Abstract
Tendon-to-bone healing occurs by formation of a fibrous, scar tissue interface rather than regeneration of a normal insertion. Because inflammatory cells such as macrophages lead to formation of fibrous scar tissue, we hypothesized immobilization would allow resolution of acute inflammation and result in improved tendon-bone healing. We reconstructed the ACL of 60 Sprague-Dawley rats using a tendon autograft. An external fixation device was used to immobilize the surgically treated knee in 30 rats. We evaluated tendon-bone interface width, collagen fiber continuity, and new osteoid formation histologically. Immunohistochemistry was used to localize ED1+ and ED2+ macrophages at the tendon-bone interface at 2 and 4 weeks. Biomechanical testing was performed at 4 weeks. Interface width was smaller and collagen fiber continuity was greater in the immobilized group. Immobilized animals exhibited fewer ED1+ macrophages at the healing interface at 2 and 4 weeks. In contrast, there were more ED2+ macrophages at the interface in the immobilized group at 2 weeks. Failure load and stiffness were similar between groups at 4 weeks. The data suggest early immobilization diminishes macrophage accumulation and may allow improved tendon-bone integration
Introduction
Despite generally favorable results with ACL reconstruction, instrumented testing of knee stability shows increased anterior laxity in 23% to 55% of patients at 3 years, which may lead to symptomatic instability [4]. Knee instability may contribute to the development of osteoarthritis in as many as 43% of patients [1, 6]. Current reconstruction techniques do not restore normal knee homeostasis and function. Although many factors determine the outcome of ACL reconstruction, including graft fixation, initial graft tension, graft material, and postoperative rehabilitation, one of the most important factors is graft healing to bone. The inability of current reconstruction techniques to restore normal knee function relates to impaired tendon-to-bone healing, and this presumption has driven our ongoing studies of tendon-to-bone healing.
The native ligament insertion site is a highly specialized transition zone that functions in the transmission of mechanical load from soft tissue to bone [30]. The normal insertion site consists of four distinct regions: ligament, unmineralized fibrocartilage, mineralized fibrocartilage, and bone [23–29]. Many experimental models show the complex structure and composition of the normal insertion site is not regenerated after ligament reconstruction [12, 15, 19, 23, 24, 26, 29]. Healing in these models occurs by formation of a fibrovascular scar tissue interface rather than reformation of a normal insertion site. This fibrous scar interface lacks the structural integrity of the native attachment site.
We found healing begins with an early influx of inflammatory cells followed by the formation of fibrovascular interface tissue between the tendon graft and bone [24–26]. As healing proceeds, there is gradual bone ingrowth into the tendon [23, 24, 26] and progressive establishment of collagen fiber continuity between tendon and bone followed by tissue remodeling and maturation [15, 17, 19]. We have identified two distinct subpopulations of macrophages that accumulate in the healing tendon-bone interface [19]. Macrophages expressing the ED1 antigen are recruited from circulating blood monocytes, accumulate in the first few days after surgery, and have a phagocytic function in débriding the wound environment, whereas macrophages expressing the ED2 antigen are derived from the local tissue environment, have maximum accumulation by 28 days, and have an anabolic role in tissue healing [22]. One of the principal functions of macrophages is secretion of growth factors and cytokines responsible for fibroblast mitogenesis and proliferation [5, 8, 13, 31], extracellular matrix and collagen synthesis [13, 22], and angiogenesis [8, 11, 13]. However, excessive levels of cytokines such as transforming growth factor β may result in excessive scar formation at the healing tendon-bone interface.
Mechanical stimulation affects healing of bone, ligament, and tendon [20]. However, whether such stimulation influences a healing tendon-bone interface is not well established. Prior study in our laboratory showed graft-tunnel motion delays tendon-to-bone healing [25]. Our findings suggest tendon-to-bone healing may be enhanced by minimizing graft-tunnel motion after tendon transplantation into a bone tunnel. We presume graft-tunnel motion causes sustained inflammation resulting from repetitive microinjury at the healing interface, preventing or delaying tendon-to-bone healing. A period of postoperative immobilization may be required to improve tendon-to-bone healing.
We therefore tested the following hypotheses: (1) immobilization after tendon-to-bone repair allows resolution of acute inflammation with reduced accumulation of phagocytic (ED1) macrophages; (2) immobilization results in a narrower scar tissue interface and greater collagen fiber ingrowth between tendon and bone; and (3) immobilization provides greater graft failure strength.
Materials and Methods
We reconstructed the ACL of 60 male Sprague-Dawley rats (weight, 275–300 g) using a flexor digitorum longus tendon graft. We used a rigid external fixation device to immobilize the surgically treated knee in 30 rats. The other 30 rats had normal cage activity postoperatively and formed the control group. We euthanized 10 animals in each group 2 and 4 weeks after surgery and prepared them for histologic analysis. Ten animals in each group were euthanized 4 weeks postoperatively and were used for biomechanical testing. Before this study, a power analysis was performed. The primary outcome was biomechanical testing of failure strength. We considered an increase in strength of 20% would be clinically important. Using these estimations, a power of 0.80 is achieved using 10 specimens per group with α = 0.05 for biomechanical testing. The power calculation was performed using the SigmaStat® program (Systat Software Inc, San Jose, CA). The experimental protocol was approved by the Institutional Animal Care and Use Committee at our institution.
The rats were kept in soft-bedding cages with free access to food and water. We anesthetized the animals with a mixture of 80 mg/kg ketamine hydrochloride and 5 mg/kg xylazine administered intraperitoneally through a 25-gauge needle. Ampicillin (25 mg/kg subcutaneously) was used for antibiotic prophylaxis. We made a longitudinal incision on the medial aspect of the distal leg and ankle. The flexor digitorum longus tendon was identified and then cut just distal to the ankle. We harvested the full length of the flexor digitorum longus tendon (average length, 20 mm). A second incision was made over the knee and medial parapatellar arthrotomy was performed. We excised the native ACL. Using an 18-gauge needle (outer diameter, 1.27 mm), a bone tunnel was made in the proximal tibia and the distal femur entering the joint at the attachment sties of the ACL. We passed a 4–0 Ethibond® suture (Ethicon, Inc, Somerville, NJ) through each end of the previously harvested tendon graft and then the graft was passed through the bone tunnels to replace the ACL. The proximal end of the grafted tendon was secured to the proximal attachment of the lateral collateral ligament on the femur and the distal end of the grafted tendon was secured to the distal attachment of the medial collateral ligament on the tibia using 4–0 Ethibond® suture. The wounds were closed in standard fashion. We did not see any negative effects of the autologous graft harvest other than the expected swelling, and the animals resumed normal gait by 2 to 3 days after surgery.
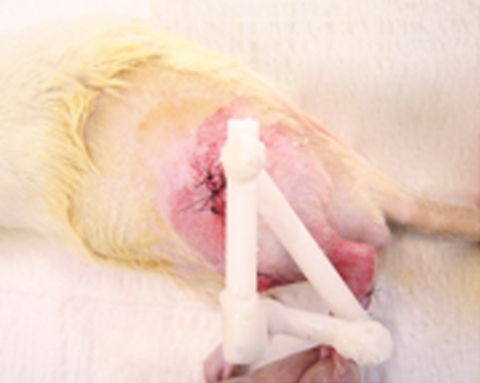
We used an external fixation device in 30 animals to immobilize the surgically treated knee. Three Kirschner wires were placed on the operated limb: one at the proximal femur, one at the proximal tibia, and one at the distal tibia. We connected these wires together by using the plastic covers of 18-gauge spinal needles (Fig. 1). Three plastic covers fixed with bone cement were used to immobilize the knee at 90º flexion. The remaining 30 animals were allowed ad libitum activity postoperatively. We sacrificed the animals by carbon dioxide inhalation at 2 and 4 weeks postoperatively. Their knees were dissected and used for histologic or biomechanical analysis.
Fig. 1.
A photograph shows placement of the external fixator on the immobilized animals.
In each group, we used 20 rats for histologic analysis. Ten of these rats were sacrificed at 2 weeks and the other 10 at 4 weeks postoperatively. At each time, five specimens were decalcified and embedded in paraffin for hematoxylin and eosin staining or immunohistochemistry. The five remaining specimens from each time were undecalcified and embedded in methacrylate for Goldner’s staining.
Harvested tissues used for decalcified histology were fixed for 3 days in 10% neutral buffered formalin at 4ºC and then decalcified in 10% ethylenediamine tetraacetic acid for 24 hours at 4ºC. We cut perpendicular sections to the bone tunnels in the tibia and the femur. The tissues then were dehydrated in serial alcohol concentrations (70%–100%) and cleared in three changes of xylene. We embedded the tissues in paraffin at 60ºC. Five-micrometer-thick sections were cut perpendicular to the bone tunnels and stained with hematoxylin and eosin. We analyzed sections from the midpoint of the bone tunnels (midway between the intraarticular entrance and the extraarticular exit of the tunnels).
We treated alternate sections used for immunohistochemistry with 3% H2O2 to quench endogenous peroxidase activity and nonspecific antibody binding was blocked with 5% goat serum. Each primary antibody was applied to separate serial sections for 60 minutes at 37ºC. Bound antibodies were observed using a goat avidin-biotin peroxidase system with 3,3′-diaminobenzidine (Dako Corp, Carpinteria, CA) as a substrate. We used mouse anti-rat ED1 macrophage and anti-rat ED2 macrophage antibodies (Serotec Inc, Raleigh, NC). The sections were counterstained with Mayer’s hematoxylin. We processed negative controls in an identical manner except for incubation with bovine serum albumin rather than the primary antibody.
We dehydrated tissues used for undecalcified histology in serial alcohol concentrations (70%–100%). Sections containing the full length of the grafted tendon and bone tunnel were cut in the coronal plane in the tibia and the femur and then embedded in methacrylate. Five-micrometer-thick sections were cut in the same plane and used for Goldner’s staining.
Microscope images, captured on a personal computer using a light microscope (Nikon Optiphot; Nikon USA, Melville, NY) connected to a CCD camera (Nikon DXM1200; Nikon USA), were used to evaluate the healing tendon-bone interface. We analyzed sections from the midpoint of the bone tunnels from the coronal plane sections (these sections contained the full length of the grafted tendon and bone tunnel). We (ED, PLH) used the ImageJ (NIH, Bethesda, MD) computerized image analysis program to measure the following parameters: (1) the tendon-bone interface width using the hematoxylin and eosin slides; (2) the ED1+ and ED2+ macrophage count using immunohistochemistry slides; (3) new osteoid formation at the interface using the Goldner’s-stained slides; and (4) collagen fiber continuity between grafted tendon and bone (measured as birefringent area per high-power field [hpf]) using polarized light microscopy. The widths of the tendon-bone interface and new osteoid formation were measured at 10 randomly selected areas along the tunnel, and three sequential sections of each specimen were examined to reduce sampling error. All histomorphometric measurements were made by consensus of the two viewers and were performed in a blinded fashion.
To evaluate the organization of collagenous tissue, picrosirius red-stained sections were illuminated with monochromatic polarized light at ×320 magnification. To control for variations in specimen orientation on the slide, measurements were obtained by rotating the polarization plane until we observed maximum brightness. To facilitate comparisons between groups, all tissues were embedded and cut to a uniform thickness. After the picture of the slide had been captured, it underwent 8-bit digitization with ImageJ software with a resolution of 640 (horizontal) by 480 (vertical) pixels. Three or four 50-μm × 50-μm (2500-μm2) areas were randomly selected along the tendon-bone interface of each section. Using the ImageJ software, individual collagen bundles in these random fields were free-hand outlined, and the total area was recorded as mm2/hpf. The slides for all specimens were analyzed during one sitting under the same light intensity to ensure consistent analysis conditions.
The limbs used for biomechanical testing were disarticulated at the hip. We removed all the muscles leaving the joint capsule and the ligaments intact to protect the ACL graft until testing. The bones were potted in bonding cement at the proximal femur and the distal tibia to allow secure fixation in the testing machine, but the graft ends were left free of cement. We stored the specimens in a freezer at −80°C until testing. Each limb was defrosted overnight at 4°C and thawed at room temperature the day of testing. We removed the joint capsule, patellar tendon, collateral ligaments, posterior cruciate ligament, and the suture material at the graft ends just before positioning the specimen on the testing machine. Nothing but the graft was left between the femur and the tibia.
We positioned the specimens on a device especially designed for rat knees. It allows knee flexion of 45° with the tensile load passing parallel to the ACL graft and the bone tunnels. The specimens were loaded on a materials testing machine (MTS Systems Corp, Eden Prairie, MN). First, we applied a preload of 15 g to the graft for five cycles and then the tensile load to failure test was performed at a displacement rate of 0.17 mm/second. The load-deformation curve was recorded on a personal computer. The primary site of failure was recorded as pullout from the femoral or tibial tunnel or midsubstance graft rupture. We recorded ultimate load-to-failure (N) and stiffness (N/mm) was calculated from the linear portion of the load-deformation curve using SigmaPlot® 8.0 (SPSS Inc, Chicago, IL).
All data are presented as mean ± standard deviation. The independent variable was the postoperative treatment (immobilized or not). We compared the histologic measures (macrophage numbers, width of fibrous interface tissue and newly formed osteoid, collagen fiber continuity), and biomechanical measures (failure strength, stiffness) between the two groups using Wilcoxon rank sum test with nonnormally distributed data and Student’s t test with normally distributed data (SigmaPlot® 8.0).
Results
The external fixator was well tolerated by immobilized animals. All animals resumed normal cage activity by the first postoperative day. Careful inspection of the joints at time of euthanasia showed all grafts were intact. The articular cartilage surfaces appeared normal with no evidence of advanced joint degeneration.
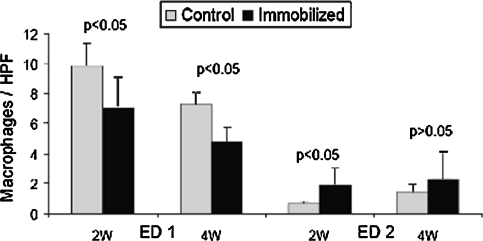
Compared to control specimens, immobilized animals exhibited fewer ED1+ macrophages at the tendon-bone interface at 2 weeks (7.1 ± 2.1 versus 9.9 ± 1.5 m/hpf, respectively (p = 0.046)) and 4 weeks (4.9 ± 0.9 versus 7.3 ± 0.8 m/hpf, respectively (p = 0.003); Figs. 2, 3). In contrast, there were more ED2+ macrophages at the interface (p = 0.033) in the immobilized group 2 weeks postoperatively compared to controls (1.9 ± 1.1 versus 0.7 ± 0.2 m/hpf, respectively; Fig. 3). There were no differences between groups in ED2+ macrophage counts at 4 weeks (2.3 ± 1.8 versus 1.5 ± 0.5 m/hpf, respectively; Fig. 3).
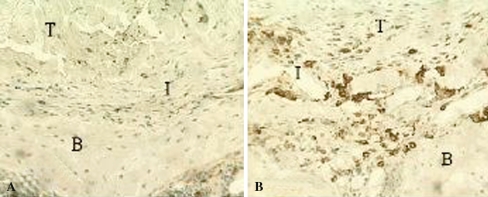
Fig. 2A–B.
There were fewer (p < 0.05) ED1+ macrophages at the tendon-bone interface in (A) the immobilized specimens compared with (B) control specimens at 2 and 4 weeks after ACL reconstruction (Stain, immunostain for ED1 antigen; original magnification, ×160). T = tendon; I = interface; B = bone.
Fig. 3.
Immobilized specimens exhibited fewer (p < 0.05) ED1+ macrophages than controls at the tendon-bone interface at both 2 weeks and 4 weeks. In contrast, there were more (p < 0.05) ED2+ macrophages at the interface in the immobilized group 2 weeks postoperatively, but no differences between groups in ED2+ macrophage counts at 4 weeks. Data presented as mean ± standard deviation.
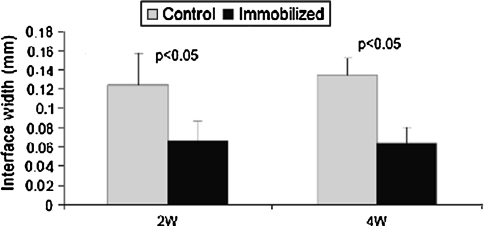
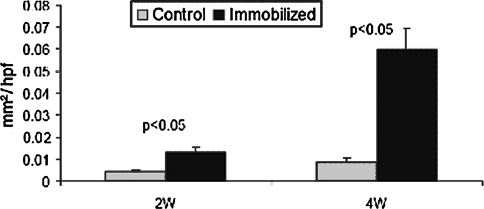
We observed highly cellular fibrovascular granulation tissue at the healing tendon-bone interface. The width of this interface was smaller in the immobilized group when compared with the control group at 2 weeks (0.07 ± 0.02 mm versus 0.12 ± 0.03 mm, respectively (p = 0.021)) and 4 weeks postoperatively (0.06 ± 0.02 mm versus 0.14 ± 0.02 mm, respectively (p = 0.001); Figs. 4, 5). There were no differences in new osteoid formation at the tendon-bone interface between the experimental and control groups (4.1 versus 5.4 μm, respectively, at 2 weeks and 4.0 versus 3.6 μm, respectively, at 4 weeks). Collagen fiber continuity between grafted tendon and bone increased over time and was greater among the immobilized groups at 2 and 4 weeks postoperatively when compared with control specimens (0.014 ± 0.002 versus 0.005 ± 0.0005 mm2/hpf, respectively (p = 0.0003), at 2 weeks and 0.06 ± 0.009 versus 0.009 ± 0.002 mm2/hpf, respectively (p < 0.0001), at 4 weeks; Fig. 6).
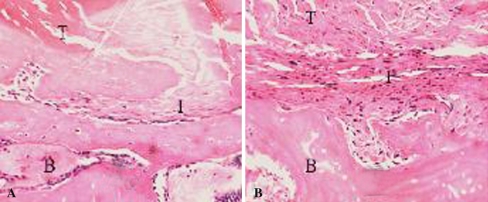
Fig. 4A–B.
Tendon-bone interface width was smaller among (A) immobilized specimens compared with (B) control specimens 2 weeks after surgery (Stain, hematoxylin and eosin; original magnification, ×160). T = tendon; I = interface; B = bone.
Fig. 5.
Tendon-bone interface width was smaller (p < 0.05) in the immobilized group when compared with the control group at 2 and 4 weeks postoperatively. Data presented as mean ± standard deviation.
Fig. 6.
Collagen fiber continuity between grafted tendon and bone increased with time and was greater (p < 0.05) in the immobilized groups at 2 and 4 weeks postoperatively when compared with the control group. Data presented as mean ± standard deviation.
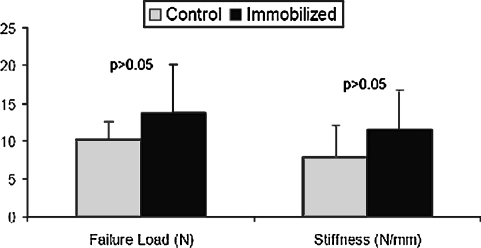
We observed no difference in failure load or stiffness of the ACL grafts in the immobilized knees compared with the controls 4 weeks postoperatively (failure load: 13.7 ± 6.5 versus 11.0 ± 2.4 N, respectively; stiffness: 11.6 ± 5.3 versus 7.9 ± 4.2 N/mm, respectively; Fig. 7).
Fig. 7.
Failure load and stiffness of the femur-ACL graft-tibia constructs were similar between immobilized specimens and control specimens at 4 weeks. Data presented as mean ± standard deviation.
Discussion
Previous studies have shown graft-tunnel motion delays tendon-to-bone healing [25, 27, 30]. We therefore tested the hypothesis that immobilization after tendon-to-bone repair leads to diminished macrophage accumulation and improved tendon-to-bone healing based on structural and biomechanical measures.
Our study has several limitations. First, we evaluated animals only to 28 days after surgery. Longer times would allow evaluation of the histologic and biomechanical differences with time. However, our goal was to study the early inflammatory response. Additional study is required to examine the influence of prolonged immobilization on late remodeling. Second, additional histologic analyses would help better characterize the inflammatory healing process by localizing other inflammatory cells, including neutrophils and lymphocytes. Third, although all graft-tunnel motion was eliminated in our model, there was likely static tension on the ACL graft, which may induce different molecular signals from those induced by a completely unloaded tendon graft. Fourth, the load applied to the healing tendon-bone interface was uncontrolled in animals allowed cage activity. We feel these limitations do not jeopardize our conclusions as the different postoperative regimens (immobilization with the external fixator versus cage activity) likely resulted in differences in mechanical load on the healing tendon-bone interface between groups. Finally, despite our power analysis, our study may be underpowered to detect a difference in the biomechanical outcomes.
Our previous work with this rat model of ACL reconstruction characterized the inflammatory cell infiltrate present during the early healing period and suggested an important role of macrophages in the early healing process between tendon and bone [19]. Because studies by us [17, 19, 24–26] and others [15, 23] have shown a scar tissue interface forms between tendon and bone rather than reformation of a normal insertion site, we therefore hypothesized the presence of abundant macrophages plays an important role in this scar formation. In other experimental models, the presence of macrophages and overexpression of transforming growth factor-β has been linked to states of chronic inflammation and tissue fibrosis [5, 7, 8, 16, 18, 21, 28]. In contrast, embryonic wounds heal in the absence of an inflammatory cell infiltrate with recapitulation of normal, scar-less tissue morphology. Studies of macrophage depletion have shown major reductions in the degree of fibrosis, and accelerated tissue healing [7, 9, 10, 31]. Hays et al. recently reported macrophage depletion resulted in less fibrous tissue formation and accelerated reestablishment of collagen fiber continuity, new bone formation, and improved biomechanical properties in the same ACL reconstruction model [17]. The results of our current study confirm those in macrophage-depleted rats [17] and support the suggestion that reducing excessive inflammation may result in more effective healing.
In this study, the independent variable was mechanical load on the healing tendon-bone interface attributable to knee motion. Few studies have examined the effect of mechanical stimulation on tendon-to-bone healing and there is little evidence to guide postoperative rehabilitation recommendations after knee ligament reconstruction. Immobilization reduces the ultimate load and energy-absorbing capabilities of the native bone-ligament complex with concomitant bone resorption at the ligament insertion site [2]. In contrast, rotator cuff tendon healing to bone was impaired by early exercise and the best healing occurred in rats immobilized postoperatively [30]. Similarly, postoperative immobilization of the limb in a rabbit model of ACL reconstruction resulted in improved healing and graft attachment strength compared with animals allowed normal cage activity postoperatively [27]. Prior work from our laboratory found graft-tunnel motion seems to impair early graft incorporation [25]; ingrowth of new bone around the tendon graft was inversely proportional to the magnitude of graft-tunnel motion with slower ingrowth and a wider tendon-bone interface at the tunnel aperture where graft-tunnel motion is highest. Our finding of improved collagen fiber ingrowth in the immobilized animals is consistent with these prior studies.
We evaluated the effect of mechanical load on accumulation of macrophages. ED1+ and ED2+ macrophages were quantified because they play a major role in early healing and reflect the inflammatory healing process. ED1+ macrophages are derived from circulation and play a phagocytic role after tissue injury [9], whereas ED2+ macrophages are derived from resident cells and reportedly have an anabolic role in tendon healing [22]. Although both are macrophage subtypes, they have disparate functions owing to differences in cytokine signaling and responsiveness to cytokines and other factors in the local wound environment, among other differences [9, 10]. Our findings support our hypothesis that immobilization leads to reduced macrophage accumulation and improved healing at the tendon-bone interface. These results confirm those in macrophage-depleted rats [17] and support the hypothesis that reducing excessive inflammation may result in more effective healing.
Despite improved collagen fiber continuity in the immobilization group, the mechanical properties of the grafted ACL were not improved 4 weeks postoperatively. Similar findings were reported in a rotator cuff repair model in which immobilization improved the collagen organization at 4 weeks, but 16 weeks was necessary to improve the mechanical properties [14]. Although a longer period of immobilization may be required to improve the failure strength, the structural properties of the bone-graft-bone construct are determined by a complex interaction between the strength of the graft-bone interface and the graft. Stress deprivation secondary to prolonged immobilization may result in weakening of the intraarticular part of the graft [3] and also can result in stiffness and detrimental effects on bone and articular cartilage.
Our data suggest early postoperative immobilization diminishes macrophage accumulation and may allow resolution of acute inflammation. These findings were associated with decreased fibrous scar tissue in the healing tendon-bone interface and improved collagen fiber ingrowth into the tendon graft. Despite improved collagen fiber continuity in the immobilization group, the mechanical properties of the grafted ACL were not improved. These findings suggest immobilization alone does not provide the appropriate signals to improve the structural properties of the healing graft-bone interface. These findings have generated the hypothesis that healing can be improved by controlled mechanical loading after an initial period of immobilization (delayed mobilization) to allow resolution of acute inflammation.
Acknowledgments
We thank Liang Ying for preparation of the tissues for histologic analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents: a prospective review. J Bone Joint Surg Br. 2002;84:38–41. [DOI] [PubMed]
- 2.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop Relat Res. 1987;219:28–37. [PubMed]
- 3.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in-vitro experimental study. Am J Sports Med. 2007;35:763–769. [DOI] [PubMed]
- 4.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renström P. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts: a prospective, randomized study. J Bone Joint Surg Am. 2002;84:1503–1513. [DOI] [PubMed]
- 5.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. [DOI] [PMC free article] [PubMed]
- 6.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH. The natural history of the anterior cruciate ligament-deficient knee: changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. [DOI] [PubMed]
- 7.Choi BM, Kwak HJ, Jun CD, Park SD, Kim KY, Kim HR, Chung HT. Control of scarring in adult wounds using antisense transforming growth factor-beta 1 oligodeoxynucleotides. Immunol Cell Biol. 1996;74:144–150. [DOI] [PubMed]
- 8.Connors D, Gies D, Lin H, Gruskin E, Mustoe TA, Tawil NJ. Increase in wound breaking strength in rats in the presence of positively charged dextran beads correlates with an increase in endogenous transforming growth factor-beta1 and its receptor TGF-betaRI in close proximity to the wound. Wound Repair Regen. 2000;8:292–303. [DOI] [PubMed]
- 9.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond). 2003;104:27–38. [DOI] [PubMed]
- 10.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. [DOI] [PMC free article] [PubMed]
- 11.Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–1988. [PubMed]
- 12.Evans C. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999;28:71–76. [DOI] [PubMed]
- 13.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. [DOI] [PubMed]
- 14.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–404. [DOI] [PubMed]
- 15.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–351. [DOI] [PubMed]
- 16.Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Dev Dyn. 2003;226:268–279. [DOI] [PubMed]
- 17.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, Rodeo SA. The role of macrophages in early healing of a tendon graft in a bone tunnel: an experimental study in a rat anterior cruciate ligament reconstruction model. J Bone Joint Surg Am. 2008;90:565–579. [DOI] [PubMed]
- 18.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159–1167. [DOI] [PubMed]
- 19.Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425–1432. [DOI] [PubMed]
- 20.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. [DOI] [PubMed]
- 21.Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg. 1995;222:146–154. [DOI] [PMC free article] [PubMed]
- 22.Marsolais D, Cote CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001;19:1203–1209. [DOI] [PubMed]
- 23.Panni AS, Milano G, Lucania L, Fabbriciani C. Graft healing after anterior cruciate ligament reconstruction in rabbits. Clin Orthop Relat Res. 1997;343:203–212. [DOI] [PubMed]
- 24.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. [DOI] [PubMed]
- 25.Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34:1790–1800. [DOI] [PubMed]
- 26.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. [DOI] [PubMed]
- 27.Sakai H, Fukui N, Kawakami A, Kurosawa H. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci. 2000;5:43–51. [DOI] [PubMed]
- 28.Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213–214. [DOI] [PubMed]
- 29.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed]
- 30.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. [DOI] [PubMed]
- 31.Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004;39:878–888. [DOI] [PubMed]