facing down to the margins of the lesion and the seam then was sealed with fibrin glue. Through a small opening, we injected the suspension of cultured chondrocytes, approximately 106/cm2 of the lesion, underneath the periosteum.
One year later, assessment arthroscopy was performed and one of us (IK) evaluated the cartilage repair tissue using the ICRS arthroscopic grading [27] system. The scoring evaluates filling of the defect (1–4 points), integration of the graft to the borders (1–4 points), and macroscopic appearance (1–4 points) A total score of 12 means normal repair tissue, 11 to 8 is nearly normal tissue, 7 to 4 is abnormal tissue, and 3 to 0 is severely abnormal tissue.
We harvested the preoperative synovial fluid sample either before the arthroscopic cartilage biopsy or before ACT. The sample was taken at the beginning of the operation during spinal anesthesia with blind needle aspiration. Another sample was taken during the 1-year followup arthroscopy. We stored the synovial fluid samples at −70°C. No anticoagulation with EDTA was used. The cells were not removed and no cell count was made. MMP-3 activity was measured using an MMP-3-specific immunocapture activity assay (Amersham Biosciences, Buckinghamshire, UK). This assay measures active MMP and total MMP (active plus latent pro-MMP) activity but is insensitive to proteinase inhibitor complexes. As a substrate, modified prourokinase was used [11]. We measured the IGF-I concentration of the synovial fluid using an enzyme-linked immunosorbent assay for human IGF-I immunoassay (R&D Systems, Minneapolis, MN). According to the manufacturer, no significant interference of IGF-binding proteins 2, 3, and 4 (IGFBP-2, IGFBP-3, and IGFBP-4) has been detected with the method.
We evaluated the normality of variables by the Shapiro-Wilk test. However, as variables were skewed, confidence intervals (CIs) for the means were obtained by bias-corrected bootstrapping (5000 replications) [7]. The bootstrap method works by resampling from the observed data and thus makes no assumptions on distribution [7]. We compared changes within and between groups using the permutation test with general scores and exact p values. The permutation test can provide very accurate p values, regardless of the shape and size of the study population. The statistical calculations were performed with Stata® 9.1 software (StataCorp LP, College Station, TX).
Results
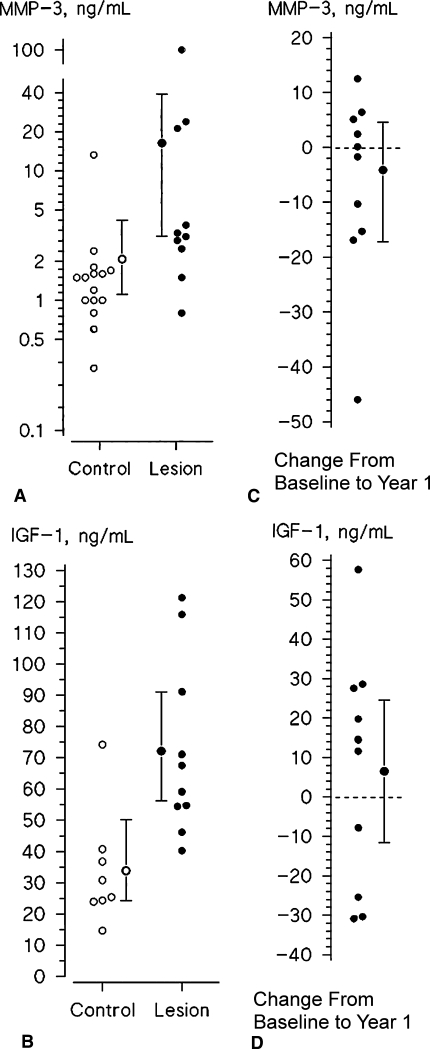
The synovial fluid analysis showed higher (p = 0.017) values of total MMP-3 (mean, 16.3 ng/mL; 95% CI, 3.4–39.0) (Fig. 1A) and IGF-I (mean, 72.1 ng/mL; 95% CI, 56.3–91.1; p = 0.0038) (Fig. 1B) in patients with cartilage lesions than in the control group with no cartilage lesions (MMP-3: mean, 2.1 ng/mL; 95% CI, 1.1–4.2; IGF-I: mean, 33.8 ng/mL; 95% CI, 24.2–50.1). The levels of active MMP-3 were very low or below the detection level and the values consisted almost solely of pro-MMP-3.
Fig. 1A–D.
The graphs show the values of (A) MMP-3 and (B) IGF-I in the synovial fluid of control subjects (open circles) and patients with cartilage lesions (closed circles); the means with 95% CIs also are shown. The changes of (C) MMP-3 and (D) IGF-1 from baseline during 1-year followup after ACT in individual patients are shown; the means with the 95% CIs also are shown. The data show persisting high levels of IGF-I and MMP-3 after ACT.
One year after ACT, the levels of MMP-3 (mean, 12.2 ng/mL; 95% CI, 6.7–27.8) (Fig. 1C) and IGF-I (mean, 78.6 ng/mL; 95% CI, 61.8–98.3; p = 0.5) (Fig. 1D) were similar (p = 0.6) compared with those at baseline. At 1 year, all 17 patients had cartilage lesions filled with ICRS Grades 9 to 12 or nearly normal to normal repair tissue, indicating successful cartilage resurfacing when evaluated by arthroscopy.
Discussion
The biochemical environment has major effects on the development and maturation of cartilage repair tissue. The substances secreted by the cells in the synovial lining into the synovial fluid affect the whole environment, including cartilage. However, degradation products from the cartilage can induce secretion of substances from synoviocytes and chondrocytes. We hypothesized levels of synovial fluid markers would be higher in patients with cartilage lesions than in patients with other knee complaints but no cartilage lesions. Second, we hypothesized the levels of synovial fluid markers would decrease after successful cartilage repair.
As a preliminary study, the number of patients in the study was limited and the control group was small. The control group did not consist of healthy volunteers, but patients coming for an arthroscopy, who did not have visible cartilage lesions during the arthroscopy. During arthroscopy, it is common to find asymptomatic cartilage lesions [1]. These lesions would have been unrecognized in any group of control subjects without arthroscopy; therefore, we chose patients undergoing arthroscopy as control subjects. It is still possible some cartilage lesions would not be observed during arthroscopy either because the area of the cartilage was not observed or because the changes were below the surface. Many of the knees (3 of 35) in this study produced a very small amount of synovial fluid and no sample was obtained from many patients (7 of 35) . Despite these limitations in the control group, we found a difference between patients with and without cartilage lesions. The cells were not removed from the samples; this can affect the actual values of MMP-3 and IGF-I, and therefore, direct comparisons with values obtained in other studies are not reliable. It is possible, for example, that any neutrophil infiltrate could produce elevated values of MMP-3. However, the values in the control group were actually lower than reported in normal control subjects (177 ng/mL) where the synovial fluid was centrifuged [30]. A similar procedure was used in all patients, and therefore, comparisons within our patient population can be made. This was a pilot study to find possible hypotheses for further research. Therefore, no power analysis was performed beforehand. Despite this, we found differences in MMP-3 and IGF-I concentrations between groups.
IGF-I levels are influenced by the amounts of IGF-binding proteins. We detected no interference of IGFBP-2, IGFBP-3, and IGFBP-4 with the analysis method of IGF-I used in this study. However, without the knowledge of IGFBP-3 and IGFBP-4 concentrations, conclusions regarding IGF-I concentrations should be made with caution [16]. Both study groups include only patients in whom an aspiration sample of synovial fluid was obtainable. This makes it impossible to make comparisons of the concentrations to concentrations from general populations.
The predominant forms of MMP-3 in the synovial fluid usually are the latent proenzymes [32]. We found the levels of active MMP-3 were very low or below the detection level and the values consisted almost solely of pro-MMP-3. Trauma induces prolonged elevation of MMP-3, which may lead to proteoglycan degradation. In chronic meniscus lesions or ligamentous knee injuries, synovial fluid levels of MMP-3, tissue inhibitor of metalloproteinase 1 (TIMP-1), and proteoglycan fragments are elevated. The levels of MMP-3 in rheumatoid arthritis are much higher, usually at least 10-fold, compared with that of patients with chronic injury or OA [30, 35]. There is a marked and transient elevation during the first weeks after the injury until a steady state of slightly elevated values is reached [5, 14, 29]. Bobacz et al. [2] reported observing elevated pro-MMP-3 levels early after trauma that decreased within 8 weeks. The proMMP-3 levels had no correlation with the extent of cartilage damage in their study, but the wide range of different diagnoses and times of arthroscopic evaluation might be a confounding factor. In the ACT group there were two patients with previous ACL reconstruction and one with a meniscal tear. Based on the current study in which many patients in the control group had meniscus or ACL tears, it seems that a large cartilage lesion has a more significant effect on the levels of MMP-3 than meniscus or ACL tears. Our observations extend the earlier findings by showing that MMP-3 levels remain high after treatment of large cartilage lesion with ACT.
This preliminary data supported our hypothesis because the catabolic (MMP-3) and anabolic (IGF-I) markers were higher in patients with cartilage lesions than in control subjects. However, the data did not support the second part of our hypothesis, because these values had not normalized at 1 year. Instead, there is a persistent state of elevated cartilage turnover 1 year after ACT. However, by arthroscopy, the lesions were macroscopically well healed according to the ICRS grading. This apparent discrepancy between the good arthroscopic results and the molecular markers of cartilage remodeling may suggest either early degradation or continuing remodeling of the repair tissue. Whether it is the chondrocytes or the synoviocytes that produce this effect is not known. Good long-term outcome of the patients after ACT suggests the increased turnover is not necessarily a sign of repair tissue degradation but represents remodeling [17, 20, 28]. Some catabolic activity might actually be essential for remodeling of the repair tissue. There is evidence from animal studies that the repair tissue histology improves with time [4, 22] and human biopsy specimens show newly synthesized collagen Type II [23]. In a previous study, we suggested the indentation stiffness of the ACT graft reached an average of 62% of the stiffness of the adjacent cartilage [31]. Delayed gadolinium-enhanced MRI, a quantitative MRI technique, suggests normal levels of glycosaminoglycans in the graft are reached between 12 and 24 months [10, 12]. It is possible thicker osteochondritis dissecans grafts take longer to mature, and in this study, the large number of osteochondritis dissecans lesions may partly explain the persisting high levels of MMP-3 and IGF-I. Schneider et al. [25] reported MMP-3 elevated 6 weeks after cartilage repair but returned to the level of the preoperative values at 52 weeks. There was no control group with normal values. Therefore, their MMP-3 results are in accordance with our results.
IGF-I enhances chondrocyte-mediated cartilage repair and matrix production and prevents chondrocyte apoptosis [8, 13, 28]. The upregulation of IGF-I in patients with cartilage lesions may reflect an attempt to repair the defect. After ACT, this upregulation may contribute to the production of extracellular matrix and remodeling. In osteoarthritic cartilage, the synthesis and expression of IGF-I are elevated and the levels in the synovial fluid also are elevated, but the chondrocyte response to IGF-I is decreased [6, 16]. In contrast to the osteoarthritic low-responder cells, the transplanted chondrocytes have been obtained from healthy-looking cartilage. It can be speculated these chondrocytes are responsive to IGF-I stimulation and therefore the elevation of IGF-I could be advantageous to the repair.
Whereas an increase in degradative enzymes normally has been considered detrimental, we suggest the possibility that persisting MMP-3 activity is important to repair tissue remodeling and cartilage turnover. This hypothesis needs to be verified by further studies. Synovial fluid MMP-3 and IGF-I levels remain elevated after ACT, suggesting ongoing graft remodeling. Longer followup is needed to determine if there is normalization of the elevated values of MMP-3 and IGF-I with time.
Acknowledgments
We thank Hannu Kautiainen, BA, from Rheumatism Foundation Hospital, Finland, for statistical analysis and Roeland Haanemaaijer, MD, PhD, TNO Prevention and Health, Leiden, The Netherlands, for expertise in MMP-3 measurements.
Footnotes
One or more of the authors (AIV, IK) have received funding from the Instrumentarium Science Foundation and Jyväskylä Central Hospital (Söderholm grant and grant B51).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Åroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. [DOI] [PubMed]
- 2.Bobacz K, Maier R, Fialka C, Ekhart H, Woloszczuk W, Geyer G, Erlacher L, Smolen J, Graninger WB. Is pro-matrix metalloproteinase-3 a marker for posttraumatic cartilage degradation? Osteoarthritis Cartilage. 2003;11:665–672. [DOI] [PubMed]
- 3.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed]
- 4.Brittberg M, Nilsson A, Lindahl A, Ohlsson L, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin OrthopRelat Res. 1996;326:270–283. [DOI] [PubMed]
- 5.Dahlberg L, Friden T, Roos H, Lark MW, Lohmander LS. A longitudinal study of cartilage matrix metabolism in patients with cruciate ligament rupture: synovial fluid concentrations of aggrecan fragments, stromelysin–1 and tissue inhibitor of metalloproteinase-1. Br J Rheumatol. 1994;33:1107–1111. [DOI] [PubMed]
- 6.Doré S, Pelletier JP, DiBattista JA, Tardif G, Brazeau P, Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation: possible role of IGF-1-binding proteins. Arthritis Rheum. 1994;37:253–263. [DOI] [PubMed]
- 7.Efron B, Tibshirani R. An Introduction to Bootstrap. New York, NY: Chapman and Hall; 1993.
- 8.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. [DOI] [PubMed]
- 9.Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430–441. [DOI] [PubMed]
- 10.Gillis A, Bashir A, McKeon B, Scheller A, Gray ML, Burstein D. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36:743–748. [DOI] [PubMed]
- 11.Hanemaaijer R, Visser H, Konttinen YT, Koolwijk P, Verheijen JH. A novel and simple immunocapture assay for determination of gelatinase-B (MMP-9) activities in biological fluids: saliva from patients with Sjogren’s syndrome contain increased latent and active gelatinase-B levels. Matrix Biol. 1998;17:657–665. [DOI] [PubMed]
- 12.Kurkijärvi JE, Mattila L, Ojala RO, Vasara AI, Jurvelin JS, Kiviranta I, Nieminen MT. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthritis Cartilage. 2007;15:372–378. [DOI] [PubMed]
- 13.Lo MY, Kim HT. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. J Orthop Res. 2004;22:140–144. [DOI] [PubMed]
- 14.Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res. 1994;12:21–28. [DOI] [PubMed]
- 15.Mankin HJ, Jennings LC, Treadwell BV, Trippel SB. Growth factors and articular cartilage. J Rheumatol Suppl. 1991;27:66–67. [PubMed]
- 16.Martel-Pelletier J, Di Battista JA, Lajeunesse D, Pelletier JP. IGF/IGFBP axis in cartilage and bone in osteoarthritis pathogenesis. Inflamm Res. 1998;47:90–100. [DOI] [PubMed]
- 17.Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;391(suppl):S349-S361. [DOI] [PubMed]
- 18.Murphy G, Knäuper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4(suppl 3):S39-S49. [DOI] [PMC free article] [PubMed]
- 19.Peterson L, Brittberg M, Kiviranta I, Åkerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. [DOI] [PubMed]
- 20.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin OrthopRelat Res. 2000;374:212–234. [DOI] [PubMed]
- 21.Plaas AHK, Sandy JD. Proteoglycan anabolism and catabolism in articular cartilage. In: Kuettner KE, Goldberg VM, eds. Osteoarthritic Disorders. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:103–116.
- 22.Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der Mark K, Bräuer R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage. 1998;6:50–65. [DOI] [PubMed]
- 23.Roberts S, Hollander AP, Caterson B, Menage J, Richardson JB. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis Rheum. 2001;44:2586–2598. [DOI] [PubMed]
- 24.Roughley PJ, Nguyen Q, Mort JS, Hughes CE, Caterson B. Proteolytic degradation in human articular cartilage: its relationship to stromelysin. Agents Actions Suppl. 1993;39:149–159. [DOI] [PubMed]
- 25.Schneider U, Schlegel U, Bauer S, Siebert CH. Molecular markers in the evaluation of autologous chondrocyte implantation. Arthroscopy. 2003;19:397–403. [DOI] [PubMed]
- 26.Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, Maroudas A. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173–188. [DOI] [PubMed]
- 27.Smith GD, Taylor J, Almqvist KF, Erggelet C, Knutsen G, Garcia Portabella M, Smith T, Richardson JB. Arthroscopic assessment of cartilage repair: a validation study of 2 scoring systems. Arthroscopy. 2005;21:1462–1467. [DOI] [PubMed]
- 28.Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, Robbins PD, Evans CH. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. [DOI] [PubMed]
- 29.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, DeGroot J. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–698. [DOI] [PMC free article] [PubMed]
- 30.Tchetverikov I, Ronday HK, Van El B, Kiers GH, Verzijl N, TeKoppele JM, Huizinga TW, DeGroot J, Hanemaaijer R. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–883. [DOI] [PMC free article] [PubMed]
- 31.Vasara AI, Nieminen MT, Jurvelin JS, Peterson L, Lindahl A, Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005;433:233–242. [DOI] [PubMed]
- 32.Walakovits LA, Moore VL, Bhardwaj N, Gallick GS, Lark MW. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992;35:35–42. [DOI] [PubMed]
- 33.Wang J, Elewaut D, Veys EM, Verbruggen G. Insulin-like growth factor 1-induced interleukin-1 receptor II overrides the activity of interleukin-1 and controls the homeostasis of the extracellular matrix of cartilage. Arthritis Rheum. 2003;48:1281–1291. [DOI] [PubMed]
- 34.Wu JJ, Lark MW, Chun LE, Eyre DR. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:5625–5628. [PubMed]
- 35.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. [DOI] [PMC free article] [PubMed]